Glycemic Index of Commonly Consumed Foods in Benin (West Africa)
Carmelle Mizéhoun-Adissoda1,2*, Halimatou Alaofè3, Jules Gninkoun4, Berenice Nicoué1, Achille Yémoa2, Bonaventure Awèdé5
1School of Nutrition and Dietetics, Faculty of Health Sciences, University of Abomey-Calavi (UAC), Cotonou, Benin
2Unit of Training and Research in Pharmacy, Faculty of Health Sciences/ UAC, Cotonou, Benin.
3Health Promotion Sciences Department, Mel & Enid Zuckerman College of Public Health University of Arizona, USA
4Faculty of Health Sciences, University of Abomey-Calavi (UAC), Cotonou, Benin
5Department of human physiology. Faculty of Health Sciences/ UAC, Cotonou, Benin
*Corresponding Author: Carmelle Mizéhoun-Adissoda, School of Nutrition and Dietetics, Faculty of Health Sciences, University of Abomey-Calavi (UAC), Cotonou, Benin
Received: 24 August 2023; Accepted: 04 September 2023; Published: 30 October 2023
Article Information
Citation: Carmelle Mizéhoun-Adissoda, Halimatou Alaofè, Jules Gninkoun, Berenice Nicoué, Achille Yémoa, Bonaventure Awèdé. Glycemic Index of Commonly Consumed Foods in Benin (West Africa). Journal of Food Science and Nutrition Research. 6 (2023): 171-178.
DOI: 10.26502/jfsnr.2642-110000143
View / Download Pdf Share at FacebookAbstract
Background: Consistent consumption of a diet with a high glycemic index (GI) increases the risk of type 2 diabetes and associated chronic diseases. As such, it is critical to know the GI of foods and make informed choices to prevent them. Unfortunately, the GI of commonly consumed foods in Benin has not been determined.
Objective: To determine the GI of six commonly consumed foods in Benin among healthy young adult subjects.
Methods: This study involved 18 healthy adult students from the Faculty of Health Sciences in Cotonou, southern Benin. Six local foods, namely local white rice, imported white rice, recycled corn paste, fried potatoes, corn paste, and red corn paste, were tested and compared to bread (reference food). The subjects' sociodemographic and anthropometric information was collected. Blood glucose levels were also measured 15, 30, 45, 60, 90, and 120 min after ingestion through venous blood samples. The GI was determined using the standard method.
Results: The study population's mean age and body mass index were 22.1 years and 21.3 kg/m2, respectively. The GI of the selected foods: local white rice, imported white rice, recycled corn paste, fried potatoes, corn paste, and red corn paste were 148.4%, 136.5%, 94.3%, 89.9%, 85.3%, and 77.8%, respectively.
Conclusion: All foods tested had a GI of over 70%, which is high, indicating that moderate consumption or small portions should be recommended to minimize their potential adverse health impacts.
Keywords
<p>Glycemic index, Area under the curve, Corn paste, Rice, Fries, Benin</p>
Article Details
Abbreviations used:
BS: Blood sugar
BMI: Body mass index
GI: Glycemic index
T2D: Type 2 diabetes
SSA: Sub-Saharan Africa
NCDs: Non-Communicable Diseases
WHO: World Health Organization
Introduction
Although type 2 diabetes (T2D) was once uncommon in sub-Saharan Africa (SSA), it has emerged as a significant public health issue [1,2]. According to the International Diabetes Federation (IDF), the number of adults with T2D worldwide is expected to rise by 51% from 463 million in 2019 to 700.2 million in 2045, with the African region experiencing the most significant increase (143%) from 19.4 million (2019) to 47.1 million (2045) [3]. As SSA undergoes a rapid and diverse change in disease burden profiles from infectious to non-communicable diseases (NCDs), T2D substantially burdens the region's healthcare systems, societies, and individuals. This shift is attributed to urbanization, which includes increased longevity, lifestyle, dietary changes, and economic development [4]. Therefore, addressing T2D’s high morbidity and mortality rates is crucial to alleviate the burden on the region [5,6].
When managing T2D, it is vital to modify lifestyle by implementing dietary changes [7,8]. It is crucial to consume foods rich in high-quality nutrients such as carbohydrates, proteins, fats, minerals, and vitamins for optimal health. Low-GI foods, such as legumes, lentils, and oats, contain slowly digesting carbohydrates that have a minimal impact on blood glucose and insulin levels. In contrast, high-GI foods like white bread can raise blood glucose and insulin levels rapidly due to their quick digestion. Therefore, consuming low-GI foods can promote insulin sensitivity, minimize fluctuations, and improve glycemic control compared to high-GI foods [9,10]. Recent studies also found that a low-carbohydrate, high-fat, calorie-unrestricted diet led to better weight loss and glucose control over a 6-month intervention compared to a high-carb, low-fat diet [11]. As such, incorporating a low-glycemic index diet may help manage T2D and improve glycemic control.
However, using food GI as a guide in selecting foods for diabetes patients has sparked inconsistencies and controversies. Research into low-GI diet effects on health and related outcomes has yielded mixed results. Short-term studies have shown that a low-GI diet can improve glucose control in patients with type 2 diabetes, as demonstrated by Jung and Choi [12] however, the long-term effects of low-GI diets remain uncertain. Thomas and Elliott's review supports this view, stating that while low-GI diets can have small but valuable effects on medium-term glycemic control in diabetes, they only offer modest secondary benefits [13]. Additionally, some studies suggest that high-GI diets may lead to poorer short-term metabolic outcomes, increased hunger, food intake, and decreased satiety. However, other studies have not found the same association or an inverse relationship [9,14].
Furthermore, the relevance of dietary GI is still debated, and having a reliable GI table is crucial to resolving this issue. The recent update includes over 4,000 items, a 61% increase compared to the 2008 edition. These values come from verified sources, both published and unpublished. However, the vast majority of published GI values are of Western origin, mainly European, Australian, and North American. African foods are limited, with 56 entries [15]. African foods differ significantly from Western foods, as they are locally grown and processed. Therefore, more research is needed to determine the GI of commonly consumed African foods to enable GI and other dietary recommendations in the region's treatment, management, and prevention of T2D. Relying on international GI tables or consulting experts to assess local foods' impact is unreliable. This study aims to determine the GI values of six commonly consumed foods in Benin, West Africa, to supplement the GI database for traditional African foods. This data will help design diabetes diets and enhance metabolic functioning in individuals with metabolic syndrome, potentially reducing T2D occurrence in the general population.
Materials and methods
2.1 Study setting and design
This prospective and descriptive research study was conducted from November 2016 to January 2017 in the Nutrition Laboratory at Faculty of Health Sciences, University of Abomey-Calavi, Benin.
2.2. Study population and sampling
The study involved students from the Faculty of Health Sciences over 18 years old. It included healthy individuals with a fasting blood glucose level below 1 g/l and non-diabetic. Additionally, they needed to be reachable by phone during the study, not undergoing hormonal treatment, and refrain from drinking alcohol the day before the test. All participants provided informed consent. To determine the glycemic index (GI), the World Health Organization (WHO) recommends a minimum sample size of six healthy volunteers of any gender [16]. For our study, we enrolled 18 healthy student volunteers who were available during the test period and willingly agreed to participate.
2.3. Preparation of foods
Dry food samples were purchased from Dantokpa Market in Cotonou, Southern Benin. The tested foods included corn paste, recycled corn paste, red corn paste (consisting of corn flour and tomato sauce), imported white rice, local white rice, and fried potato. The quantities tested were 180g, 180g, 180g, 213g, 166g, and 226g, respectively. Vegetables soups and tomato stews were served with the tested food to reflect local consumption habits. The amount of food consumed for each recipe was calculated using the West African food composition table [17] and the Beninese food guide helped determine the appropriate food servings [18]. Table 1 outlines the processing and preparation of the tested foods.
* Corn paste prepared the day before and warmed the next day with a harder consistency than freshly prepared corn paste.
** Stew= tomato sauce obtained by coarsely slicing tomatoes, onion and green pepper then seasoning.
Table 1: Processing and preparation of tested foods
Data collection
A standardized questionnaire containing information on age, sex and socio-demographic characteristics was used. The participants had their weight and height taken using a scale and a stadiometer without shoes or heavy objects. These measurements were averaged for analysis. After the weights and heights were checked, participants were asked about their last meal to ensure they fasted for 10-14 hours before testing. Venous blood was drawn from each participant to measure their blood sugar (BS) using a glucometer. Following this,100g of white bread (reference food), containing 50g of carbohydrate was given to each participant. The stop watches were started when subjects started to eat white bread. The time each participant began to eat the bread was recorded. Fifteen (15) min after consuming the reference food, participants had their blood glucose levels tested. Samples were subsequently taken from all subjects at the 15th, 30th, 45th, 60th, 90th, and 120th min intervals to check their glucose concentration in mmol/L. This process continued for two hours. Once completed, the BS of each respondent was measured, followed by an estimated intake of test foods containing a 50g available carbohydrate portion. The nutritional values of the tested foods are shown in table 2. The investigation team consisted of a nutritionist for anthropometric measurements, laboratory technicians for blood samples, and a cook for the preparation of dishes to be ingested.
|
Foods |
Values for a serving of 100g |
||
|
Energy (kcal) |
Carbohydrates (g) |
Fibers (g) |
|
|
Corn paste |
368.8 |
65.2 |
4.3 |
|
Recycled corn paste |
368.8 |
65.2 |
4.3 |
|
Red paste |
355.8 |
42.7 |
3.3 |
|
Imported white rice |
139 |
25.2 |
1.7 |
|
Local white rice |
529.7 |
57.9 |
2.8 |
|
Fried potato |
161.7 |
20.6 |
2.5 |
Table 2: Nutritional values of tested foods
Anthropometric measurements
The weight of the subjects was measured with a SECA® mechanical scale with a capacity of 120 kg of 0.1 kg precision. The height was measured using a SECA® portable measuring rod with a 2-meter capacity and centimeter graduations. The body mass index (BMI) was calculated as weight in kg divided by squared height in meter and compared with the WHO scale [16].
Determination of blood glucose
To measure blood glucose levels, an enzyme method was used with glucose oxidase and peroxidase. A spectrophotometer at a wavelength of 520 nanometers was used to take readings. The process involved collecting 100 μl of reagent (glucose kit) and adding it to test tubes with an anticoagulant. The number of test tubes needed equals the number of samples plus three additional tubes for the reagent white, the standard, and the control serum. After centrifuging the samples at 300 nm for 15 min, 10 μl of serum from each sample was added to the corresponding test tube.
Glycemic index calculations
Changes in blood glucose concentration were calculated separately for each post meal period by using the blood concentration before meal (time 0) as a baseline. Postprandial responses were compared for maximum increase and incremental area under the glucose curves for each food. The integrated area under the postprandial glucose curve was calculated by the trapezoidal method [19]. Area increments under the curves for a given food were determined for the 2-hour period after the meal. The relative glycemic index of each food was calculated as percent of the mean of individual areas under the glucose response curves [19] using the following formula:
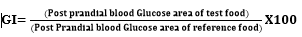
Foods were classified as low, medium, or high GI according to the following: GI values ≤ 55, Low GI; GI of 56-69, Medium and GI ≥ 70, High GI [20].
Statistical analysis
Data were entered in Microsoft Excel 2022 and the softwares MATLAB [21] and AIRES [22] were used to perform data analysis and processing. The findings were presented as ratios, mean values ± standard deviation, and medians. The student’s t test helped to compare the mean values of incremental areas under the curve calculated for each food and reference food. The analysis of variance (ANOVA) permitted to compare the calculated mean values of incremental areas under the curve, and the GIs and loads of the four fruits. Difference was significant if p < 0.05.
Ethical considerations
The study's research protocol received approval from the Local Ethics Committee of the Faculty of Health Sciences in Cotonou. The researchers also ensured that participants were fully informed about the study's aims and procedures and obtained written informed consent from all participants before enrollment.
Results
General characteristics of study subjects
Table 3 shows the general characteristics of the subjects involved in the study. The age of the volunteers ranged from years 19-26 years with equal proportions of male and female. The weight was found to be between 52 kg - seventy-six 76 kg. The height of the subjects ranged from 1.6 - 1.8 m. The BMI of participating subjects ranged from 18.2-24.4 kg/m2 while 62% of them were in normal range, 25% underweight, and 12,5% in overweight range. The volunteers BS ranged from 59 mmol-87 mmol. Levels were within the normal range.
|
Men |
Women |
Men & Women |
||
|
Mean ± SD |
Mean ± SD |
Mean ± SD |
N (%) |
|
|
Age (ans) |
24.4 ± 4 |
20.2 ± 0.9 |
22.1 ± 3.4 |
|
|
Weight (kg) |
66.2 ± 4.2 |
57.0 ± 10.6 |
61.6 ± 8.9 |
|
|
Height (m) |
1.76 ± 0.0 |
1.64 ± 0.1 |
1.7 ± 0.1 |
|
|
BMI (kg/m2) |
21.3 ± 1.1 |
21.2 ± 4.5 |
21.3 ± 3.1 |
|
|
<18.5 |
2 (25) |
|||
|
18.5-24.9 |
5 (62.5) |
|||
|
25-29.9 |
1 (12.5) |
|||
BMI: Body Mass Index; SD = Standard Deviation
Table 3: Description of the study population (N = 8, sex-ratio (H/F) =1)
Variation in blood glucose during 2 hours after consumption of each of the six tested foods
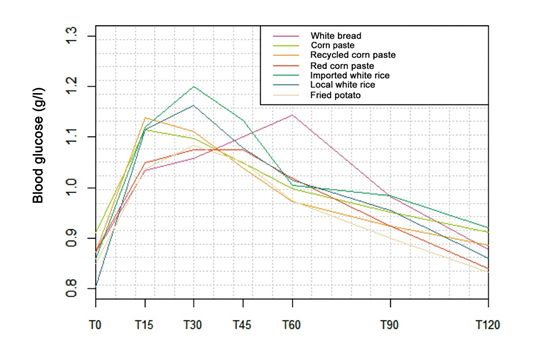
Changes in blood glucose (g/l) at different measurement times (min) for white bread and the six foods tested (corn paste, recycled corn paste, red paste, imported white rice, local white rice, fried potato) is shown in figure 1. Peak blood glucose is reached at T = 15 min for corn paste and recycled corn paste in contrast to imported white rice, local white rice and French fries whose peak blood glucose levels are observed at T = 30 min. As for the red paste, its peak was observed at T = 45 min. Finally, the peak in blood glucose related to the consumption of white bread (reference food) was obtained at T = 60 min.
Glycemic indexes of the six texted foods
The glycemic index of the foods tested is presented in table 4. The GI values obtained for all of these foods show that they belong to the group of foods with a high glycemic index (GI > 70). The GI variance analysis did not show any significant difference (p = 0.139) between the values for all tested foods.
|
Foods |
IG (%) ± SD |
|
White bread |
100 (Reference) |
|
Corn Paste |
85.35 ± 0.4 |
|
Recycled Corned Paste |
94.32 ± 0.8 |
|
Red Corn Paste |
77.76 ± 0.3 |
|
Imported white rice |
136.46 ± 0.8 |
|
Local white rice |
148.40 ± 0.7 |
|
Fried potatoes |
89.87 ± 0.5 |
Table 4: Résultats des index glycémiques obtenus pour chacun des aliments testés
Discussion
After the consumption of each of the six staple foods, blood glucose level increased over time, reaching its peak at 30 min for imported white rice, local white rice, and fried potatoes, whereas for corn paste and recycled corn paste, the increase came earlier at 15 minutes; later at 45 min for the red paste, then blood glucose lowering was observed over time. Among ten healthy, non-diabetic human subjects between 20 and 50 years, all carbohydrates-rich Ghanaian staples peaked 30 min after ingestion by measuring blood glucose levels every 15 min over two hours [23]. Another study focused on the glycemic response of maize and rice among healthy subjects revealed a peak in approximately 30 min, followed by a gradual decrease in that glycemic response [24]. Our study and other studies' findings showed distinct variations in the glycemic response to fixed amounts of carbohydrates available in carbohydrate-rich staples. These findings confirm that equal servings of carbohydrates in various foods may generate different glycemic responses [25]. The study also revealed that corn, recycled, and red corn paste had very high GI values of 85.3%, 94.3%, and 76.8%, respectively. It was found that processing methods such as cooking, boiling, frying, steaming, and baking tend to increase GI values [26, 27]. Although Akanni found that corn paste's GI is 48.1% [28], which is much lower than our study's results, it should be noted that the quantity of corn paste tested was not specified, and the difference observed could be due to the corn paste accompaniment and preparation method. It is also worth noting that recycled corn paste undergoing processing usually has a slightly higher GI than hot corn paste. In our study, we used recycled corn paste or reheated hot corn paste from the previous day, which could have caused a break in the polysaccharide chains and, consequently, increased glycemic index. This could also explain why recycled paste had a higher GI value. However, red corn paste had a lower GI than the others, which could be attributed to the cooking process, mainly the action of fibers and other macronutrients contained in the tomato sauce used to prepare the paste [26]. Similarly, the GI for imported and local white rice was high, 136.5% and 148.4%, respectively, with no significant difference between both GI values (p > 0.05). It is important to note that different types of rice have varying GI values due to their inherent botanical differences. In particular, the presence of amyloidosis content can impact carbohydrate digestion and absorption. Rice structure, such as particle size and cooking method, also affects glycemic responses [29]. Furthermore, rice contains 80% starch, and its increased consumption in refined form can increase T2D risk [30,31]. For example, Asian populations who consume staple white rice have a higher risk of diabetes and metabolic syndrome [32]. Finally, Foster-Powell et al. have found that potatoes generally have a high GI value, regardless of processing. However, some varieties may have lower GI values [33]. The tested fried potatoes in this study had a GI of 89.9%, comparable to Foster-Powell et al.'s findings. Further, due to the nature of the tested foods, they were served with accompaniments instead of being consumed alone. For example, corn paste was paired with 30g of vegetable soup, while red paste and fried potatoes were served with tomato stew. Various factors, such as fat [34], protein [35], and acidic compounds [36-38], can influence a meal's glycemic index (GI). Therefore, consuming a particular food with different accompaniments can result in a different glucose response than consuming that same food alone. The amount and type of carbohydrates in a meal can impact the glucose response, and the presence of fat and protein can affect gastric emptying and insulin secretion [39]. However, fat and protein must be present in large amounts per 50g of available carbohydrate to significantly affect GI [40-42]. This was not the case in the present study, where the total quantities of fat and protein did not reach the necessary thresholds of 30g and 50g, respectively. Finally, the results of this study will play a crucial role in developing practical dietary and therapeutic objectives for individuals with T2D and other clinical conditions that require carbohydrate restriction. However, it is essential to note that this study has certain limitations. Specifically, it only involved healthy adults, so glycemic responses to starchy foods for non-healthy patients remain unknown. Therefore, this study only applies to healthy adults, not those with certain diseases. Future research should also focus on the obese adult population, which is prevalent in Benin. Food's impact on health, especially carbohydrate consumption, is a significant public health concern. Several studies have explored the positive effects of low GI (GI < 55) foods [9,10] on reducing the risk of T2D and associated chronic diseases. However, this study found that all the foods tested had a high GI (GI >70) and are commonly consumed in the Beninese diet. Therefore, nutrition education is necessary to promote better dietary practices and combat T2D and associated diseases in the country. Nevertheless, further long-term studies are required to establish the direct relationship between these diseases and high-GI foods. Additionally, more research is needed to determine the GI of other locally-consumed foods to effectively support the use of GI in conjunction with other dietary recommendations in managing and preventing T2D.
Conclusion
This study determined the glycemic index of six commonly consumed foods in Benin. The GI of all tested foods, namely local white rice, imported white rice, recycled corn paste, fried potato, corn paste, and red paste, was above 70. Therefore, these high-GI foods should be consumed with low-GI foods such as vegetables, legumes, meat, etc. Nutritional education of the population is also necessary to promote healthy diet and prevent the risk of diabetes.
Highlights
- High glycemic index (GI) foods can raise blood glucose and insulin levels.
- Low GI diet can improve glucose contrôle in patients with Type 2 Diabetes.
- Few data on african food GI is available, particulary in Benin.
- Six commonly consumed foods in Benin had GI above 70.
- Consumption of moderate portions of these food should be recommended.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contribution
Mizéhoun-Adissoda & H. Alaofè: Methodology, Writing - Original draft, Investigation, Resources, Data curation, Writing - review & editing. B. Nicoué, J. Gninkoun & A. Yemoa: Methodology, Formal analysis, Investigation, Resources, Data curation, Writing - review & editing. B. Awèdé: Conceptualization, Methodology, Supervision.
Declaration of competing interest:
No conflict of interest to declare
References
- Goedecke JH, Mendham AE. Pathophysiology of type 2 diabetes in sub-Saharan Africans. Diabetologia 65 (2022): 1967-1980.
- Motala AA, Mbanya JC, Ramaiya K, Pirie FJ, Ekoru K. Type 2 diabetes mellitus in sub-Saharan Africa: challenges and opportunities. Nat Rev Endocrinol 8 (2022): 219-229.
- Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183 (2022): 109119.
- Juma K, Juma P, Shumba C, et al. Non-communicable diseases and urbanization in African cities: a narrative review, non-communicable diseases and urbanization-a global perspective. IntechOpen (2019).
- Khan MAB, Hashim MJ, King JK, et al. Epidemiology of type 2 diabetes - Global burden of disease and forecasted trends. J Epidemiol Glob Health 10 (2020): 107-111.
- Olowoyo P, Popoola F, Yaria J, et al. Strategies for reducing non-communicable diseases in Africa. Pharmacol Res 170 (2021): 105736.
- Galaviz KI, Narayan KMV, Lobelo F, et al. Lifestyle and the prevention of type 2 diabetes: A status report. Am J Lifestyle Med 12 (2015): 4-20.
- Guo Y, Huang Z, Sang D, et al. The role of nutrition in the prevention and intervention of type 2 diabetes. Front Bioeng Biotechnol 8 (2020): 575442.
- Ojo O, Ojo OO, Adebowale F, et al. The effect of dietary glycaemic index on glycaemia in patients with Type 2 Diabetes: a systematic review and meta-analysis of randomized controlled trials. Nutrients 10 (2018): 373.
- Livesey G, Taylor R, Livesey HF, et al. Dietary glycemic index and load and the risk of type 2 diabetes: Assessment of causal relations. Nutrients 11 (2019): 1436.
- Vlachos D, Malisova S, Lindberg FA, et al. Glycemic Index (GI) or Glycemic Load (GL) and dietary interventions for optimizing postprandial hyperglycemia in patients with t2 diabetes: A review. Nutrients 12 (2020): 1561.
- Jung C, Choi KM. Impact of high-carbohydrate diet on metabolic parameters in patients with type 2 diabetes. Nutrients 9 (2017): 7869.
- Thomas DE, Elliott EJ. The use of low-glycaemic index diets in diabetes control. Br. J Nutr 104 (2010): 797-802.
- Eleazu CO. The concept of low glycemic index and glycemic load foods as panacea for type 2 diabetes mellitus; prospects, challenges and solutions. Afr Health Sci 16 (2016): 468-479.
- Atkinson FS, Brand-Miller JC, Foster-Powell K, et al. International tables of glycemic index and glycemic load values 2021: a systematic review. Am J Clin Nutr 114 (2021): 1625-1632.
- Aston LM, Gambell JM, Lee DM, et al. Determination of the glycaemic index of various staple carbohydrate-rich foods in the UK diet. Eur J Clin Nutr 2 (2008): 279-285.
- Stadlmayr B, Charrondiere UR, Enujiugha VN, et al. West african food composition table. Food and Agriculture Organization of the United Nations (FAO): (2012).
- Benin's food guide. FAO: (2015).
- Jenkins DJ, Wolever TM, Taylor RH, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr 34 (1981): 362-366.
- Foster-Powell K, Holt SH, Brand-Miller JC. International table of Glycemic index and Glycemic load values. Am J Clin Nurt 76 (2002): 5-56.
- MATLAB R2016a 9.0. The MathWorks, Inc., Natick, Massachusetts, United States (2016).
- Spataro E. Logiciel aire (2006).
- Eli-Cophie D, Agbenorhevi JK, Annan RA. Glycemic index of some local staples in Ghana. Food Sci Nutr 5 (2016): 131-138.
- Akinlua O, Sedodo NS, Victoria AJ. Glycemic index of selected nigerian foods for apparently healthy people. J obes wt loss ther 3 (2013): 160.
- Francis RD, Bahado-Singh PS, Smith AM, et al. Glycemic index of traditional foods in Jamaica. Eur J Exp Biol 8 (2018): 15.
- Bahado-Singh PS, Riley CK, Wheatley AO, et al. Relationship between Processing Method and the Glycemic Indices of Ten Sweet Potato (Ipomoea batatas) Cultivars Commonly Consumed in Jamaica. J Nutr Metab (2011): 584832.
- Adedayo BC, Adebayo AA, Nwanna EE, et al. Effect of cooking on glycemic index, antioxidant activities, α-amylase, and α-glucosidase inhibitory properties of two rice varieties. Food Sci Nutr 6 (2018): 2301-2307.
- Akanni M. Index glycemique des aliments les plus couramment consommés au Benin [Internet] [Thesis]. Cotonou: Université d’Abomey-Calavi (2007).
- Khosravi-Boroujeni H, Sarrafzadegan N, Mohammadifard N, et al. White rice consumption and CVD risk factors among Iranian population. J Health Popul Nutr 31 (2013): 252-261.
- Misra A, Singhal N, Khurana L. Obesity, the metabolic syndrome, and type 2 diabetes in developing countries: role of dietary fats and oils. J Am Coll Nutr 29 (2010): 289-301.
- Miller JB, Pang E, Bramall L. Rice: a high or low glycemic index food? Am J Clin Nutr 56(1992):1034-6. doi: 10.1093/ajcn/56.6.1034.
- Ramachandran A, Ma RCW, Snehalatha C. Diabetes in Asia. Lancet 375 (2010): 408-418.
- Foster-Powell K, Holt SHA, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr 76 (2002): 5-56.
- Collier G, O’Dea K. The effect of coingestion of fat on the glucose, insulin, and gastric inhibitory polypeptide responses to carbohydrate and protein. Am J Clin Nutr 37 (1983): 941-944.
- Granfeldt Y, Bjorck I, Hagander B. On the importance of processing conditions, product thickness and egg addition for the glycaemic and hormonal responses to pasta: a comparison with bread made from ‘pasta ingredients. Eur J Clin Nutr 45 (1991): 489-99.
- Liljeberg H, Bjorck I. Delayed gastric emptying rate may explain improved glycaemia. European Journal of Clin. Nutr 52 (1998): 368-71.
- Liljeberg HG, Lonner CH, Bjorck IM. Sourdough fermentation or addition of organic acids or corresponding salts to bread improves nutritional properties of starch in healthy humans. J Nutr 125 (1995): 1503-1511.
- Liljeberg HG, Bjorck IM. Delayed gastric emptying rate as a potential mechanism for lowered glycaemia after eating sourdough bread: studies in humans and rats using test products with added organic acids or an organic salt. Am J Clin Nutr 64 (1996): 886-93.
- Wolever TMS, Bolognesi C. Prediction of glucose and insulin responses of normal subjects after consuming mixed meals varying in energy, protein, fat carbohydrate and glycaemic index. J Nutr 126 (1996): 2807-2812.
- Wolever TM, Katzaman-Relle L, Jenkins AL, et al. Glycaemic index of 102 complex carbohydrates in patients with diabetes. Nutr. Res 4 (1994): 651-669.
- Willet W, Manson J, Liu S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am J Clin Nutr 76 (2002): 274-280.
- Mcmillan J, Brand-Miller JC. Low glycemic index diets and body weight regulation. Int J Obes 30 (2006): 40-46.


 Impact Factor: * 3.8
Impact Factor: * 3.8 Acceptance Rate: 77.96%
Acceptance Rate: 77.96%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 