Mycobacterium Bovis Sternoclavicular Joint Septic Arthritis in Patient on Avelumab for Metastatic Urothelial Cell Carcinoma
Karim Germanos3, Changsu L. Park1, Ramy R. Saleh1*, Feras A. Moria1,2
Affiliation:
1Department of Medical Oncology, McGill University Health Center, McGill University, Montreal, Quebec H4A 3J1, Canada.
2Department of Medicine, Division of Oncology, King Abdulaziz University Hospital, King Abdulaziz University, Jeddah, Saudi Arabia
3Department of Medicine, University of Balamand, Balamand, Lebanon
*Corresponding author:
Ramy R. Saleh, MD, Department of Medical Oncology, McGill University Health Center, McGill University, Montreal, Quebec H4A 3J1, Canada.
Received: December 04, 2023;Accepted: December 11, 2023;Published: December 29, 2023
Article Information
Citation: Karim Germanos, Changsu L. Park, Ramy R. Saleh, Feras A. Moria. Mycobacterium Bovis Sternoclavicular Joint Septic Arthritis in Patient on Avelumab for Metastatic Urothelial Cell Carcinoma. Journal of Cancer Science and Clinical Therapeutics 7 (2023): 277-282.
View / Download Pdf Share at FacebookAbstract
Bladder cancer is the sixth most common cancer worldwide intravesical BCG has been the standard of care of treating non-muscle-invasive bladder cancer (NMIBC) following TURBT since late 70s and early 80s. Despite of is efficacy and safety it has been associated with non-negligible risk of recurrence and about 1-5% risk of infectious complications. The current recommendation for treatment of advanced urothelial cancer (aUC) is combined platinum doublets followed by maintenance avelumab in patient who didn’t progress on chemotherapy. We report a case of a 69-year-old male with aUC who progressed following adjuvant intravesical therapy and subsequently developed right sternoclavicular joint (SCJ) septic arthritis 16 months following finishing BCG treatment and while receiving immunotherapy.
Keywords
<p>Aveulmab; Septic arthritis, Mycobacterium Bovis, Immunotherapy, Immune checkpoint inhibitors, ICI, sternoclavicular joint.</p>
Article Details
Introduction
Bladder cancer is the sixth most common cancer worldwide [1]. In Canada, the estimated incidence for 2022 was 13,300 new cases, with 2,500 disease-related deaths during the year [2]. In alignment with the national and international guidelines, the treatment of bladder cancer is stratified based on histologic subtype and extent of the disease distinguishing between NMIBC and muscle-invasive bladder cancer (MIBC) [3-5]. NIMBC is defined when the tumor growth doesn’t extend beyond the lamina propria while MIBC invades through the muscular wall of the bladder or beyond [6]. The approach to NMIBC typically involves a tailored regimen of intravesical BCG instillations, contingent on the patient’s risk profile, following a transurethral resection of bladder tumor (TURBT) [3-5]. While intravesical BCG immunotherapy remains safe and effective approach in the treatment of NMIBC [7-9]; infectious complication can occur in about 1-5% of the cases [10], including disseminated BCG infection, which occurs in about 0.4% of the patients [11]. Despite the rarity of disseminated BCG, it is associated with high risk of mortality (5.4%) and morbidity (7.4%) [7]. Septic arthritis from BCG is extremely rare with an estimated incidence of 30 in 1,000,000 and only case reports describing this complication following intravesical BCG [12-14]. We are reporting an unusual case of BCG associated septic arthritis of the SCJ 16 months following intravesical BCG and while receiving systemic immunotherapy for aUC.
Case Presentation:
A 69-year-old man with a past medical history of severe eczema, hypothyroidism, hypertension, dyslipidemia, asthma, and obstructive sleep apnea presented with hematuria. He was diagnosed with T1 high grade (HG) urothelial cell carcinoma (UCC) and underwent TURBT. Subsequently, he underwent BCG induction and maintenance therapy. Surveillance cystoscopy revealed recurrent disease; pathology was suspicious for muscle invasive UCC. Staging scans with computed Tomography (CT) revealed metastasis to the retroperitoneal lymph nodes, and no other distant metastasis. He was started on dose dense (every 2 weeks) Metho-trexate, Vinblas¬tine, Adria¬mycin and Cisplatin (dd-MVAC). Treatment was tolerated well with no grade 2 or above toxicity. He received a total of 4 cycles. Six weeks following chemotherapy, maintenance avelumab every 2 weeks was started. Treatment was tolerated, with only grade 1 immune related dermatitis treated with topical low potency steroid. Following 10 cycles of treatment; he developed grade 2 polyarthritis and required 2 months of oral 10 mg / day of prednisone with a slow taper over 1 month.
A month later, the patient noticed a painful swelling at the base of his neck, it was not tender to touch. Laboratory work up revealed white blood cell count (WBC) 6.9 × 109 cells/ mm3 [4.5-11× 109] with normal differentiation, otherwise complete blood count (CBC) was unremarkable, C-reactive protein (CRP) 6.6 mg/L [0-5]. Ultrasound of that area revealed significant thickness (Figure 1A) of the right SCJ capsule which measures 4.5 x 3.2 x 1.5 cm with no significant internal flow with doppler (Figure 1B) these changes were thought to be secondary to osteoarthritis rather than septic arthritis as the patient was denying any fever, night sweats, weight loss, cough or shortness of breath and no previous history of Tuberculosis (TB) exposure.
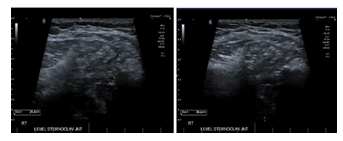
Figure 1A-B: significant thickening of the joint capsule, the capsular thickening and bulge measures up to 4.5 cm transverse by 3.2 cm craniocaudally with a thickness of up to 1.5 cm.
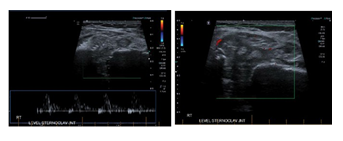
Figure 1C-D: No significant internal flow at Doppler.
Subsequently the patient had CT scan which showed right clavicular head erosion with cortical destruction and soft tissue pannus, there was also a new heterogenous subcutaneous opacity abutting the right pectorals muscle measuring 1.5 x 1.9 cm and new focal erosion in the manubrium which was associated with right anterior chest wall subcutaneous fat stranding (Figure 2 A-B). At that time, the pain had subsided with minimal intake of non-steroidal anti-inflammatory drugs.
The patient was assessed by rheumatology, but laboratory work up including blood cultures, rheumatoid factor, and TB QuantiFERON was negative, WBC 6.2× 109 cells/mm3 otherwise complete blood count (CBC) was unremarkable and CRP 9.10 mg/L. Fluid aspiration was sent for bacterial and fungal cultures which showed no growth after 7 days of incubation. Repeated CT scan a month later was done giving worsening patients symptoms showed slightly increased size of the heterogenous, enhancing/hyperdense amorphous soft tissue surrounding right SCJ (Figure 3A) now measuring 4.8 x 2.8 cm and the right pectoralis major muscle collection (Figure 3B) now measuring 2.1 x 2 cm with increased surrounding fat stranding, no evidence of enlarged mediastinum nor hilar lymph node, no evidence of parenchymal lung disease. Notably the CT scans showed complete response (CR) from the cancer perspective. Repeated infectious work up including bacterial and fungal culture was negative, imaging guided biopsy was done, and acid-fast bacilli staining was also negative, TB culture was pending, CRP 23.10 mg/L, CBC was unremarkable. It was decided at the time to interrupt Avelumab and to initiate colchicine.
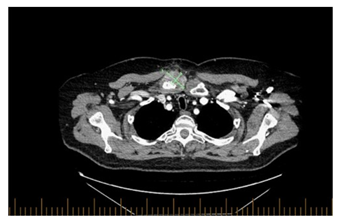
Figure 2A: Axial view of the chest, right clavicular head erosions with cortical destruction and increased surrounding soft tissue pannus at the right SCJ measuring 4.3 x 2.7 cm.
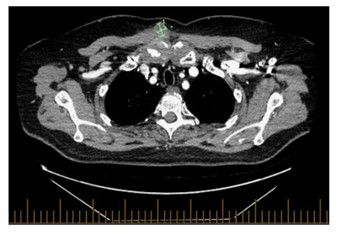
Figure 2B: Axial view of the chest, A new small heterogeneous subcutaneous opacity is noted abutting right pectoralis muscle measuring 1.9 x 1.5 cm.
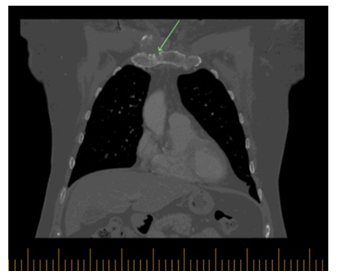
Figure 2C: Coronal view of the chest, there is also new focal erosion involving the right manubrium associated right anterior chest wall subcutaneous fat stranding.
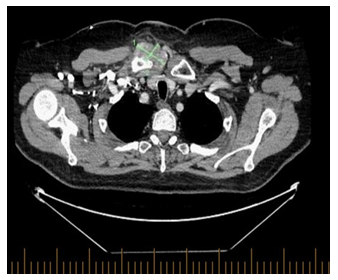
Figure 3A: Axial view of the chest, increased size of the heterogenous, enhancing/hyperdense amorphous soft tissue surrounding right SCJ, in keeping with pannus/phlegmonous joint effusion now measuring 4.8 x 2.8 cm
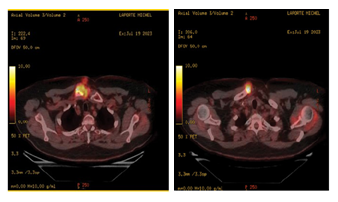
Figure 4A-B: Intense heterogeneous radiotracer accumulation projected in the right SCJ involving both the clavicular side and the sternal side which is also associated with a significant soft tissue components anteriorly and posteriorly to that SCJ including a soft tissue linear distribution from the skin to the main dominant hypermetabolic activity. Maximum SUV 13.6
A couple of weeks later, giving the improvement in symptoms and stability of the lesion following the previous measures it was thought to be secondary to Immune-related arthritis. 18 F-fluorodeoxyglucose (FDG) PET/CTscanshowed intense heterogeneous radiotracer accumulation projected in the right SCJ involving both the clavicular side and the sternal side (Figure 4A-B) which is also associated with a significant soft tissue components anteriorly and posteriorly to that SCJ including a soft tissue linear distribution from the skin to the main dominant hypermetabolic activity, maximum SUV 13.6 projected inside the medial end of the right clavicle, there was no other abnormal tracer uptake elsewhere. In the meantime, TB culture was flagged positive for Mycobacterium bovis (BCG), the patient was started on Rifampicin, Isoniazid, and pyridoxine, avelumab was resumed 2 weeks later. Currently the patient is still on maintenance avelumab with continuous response from immunotherapy and TB treatment.
Discussion
Bacillus Calmette-Guerin vaccine is made from a live attenuated strain of Mycobacterium bovis. It was developed as a prevention against tuberculosis [15]. For decades, patients with NMIBC have been treated with intravesical BCG for 1-3 years depending on risk features seen at the time of cystoscopy and on pathological examination following TURBT [16-18]. It is uncertain whether BCG directly targets tumor cells or if its general immune response is responsible for the anti-tumor activity [19]. Nevertheless, it is thought that the major impact lies in stimulating local cytokine production and the inflammatory response particularly within the bladder, recruiting CD4+ T cells by binding of urothelial cells and dendritic cells to BCG through MHC class II, and CD8+ T-cells through MHC class I leading to their activation and recognition of tumor cells via T helper cells and interferon gamma (IFN-γ). The activation of TH1 and TH2 leads to the production of different cytokines including IL-2, IL-12, TNF,
TNF – β [20]. Despite the safety of BCG immunotherapy, about 1-5% of cases is associated with an infectious complication [10] and approximately 2.9% of patients only will discontinue treatment due to BCG related infection [21]. Risk of disseminated BCG increases with traumatic catheterization, active cystitis, immunosuppression, TURBT within 2 weeks of instillation [22]. Clinical presentation of BCG infection is classified into early onset manifestation (usually within 3 months of instillation) and delayed onset which is more than 3 months of instillation [21]. Early onset manifestations are characterized by systemic symptoms, most commonly involving lung and liver. Achieving a microbiological diagnosis is usually low (31-35%). On the other hand, late onset manifestations are characterized by localized infection usually involving joint or bone, soft tissue abscess or central nervous system, with usually a higher rate of microbiological diagnosis (83-91%) [21]. Osteoarticular complications can be divided into: i) infectious manifestation, it is thought that the vesical venous plexus draining directly to the prostatic venous plexus offers a direct path to the vertebral venous plexus explaining the relatively higher incidence of disseminated BCG and osteoarticular side effects in men [23]. ii) non-infectious - osteoarticular side effects from intravesical BCG is include Reiter’s syndrome, which has been reported in about 0.5-1% of the cases [26]. Other infectious complication that are even less common like granulomatous prostatitis (0.9%), epididymitis (0.4%), and cytopenia (0.1%) due to bone marrow involvement [24, 25] have been reported. Despite the efficacy of adjuvant intravesical, about 40 to 50% of patients progress over 2-year period [26]. Additionally, about 15-20% of the patient who received intravesical BCG will progress to MIBC, regional or invasive disease which confer a worse prognosis [27- 29]. The treatment of aUC is consistent of cisplatin-based chemotherapy, for patients who are not eligible for cisplatin, carboplatin is a good alternative [5]. With the emerging data from the Javalin bladder 100, maintenance immunotherapy has become a cornerstone in the management of aUC [30].
Avelumab is a fully human IgG1 monoclonal antibody that specifically binds to PD-L1, preventing the interaction between PD-L1 and the inhibitory T cell receptors, PD-1 and B7.1 resulting in T cell-mediated, adaptive antitumor immune responses and T cell reactivation and cytokine production [31]. Data is controversial regarding the risk of Tuberculosis activation in patients receiving checkpoint inhibitors. In a national based study in South Korea in 5037 patients receiving Immune checkpoint inhibitors (ICI) despite the incidence of TB in cancer patients exposed to ICIs was eightfold higher than in the general population, the risk of patients with cancer developing TB did not significantly differ according to ICI exposure [32]. Additionally, in a large retrospective study at Mayo Clinic it was found no patients developing TB during ICI [33]. While there is no data in the literature have prescribed
Mycobacterium bovis reactivation with Avelumab, there have been mentioned of multiple case reports suggesting that ICI maybe contributing to TB activation. Anastasopoulou A Et al. described few case reports about TB activation in the context of ICI, all these cases occurred within 6 months of treatment not like our case which happened about 9-10 months of treatment and most of the patient (86%) did not receive concurrent immunosuppressive medications to treat IrAE. Also, most of the patients received single agent ICI and not a combined therapy with another ICI or other cancer treatment [34, 35]. The mechanism of TB activation is controversial and data from pre-clinical models shows that PD-1 knockout (PD-1−/−) mice are having aggressive clinical presentation and higher mortality, The inability of (PD-1−/−) mice to control mycobacterial infection is thought to be secondary to increased Th1-mediated responses and continues activation of T cell due to overproduction of interferon-gamma (IFN-γ), leading to T cell exhaustion [36-38].
Conclusion
To the best of our knowledge, this is the first case of septic arthritis involving the SCJ in a bladder cancer patient undergoing treatment with avelumab post intravesical BCG. A small number of case reports mentions reactivation of TB in patient undergoing immunotherapy, but this is the first case of disseminated BCG in a patient receiving intravesical BCG for the treatment of bladder cancer.
Acknowledgements
Conceptualization, K.G, F.A.M and R.S.; writing— original draft preparation, K.G and F.A.M, writing—review and editing, and F.A.M, C.L.P. and R.S.; supervision, F.A.M and R.S. All authors have read and agreed to the published version of the manuscript.
Funding:
None
Conflicts of interest: The authors declare no conflict of interest.
References
- Bethesda SEER Cancer Statistics Factsheets: Common Cancer Sites. National Cancer Institute.
- Brenner DR, Abbey P, Ryan R W, et al. Projected estimates of cancer in Canada in Canadian Medical Association Journal 194 (2022): E601-E607.
- Bhindi B, Ronald K, Girish S K, et Canadian Urological Association guideline on the management of non-muscle- invasive bladder cancer – Abridged version. Canadian Urological Association Journal 15 (2021): 230-239.
- Flaig TW, Philippe E S, Michael A, et al. NCCN Guidelines® Insights: Bladder Cancer, Version 2022. Journal of the National Comprehensive Cancer Network 20 (2022): 866-878.
- Powles T, J Bellmunt, E Comperat, et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Annals of Oncology 33 (2022): 244-258.
- Amin MB, Stephen BE, Frederick LG, et AJCC cancer staging manual 1024 (2017): 1032.
- Pérez-Jacoiste Asín MA, Fernández-Ruiz M, López- Medrano F, et al. Bacillus Calmette-Guérin (BCG) infection following intravesical BCG administration as adjunctive therapy for bladder cancer: incidence, risk factors, and outcome in a single-institution series and review of the literature. Medicine (Baltimore) 93 (2014): 236-254.
- Shelley M, Court JB, Kynaston H,et al. Intravesical Bacillus Calmette-Guérin in Ta and T1 bladder cancer. Cochrane Database of Systematic Reviews (2000).
- Sylvester RJ, J Alfred Witjes, Karlheinz K, et Bacillus calmette-guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. J Urol 174 (2005): 86-91; discussion 91-2.
- Seegobin K, Maharaj S, Baldeo C, et al. Mycobacteria Bovis osteomyelitis following intravesical BCG for bladder cancer. IDCases 10 (2017): 75-78.
- Taniguchi Y, Nishikawa H, Kimata T, et al. Reactive Arthritis After Intravesical Bacillus Calmette-Guérin Therapy. J Clin Rheumatol 28 (2022): e583-e588.
- Carrega G, Riccio G, Vallerga D, et A severe Bacillus Calmette-Guérin vertebral osteomyelitis requiring spinal stabilization: a clinical and microbiological investigation. J Microbiol Immunol Infect 56 (2023): 641-643.
- Lee SC and CS Geannette. Osteomyelitis and septic arthritis after Mycobacterium Bovis BCG Therapy for Urinary Bladder Clin Imaging 68 (2020): 179-183.
- Chiu N, Lin M, Lin W, et Mycobacterium bovis BCG– Associated Osteomyelitis/Osteitis, Taiwan. Emerging Infectious Diseases 21 (2015): 539-540.
- Calmette A. Bacillary infection and tuberculosis in humans and animals: infection and defense processes, biological and experimental study (1922).
- Lamm DL, Thor DE, Harris SC, et Bacillus Calmette- Guerin immunotherapy of superficial bladder cancer. J Urol 124 (1980): 38-40.
- Morales A, D Eidinger and AW Bruce. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol 116 (1976): 180-183.
- Pinsky CM, Camacho FJ, Kerr D, et al. Intravesical administration of bacillus Calmette-Guérin in patients with recurrent superficial carcinoma of the urinary bladder: report of a prospective, randomized Cancer Treat Rep 69 (1985): 47-53.
- Larsen ES, Joensen UN, Poulsen AM, et al. Bacillus Calmette-Guérin immunotherapy for bladder cancer: a review of immunological aspects, clinical effects and BCG infections. Apmis 128 (2020): 92-103.
- Pettenati C and MA Ingersoll. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat Rev Urol 15 (2018): 615-625.
- Cabas P, Rizzo M, Giuffrè M, et al. BCG infection (BCGitis) following intravesical instillation for bladder cancer and time interval between treatment and presentation: A systematic Urol Oncol 39 (2021): 85-92.
- Patel AR, Sabanegh ES, Jones JS, et Bacillus Calmette- Guérin osteomyelitis mimicking spinal metastasis from urothelial cell carcinoma of the bladder. Eur Urol 58 (2010): 934-937.
- Mackel CE, Burke SM, Huhta T, et al. Mycobacterial Osteomyelitis of the Spine Following Intravesical BCG Therapy for Bladder Cancer. Cureus 8 (2016): e545.
- Dammert P, Boujaoude Z, Rafferty W, et al. Fever of unknown origin and pancytopenia caused by culture- proven delayed onset disseminated bacillus Calmette- Guerin (BCG) infection after intravesical instillation. Case Reports 2013 (2013): bcr2013008949-b.
- Elzein F, Albogami N, Saad M, et al. Disseminated Mycobacterium bovis Infection Complicating Intravesical BCG Instillation for the Treatment of Superficial Transitional Cell Carcinoma of the Bladder. Clin Med Insights Case Rep 9 (2016): 71-73.
- Chung R, McKiernan J, Arpaia N, et al. Neo-Adjuvant immunotherapies: Bladder cancer as a platform for drug development targeting mucosal immunity. Eur J Cancer 187 (2023): 58-64.
- Hensley PJ, Bree KK, Campbell MT, et Progression of Disease after Bacillus Calmette-Guérin Therapy: Refining Patient Selection for Neoadjuvant Chemotherapy before Radical Cystectomy. J Urol 206 (2021): 1258-1267.
- Soukup V, Capoun O, Cohen D, et al. Prognostic Performance and Reproducibility of the 1973 and 2004/2016 World Health Organization Grading Classification Systems in Non-muscle-invasive Bladder Cancer: A European Association of Urology Non-muscle Invasive Bladder Cancer Guidelines Panel Systematic Review. Eur Urol 72 (2017): 801-813.
- Davis JW, Sheth SI, Doviak MJ, et al. Superficial bladder carcinoma treated with bacillus Calmette-Guerin: progression-free and disease specific survival with minimum 10-year J Urol 167 (2002): 494-500; discussion 501.
- Powles T, Park SH, Voog E, et Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. New England Journal of Medicine 383 (2020): 1218-1230.
- Akinleye A and Z Rasool. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. Journal of Hematology & Oncology 12 (2019).
- Bae S, Kim YJ, Kim MJ, et al. Risk of tuberculosis in patients with cancer treated with immune checkpoint inhibitors: a nationwide observational study. Journal for ImmunoTherapy of Cancer 9 (2021): e002960.
- Stroh GR, T Peikert and P Escalante. Active and latent tuberculosis infections in patients treated with immune checkpoint inhibitors in a non-endemic tuberculosis Cancer Immunol Immunother 70 (2021): 3105-3111.
- Anastasopoulou A, Ziogas DC, Samarkos M, et al. Reactivation of tuberculosis in cancer patients following administration of immune checkpoint inhibitors: current evidence and clinical practice recommendations. J Immunother Cancer 7 (2019): 239.
- Kato Y, Watanabe Y, Yamane Y, et Reactivation of TB during administration of durvalumab after chemoradiotherapy for non-small-cell lung cancer: a case report. Immunotherapy 12 (2020): 373-378.
- Lázár-Molnár E, Chen B, Sweeney KA, et Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci U S A 107 (2010): 13402-13407.
- Barber DL, Mayer-Barber KD, Feng CG, et al. CD4 T cells promote rather than control tuberculosis in the absence of PD-1-mediated inhibition. J Immunol 186 (2011): 1598-607.
- Tousif S, Singh Y, Prasad DVR, et al. T cells from Programmed Death-1 deficient mice respond poorly to Mycobacterium tuberculosis infection. PLoS One 6 (2011): e19864.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 74.74%
Acceptance Rate: 74.74%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 