Nutritional Composition, Glycaemic Properties and Anti-Diabetic Potentials of Cereal-Based Soy-Fortified Flours for Functional Dough Meal in Diabetic Induced Rats
Akinjayeju O*, Ijarotimi OS, Awolu OO, Fagbemi TN
Department of Food Science and Technology, Federal University of Technology, Akure, Nigeria
*Corresponding Author: Oluwole Akinjayeju, Department of Food Technology, Yaba College of Technology, P.M.B.2011, Yaba-Lagos, Nigeria
Received: 24 March 2020; Accepted: 06 April 2020; Published: 24 April 2020
Article Information
Citation: Akinjayeju O, Ijarotimi OS, Awolu OO, Fagbemi TN. Nutritional Composition, Glycaemic Properties and Anti-Diabetic Potentials of Cereal-Based Soy-Fortified Flours for Functional Dough Meal in Diabetic Induced Rats. Journal of Food Science and Nutrition Research 3 (2020): 102-120.
DOI: 10.26502/jfsnr.2642-11000042
View / Download Pdf Share at FacebookAbstract
This study evaluated glycaemic properties and anti-diabetic potentials of blends of quality-protein-maize (QPM), soy-cake (DSF), millet (WMF) flours for functional dough meal using cassava starch (CST) as binder. Protein and crude fibre contents of blends were optimized using RSM and three blends with highest protein and fibre contents, XYZ=76.03% QPM, 16.76% DSF, 7.21% WMF, 5.00% cassava starch; BCD=72.50% QPM, 2.50% WMF, 25.0% DSF, 2.33% CST; FDT=80.52% QPM, 3.06% WMF, 16.42% DSF, 5.00%), QPM (100% QPM flour) and CER (commercial anti-diabetic flour formulation) were evaluated for glycaemic and biochemical properties and blood glucose-reducing potentials using Wistar rats. Glycaemic index and load were 34-52% and 8-15% respectively, which were lower than 55% and 20% for low glycaemic index and load. Blood glucose-reducing potentials of samples were in ascending order of BCD (57.5%) < FDT (70%) < XYZ (75%), with samples FDT and XYZ higher than 62.5% for sample STD (rats treated with synthetic anti-diabetic agent-Glibenclamide). Haematological indices, PVC, Hb, WBC and RBC, of rats fed on samples were 46.35-51.25%, 13.40-18.10g/L, 8.30-10.80 (x10/mm3) and 4.19-6.94 (x106) respectively, while kidney and liver functionality indices, aspartate amino transferases (AST), alanine amino transferases (ALT) and alkaline phosphate (ALP) were 39.5-43.00 µ/L, 48.50-55.50 µ/L and 92.80-104.20 µ/L, within normal ranges of 7-56µ/L, 45.7-80.8µ/L and 41-133 U/L, respectively. AST:ALT ratios were within standard value of <1. This study established that experimental samples, particularly XYZ, had low glycaemic properties, high anti-diabetic potentials, making them suitable for diabetes patients.
Keywords
<p>Anti-diabetic potentials, Cereal-based flours, Diabetic-induced rats, Functional dough meal, Glycaemic properties, Nutritional composition</p>
Article Details
Introduction
The increasing incidence of many generic diseases like diabetes mellitus (DM), and heart related ailments such stroke and high blood pressure is becoming alarming all over the world, not only in affluent regions but also in developing regions. According to Wild et al. [1] and Shaw et al. [2], increase in the prevalence of diabetes among adults of ages between 20 and 79 years by the year 2030 has been put at between about 5 and 8%, thereby affecting 400 m and over 600 m people out of estimated world population of 8 billion. If diabetes is not properly managed or controlled, it could lead to risks of hyper-glycaemia and atherosclerosis resulting in retinopathy, nephropathy, neuropathy, as well as cardiovascular, amongst other complications [3, 4]. In Nigeria, studies have shown that DM is one of the causes of admissions into tertiary health facilities and death [5, 6]. These diseases and their resulting complications are most often managed or controlled by use of expensive synthetic drugs, which have been implicated with serious side effects on the users, especially when used over a period of time [7, 8]. However, studies have shown that regular consumption of diets from plant based foods has the potentials to prevent or manage these diseases [8, 9].
The therapeutic properties of plant-based foods have assumed a more important dimension in recent years owing largely to the benefits that is associated with their bioactive components. Consequently, they have become indispensable components of traditional diets in many parts of world especially in Africa, Asia, Latin America [9]. Many plant-based foods are excellent sources of important nutrients and non-nutrients most especially dietary fibres, protein and phytochemicals. The importance of protein, fibre and phytochemicals in improving nutritional and health status has been highly acknowledged. Evidences have shown that regular consumption of plant-based foods containing bioactive compounds like protein, fibre and phytochemicals are associated with fewer digestive disorders, reduced rate of colon cancer, better blood-sugar control, and lower blood cholesterol levels [10-13].
In recent, several studies have reported on the production of functional foods in form of dough meal from plantain and cereal based for the management of diabetes [8, 14, 15, 16]. Dough meal, also called dumpling, is a gelatinous, non-leavened semi-solid food products prepared by mixing flours with measured amount of water and cooked, while turning with a small flat wooden ladle to consistent dough mass [17, 18]. Dough meal has been reported to be deficient in some important nutrients such as protein, minerals, vitamins, fibre and phytochemicals. However, many studies have shown that the nutritional value of dough meals may be enhanced by the addition of legume like soya flour and fibre-rich commodities like whole millet and maize bran flours [8, 16, 18, 19]. In view of this, the present study aimed to formulate and evaluate dough meals from cereals based (Quality-protein maize and pearl millet) enriched with legume (soy cake), and determine their nutrient composition, glycaemic index and anti-diabetic potential in Alloxan-induced Wistar rats.
2. Materials and Methods
2.1 Materials and Sources
The materials used in this study were Quality Protein Maize (ART/98/SW6-OB-W), which was purchased from IART, Moor Plantain, Ibadan, Pearl millet (Pennisetum glaucum), was purchased from Oyingbo Retail Market in Lagos, soy bean (Glycine max L.) cake was purchased from Adom Agro-Allied Nig. Ltd., Ibadan, while cassava starch obtained from MATNA Foods Co. Ltd., Akure, Ondo State, in Nigeria.
2.2 Preparation of Quality Protein Maize (QPM) and whole millet flours
The raw seeds were separately processed into flour using modified method of Olaoye et al. [20]. The seeds were sorted to remove unwanted materials like stones, pebbles and other foreign seeds, washed with distilled water and drained. The drained seeds were oven dried at 60°C for 20 h using a hot-air oven (Plus11 Sanyo Gallenkamp PLC, Loughborough, Leicestershire, UK) and milled with a laboratory blender (Model KM 901D; Kenwood Electronic, Hertfordshire, UK). Milled QPM was passed through a 60 mm mesh sieve (British Standard) to obtain QPM flour while the pearl millet flours was not sieved to obtain whole flour. The flour samples were packed in a plastic container, sealed and stored at room temperature (~27°C) until formulation.
2.3 Preparation of Soy cake Flour
Defatted soybean flour was processed using method described by Ijarotimi and Owoeye [21]. The defatted soybean cake was oven dried at 60°C for 4 h using a hot-air oven (Plus11 Sanyo Gallenkamp PLC, Loughborough, Leicestershire, UK), milled with a laboratory blender (Model KM 901D; Kenwood Electronic, Hertfordshire, UK) and passed through a 60 mm mesh sieve (British Standard) to obtain fine defatted defatted soybean flour. The flour was packed into plastic zip lock bag, sealed and stored at room temperature (~27°C) until analysis.
2.4 Composite Flour Formulations
Blends of the flours were produced using the central composite design of Design Expert using variables QPM flour (65-88%), soy cake flour (10-25%) and whole millet flour (2.5-12.5%) which targeted protein content of 10-20% and fibre of 5-10% in final product as recommended by FAO/WHO [22]. The flour samples were blended to obtain the following food sample combinations, that is, XYZ (QPM=76.03%; WMF=7.21%, DSF=16.42% and CST=5.00%); BCD (QPM=72.50%, WMF=2.50%, DSF=25.00%, CST=2.33%); FDT (QPM=80.52%, WMF=3.06%, DSF=16.42%, CST 5.00%); QPM (100% Quality Protein Maize flour). Cerolina (CER) was used as control sample.
2.5 Determination of Proximate Composition Improved Breakfast Meal Samples
Proximate compositions, that is, moisture content, ash, crude fibre, crude fat and crude protein content of improved breakfast meal samples were determined using the standard methods [23]. Carbohydrate content was determined by difference as follow:
Carbohydrate (%) = 100-(%Moisture + %Fat + %Ash + % Crude fibre + %Crude protein)
2.6 Statement of Animal Rights
The study protocol was approved by the Ethical Committee for Laboratory Animals of School of Agriculture and Agricultural Technology, Akure, Nigeria (FUTA/SAAT/2019/041). The experiments on animals were conducted in accordance with the force laws and regulations as regards care and use of laboratory animals.
2.7 In Vivo Determination of Glycaemic Index and load of flour blends
Twenty-five Wistar Albino rats of body weights between 150-175 g were divided into 5 groups (5 rats/group), and each group was housed in plastic cages in a climate-controlled environment with free access to feed and water. The rats were allowed to adapt to their new environment for 7 days, after which they were reweighed and then fasted for 12 h overnight. The blood glucose concentration of each animal was taken at zero time from the tail vein, followed by feeding them with 2.0 g of available carbohydrate experimental samples and glucose (a control), which was consumed within 25 min. After the consumption, the serum glucose levels of the animals were measured using an automatic glucose analyzer (‘Accu-chek Active’ Diabetes monitoring kit; Roche Diagnostic, Indianapolis, USA) at 0, 30, 60, 90 and 120 min intervals. The glycaemic response was determined as the Incremental Area under the Blood Glucose Curve (IAUC) measured geometrically from the blood glucose concentration-time graph ignoring area beneath the fasting level [24].
2.8 Measurement of blood glucose response
Blood glucose curves were constructed from blood glucose values of animals at time 0, after 15, 30, 45, 60, 90 and 120 min intervals after consumption of the glucose (control) and experimental food samples of each group. The Incremental Area Under the Curve (IAUC) was calculated for reference food (glucose) by the trapezoidal rule in every rats in each group separately as the sum of the surface of trapezoids between the blood glucose curve and horizontal baseline going parallel to x-axis from the beginning of blood glucose curve at time 0 to the point at time 120 min to reflect the total rise in blood glucose concentration after eating the reference food (glucose). The Incremental Area Under the Curve (IAUC) from the animals fed with the formulated food samples were similarly obtained. The glycaemic Index (GI) for each diet was calculated by ratio of Incremental Area Under two hours of blood glucose response or Curve (IAUC) for each diet to the IAUC for glucose solution standard according to the method of Wolever et al. [24] using the equation 1, and classified as follows: Low GI (<55%), Medium GI (56-69%) and High GI (>70%) [25].
GI= (Incemental area under 2h blood glucose curve or food test sample (2.0g))/(Incremental area under 2h blood glucose curve for glucose (2.0g)) × 100 (1)
2.9 Calculation of glycaemic load (GL)
Glycaemic Load (GL) for each food sample was determined by the method of Salmeron et al. [26]. In each individual glycaemic load was calculated by taking the percentage of the food’s carbohydrate content in a typical serving food and multiplying it by its glycaemic index (GI) value, using the formula in equation 2.
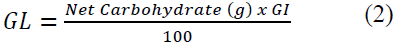
Net Carbs ¼ Total Carbohydrates in the food sample served. The GL was classified as follows: Low-GL (<10), Medium-GL (11-19) and High-GL (>20) [25].
2.10 In vivo anti-diabetic potentials of dough meals in diabetic rats
The in vivo anti-diabetic potentials of the dough meals were determined. The baseline blood glucose levels of the animals were measured before induction with Alloxan drug. The stock solution of Alloxan was prepared by dissolving 0.1 g of the power into 5 mL of freshly prepared sodium citrate buffer 0.1 M, pH 4.5 solution, to give a stock concentration of not less than 50 mg/kg body weight. Diabetes mellitus was induced by single injection intraperitoneally, of freshly prepared solution of Alloxan, dissolved in freshly prepared sodium citrate buffer in overnight fasted Wistar Albino rats [27]. The concentration of the Alloxan induced in each animal was determined based on the body weight of each animal using equation 3.
Vol.ofAlloxansolutioninduced (ml)=
(weightofanimal (kg/1000 gm) × dose(mg/kg))/concofAlloxan (mg/ml) (3)
where conc. = 50 mg/ml
The rats were then given 5% glucose solution to prevent excessive hypoglycaemic effects of the drug. The base line blood glucose levels of the animals were measured 72h after the drug administration through tail tipping using an automatic glucose analyzer (Roche ACCU-CHEK Active Model GU, Roche, Mannheim, Germany), and animals with obvious sign of diabetes (serum glucose ≥ 250 mg/dl) were selected for the study [28, 29]. The diabetic induced rats were divided into eight groups of five animals each. The animals in the first five groups were fed respectively on the three experimental flour blends (XYZ, BCD and FDT), “Cerolina”, a commercial flour formulation for diabetic patients (CER) and 100% quality protein flours (QPM).
The remaining three groups were treated as follows; first group were induced with Alloxan, treated with Gilbenclamide 5 mg B. P., an anti-diabetic drug, manufactured by the Nigerian-Chemical Co. Ltd., Lagos(STD), the second group was not induced with Alloxan, and taken as control (CNT), while the third group was induced but not treated and taken as induced not treated (INT), Animals in all the groups had access to water ad libitum, while animals in the STD, CNT and INT groups were fed on commercial rat feeds for the duration of the 28-day feeding period. The blood glucose levels of the rats were measured in the morning everyday by drawing blood from each rat through tail tipping and the blood glucose level was measured using automatic glucose analyzer (Roche ACCU-CHEK Active Model GU, Roche, Mannheim, Germany) [30].
2.11 Determination of biochemical properties of albino rats fed on dough meals
2.11.1 Collection of blood sample: After the experimental period of 28 days, the experimental animals were fasted overnightwith access to water ad libitum and sacrificed using the cervical dislocation method. The blood samples were collected via cardiac puncture with syringe and poured into heparinised and non-heparinised tubes. The non-heparinised tubes were allowed to clot and were centrifuged at 3000 x g for 25 min to obtain the sera, and the blood samples were stored in a deep freezer (-20°C) prior to haematological and biochemical analyses at the Medical Laboratory Unit of University Health Centre, Federal University of Technology, Akure [31].
2.11.2 Haematological and biochemical determination: The Automated Haematologic Analyzer (Sysmex, KX-21, Systmex Corporation, Kobe, Japan) was used to analyze the haematological parameters, that is, red blood cells (RBC), pack cell volume (PCV), haemoglobin concentration (Hbc), white blood cells (WBC), neutrophil (NEU) and lymphocytes (LYM) using methods described by Dacie and Lewis [32]. Mean corpuscular haemoglobin (MCH),mean corpuscular haemoglobin concentrated (MCHC) and mean corpuscular volume (MCV) were calculated from values obtained from RBC, PCV and Haemoglobin (Hbc) content [32]. The biochemical parameters were analyzed using methods described by Jasper et al. [33]. The blood sample was first centrifuged at 1,500 × g for 10 min at ambient temperature. The serum was then separated and used for liver function assessment employing measurements of the enzymes aspartate aminotransferase (AST), alanine aminotransferase (ALT) and Alkaline Phosphate (ALP). Renal function was evaluated using serum concentrations of urea and creatinine. These tests were performed using disposable kits obtained from LabtestDiagnostica S.A. (Lagoa Santa, MinasGerais, Brazil) [34, 35].
2.12 Statistical analysis
Determinations were made in triplicates and means of data were obtained and compared using the one-way ANOVA and separated using Duncan Multiple Range Test (DMRT) at p=0.05 significant level by SPSS [36].
3. Results and Discussion
3.1 Proximate compositions of flour samples
The proximate composition of dough meal composite flours is presented in Table 1. The moisture content (MC) of samples showed that XYZ (8.86%) had the highest value, while QPM (7.96%) had the least value. The moisture content for these samples were comparatively lower than recommended value for flour sample (<10%). This observation implies that the spoilage period of the flour would be longer, since the moisture content of the flour samples was very low for the activity of microorganisms; and this finding agreed with other reports for flour samples [37-39]. The crude protein content of the experimental samples ranged between 10.63% in QPM and 27.28% in BCD, and the value was significantly higher in experimental samples than Cerolina (CER) and QPM. This observation could be attributed to the inclusion of soy cake, which is very high in protein [38, 40, 41]. It is evident that combination of two or more varieties cereals and legumes usually increased the protein quality of the overall products [39, 42, 43]. The crude fibre of the samples ranged from 2.24% in QPM to 9.47% in XYZ. Comparatively, the crude fibre in the experimental samples was significantly (p<0.05) higher than those of CER and QPM. Nutritionally, consumption of these experimental dough meals would be of benefits to the consumers, since, evidences have shown that dietary fibres promote good health by prevent degenerative diseases like diabetes and hypertension [10, 11].
3.2 Trends of blood glucose concentration of rats fed on flour blends, 100% QPM and commercial flour sample
The in-vivo blood glucose response/concentrations of Albino Wistar rats fed with the experimental flour samples (XYZ, BCD and FDT), 100% quality protein maize flour (QPM) and formulated commercial flour sample for diabetic patients (CER), as well as 100% glucose estimated at 0, 30, 60, 90 and 120 minutes, respectively, using the oral glucose tolerance test, are shown in Figure 1. The oral glucose tolerance test (OGTT) is a widely used to evaluate apparent insulin release and insulin resistance in various clinical settings [44]. As shown in Figure 1, the blood glucose concentration of the control group (glucose, as indicated by the red curve), rose from about 100 mg/dL to 300 mg/mg/dL in the first 30 mins of being administered to the rats and thereafter reduced slowly to a little below 200 mg/dL over the subsequent 90 minute period. This indicates a high absorption and metabolizing rate of blood glucose in the rats, which may most likely result in high incidence of diabetes [45].
Conversely, the blood glucose concentration of rats fed on the experimental flour samples as well as the control sample (CER), increased gradually in the first 30 minutes and thereafter further reduced slowly. For instance, sample BCD showed the lowest blood glucose trend after administration of the sample in rat. This indicates low digestion and absorption rate of the food sample, and consequently its ability to regulate blood glucose level and consequently high potential to control type-2 diabetes. The low blood glucose increasing properties of these experimental dough meals control could be attributed to the inclusion of soy cake and whole pearl millet flours in the blends. These flour samples have known to contain certain bioactive compounds such as dietary fibres, phytochemicals and protein, which capable of lowering blood sugar concentration [8, 46].
3.3 Glycaemic Index and Load
The value of glycaemic index for the three experimental samples in this study were 35% for BCD, 51% for XYZ, and 37% for FDT sample (Figure 2). The high glycaemic index of sample XYZ compared to other two experimental food samples could be attributed to its relatively high pearl millet (7.2%) and cassava starch (5%) contents. However, the glycaemic index of sample BCD, FDT and XYZ were lower than recommended value for low glyceamic index foods (<50), which several studies have established as ideal foods for diabetic patients [16, 24, 26]. The glycaemic load (GL) of the flour samples showed similar trends like the glycaemic index, with the following values 8% for BCD sample, 11% for FDT and 13% for XYZ. These samples, particularly BCD, could be classified as low glycaemic load foods, since their values were within the recommended value (<10%) for low glycaemic load foods.
Glycaemic index of foods determines the rate at which carbohydrate is digested to increase the blood glucose level [47, 48]. It is well established that glycaemic index of a food is known to be influenced by the nature of the starch/carbohydrates granules and food processing, and that low glycaemic index foods are associated with reduced risk of diabetes [26], body weight and serum cholesterol [49, 50]. The values of glycaemic index (GI) obtained for the present study experimental food samples were comparatively lower than what reported for composite flour blends from plantain, defatted soya and tigernut flours [16]. The variations in GI values in these studies could be due to variation in the food materials used in these studies. In general, the low glycaemic properties of the flour samples used in this study have high hypoglycaemic effect, which means that the dough meal prepared from these flour samples will most probably result in gradual increase in the blood glucose concentration of consumers, thereby suitable to manage type-2 diabetes.
3.4 Haematological and Biochemical properties of Alloxan-induced Albino Wistar rats fed on formulated flour blends, 100% QPM and commercial flour sample
The haematological and biochemical properties of rats induced with diabetic and fed with the formulated dough meal flour samples are presented in Tables 2 and 3, respectively. Haematological examination of blood serum is a common and important method used for the assessment of new compounds, such as foods and drugs, and for the detection of some changes in health status of animals and their vital organs, and consequently their fitness. Hematological and biochemical parameters can be used to determine the extent of deleterious effect of foreign compounds (toxic or microorganisms) in the blood constituents of an animal [51]. There were significant (p<0.05) differences for all hematological parameters measured for all the flour blends, especially the PVC, Hb, WBC and RBC, with respect to the three experimental blends, i.e., XYZ, BCD and FDT, these values ranged as 46.35 - 51.25%; 13.40-18.10g/L; 8.30-10.80 (× 10/mm3); and 4.19-6.94 (× 106), respectively.
Sample XYZ had the highest values for these parameters except for WBC, for which sample FDT had the highest value of 10.80 × 103/mm3. Comparatively, these values were within the recommended values for PVC (37.6-50.6), Hb (11.5-16.1), WBC (6.6-12.6) and RBC (6.76-9.75) [52]. This observation indicates that the experimental flour samples in this study are of high nutritional quality and contain appreciate amount of essential nutrients to support production of blood components in rats, and besides, the food samples were free from toxic chemicals and microorganisms, which may result in heamolytic of the blood. Hence, the food samples may be suitable for the prevention of anaemia [53, 54]. This observation agreed with the observations of Roberts, who reported that foods containing high quality protein would enhance haemoglobin production in animals.
Comparatively, the haematogical indices obtained in this study were higher than that of diabetic- induced rats treated with Glibenclamide (a synthetic anti-diabetic agent), and also, values obtained by Oluwajuyitan and Ijarotimi [39] for dough meal from the blends of plantain, tigernut and defatted soy bean. This finding indicates that the experimental flour samples were without any side effects and did not cause heamolytic of the blood samples. For MCHC, MCV and MCH, the experimental samples, that is, XYZ, BCD, and FDT ranged from 31.30 to 33.70 g/dL 19.95-31.43 pg and 67.70-89.37 fl. These results showed that sample XYZ had the least values for most parameters except for MCHC which sample FDT had the lowest value, while sample BCD had the highest values. These haematological indices were lower than those values obtained for complementary foods from fermented popcorn, African locust bean and bambara groundnut flour blends [54] and flour blends for dough meal from plantain, tigernut and defatted soy bean [16]. These haematological indices (MCHC, MCV and MCH) are useful indicator for Hb concentration of the red blood cells, and their low concentrations in animals indicated haemolytic anaemia [55].
The biochemical properties of Alloxan-induced diabetic rats fed on the flour samples are presented in Table 2. These parameters measure the liver and kidney functionalities of the animals as well as their serum lipid profiles. The liver also plays a major role in regulating carbohydrate metabolism, thereby assisting in the maintenance of normal blood glucose concentrations in both fasting and post-prandial states [56]. The alanine amino transferases (ALT) and aspartate amino transferases (AST) are considered as markers of hepatic damage or injury [57]. The normal serum ALT, AST and ALP were 7-56 µ/L, 45.7-80.8 µ/L and 41-133 µ/L, respectively [52]. Higher levels of transaminase enzymes, ALT and AST, had been attributed to hepatic injury of damage [58], or extensive tissue necrosis during myocardial infarction and also in chronic liver diseases like liver tissue degeneration and necrosis [59, 60].
In this study, the ALT, AST and ALP of rats fed on XYZ, BCD and FDT were within the normal ranges for these enzymes. This could most probably due the therapeutic effects of the flour samples on the rats. However, the group of rats induced with Alloxan but not treated (INT) had higher values for these enzymes (AST, ALT and ALP) than recommended range values. For instance, the AST, ALT and ALP for this group of animals were 45.50 µ/L, 40.5 µ/L and 164 µ/L, respectively, and were not within the normal range values for these enzymes. This may be attributed to the liver damage by the Alloxan, which led to the leakage of these enzymes from the liver into the blood stream [61]. This finding agreed with the report of Mansour et al. [62], who reported on hepatic effect of Alloxan in rats. The values of AST, ALT and ALP in this study were higher than what obtained for the rats fed on dough meals from plantain, tigernut and defatted soy bean flours for dough meal [16].
The AST/ALT ratio is often seen as being of more clinical importance than each of the two transferases, especially at increased levels. A coenzyme pyridoxal-5\'-phosphate deficiency may depress serum ALT activity and consequently increases the AST/ALT ratio [63]. The standard ratio for the AST/ALT ratio is <1, and it normally increases in progressive liver functional impairment [64]. The AST/ALT ratios for the experimental samples, as well as other flour samples are within the standard, but a little higher than the values obtained by Oluwajuyitan and Ijarotimi [16] for dough meal flours from plantain, defatted soy bean and tigernut flours. This is due to the relatively higher ALT values obtained in their study compared to this present study, which may have been as a result of the different effect of streptozocin used to induce diabetes in their study, as against Alloxan used in this study. However, the ratio for animal group, induced with Alloxan but not treated (INT) was 1.12, which was higher than the standard value of <1. This is due the relatively lower value of ALT and higher of AST compared to other groups, which most probably is due to the adverse effect of Alloxan. Previous studies have attributed high concentration of ALT in the blood to possible liver mal-function and damage [65, 66]. The low values of this parameter obtained for the experimental samples are indicative of safety of these samples without any adverse effect on the liver functionality in consumers.
The serum protein and albumin are also important indices of functionality of the liver. The standard range for these parameters is, 6-8 g/dL for serum protein, and 3.5-5.0 g/dL for albumin, respectively [52]. The serum protein and albumin for all the flour samples were within these standard values, including the group of Alloxan-induced and untreated animals. This is most likely because Alloxan produced no obvious effect on the total protein and albumin of the serum in all the test groups for all the flour samples. For the three experimental samples namely, XYZ, BCD and FDT, there were significant difference (p<0.05) for serum protein, which ranged between 5.98 g/dL and 6.28 g/ dL with sample BCD having the lowest value. For albumin, there was no significant difference (p>0.05) between the experimental samples with values ranged from 4.35 to 4.47 g/dL. These values were comparatively higher than values for total protein and albumin of rats fed on dough meal flours from blends of plantain, tigernut and soy bean flours reported by Oluwajuyitan and Ijarotimi [16]. This variation is most likely as a result of the difference in food materials and quality of protein. Fujita et al. reported that serum protein and albumin depend on the quantity and quality of protein intake.
The standard values for the metabolites creatinine and urea are <1.5 mg/dL and <55 mg/dL, respectively [52]. The result showed that the values obtained for all the rat groups fed with the flour samples, including the untreated group (INT) were within the normal range values. This indicates that there was no sign of kidney damage by these experimental food samples, that is, XYZ, BCD and FDT. The creatinine and urea concentration for the rats fed on these experimental samples were 0.75-1.00 mg/dL and 40.40-41.40 mg/dL, respectively. These results agreed with the report of Okafor and Ebuehi [46], who reported that was no significant difference (p>0.05) in the urea, creatinine and total bilirubin concentration of rats fed on bread produced from wheat-soy composite flours. Oluwajuyitan and Ijarotimi [16] however obtained much higher values for urea, but lower values for creatinine in rats fed with dough meal flour blends from plantain, tiger nut and defatted soy bean flours.
With respect to electrolytes potassium (K), Calcium (Ca) and Chloride (Cl), which are indices of kidney functionality, apart from paying important role in cellular function and enzyme activities [67], their values for the three experimental samples, XYZ, BCD and FDT, are 4.75, 4.60 and 4.75 mg/dL, 10.10, 9.80 and 9.80 mg/dL and 98.00, 94.20 and 96.40 mg/dL respectively. These results are within the standard values of 3.5-5.0mg/dL, 8.5-10.5 mg/dL and 90-100 mg/dL respectively for K, Ca and Cl, which show only marginal differences between the samples for all the electrolytes. This indicates that there is little effect of substitution levels of both soy cake and whole millet flours on these parameters. Results also show that the values of these parameters for rats fed with other flour samples, apart from the three experimental samples, as well as rats in Alloxan-induced but untreated group (INT), are also within the normal ranges for these electrolytes. This most probably gives an indication that Alloxan produced little of no adverse effect on these kidney functionality parameters.
3.5 Blood Glucose Reduction Potential of Dough Meal Flour Samples, Quality Protein Maize and Commercial Flour Sample
The blood glucose reduction potentials of Alloxan-induced diabetic rats fed on the flour samples are shown in Figure 3. The percentage of blood glucose reduction in rats fed on experimental flour samples were in ascending order of BCD < FDT < XYZ with values of 57.5%, 70% and 75%, respectively.
The higher blood glucose reducing properties of XYZ (i.e., 76.03 quality protein maize, 16.76 soy cake, 7.21 whole millet and 5.000 cassava starch) in diabetic induced rats when compared with other experimental samples (BCD and FDT) could be due to its high fibre contents from the inclusion of soy cake and pearl millet flours. Nutritionally, studies have reported that higher fibre intakes have some health benefits such as promotion of normal gastrointestinal function, maintenance of normal blood glucose and blood pressure [68, 69]. The percentage blood glucose reduction of XYZ sample was comparable to that of control samples (QPM and CER), but significantly (p<0.05) higher than that of STD (group of rats treated with synthetic anti-diabetic agent- Glibenclamide). This finding agreed with the reports that long time consumption of plant-based foods, which contain bioactive compounds have therapeutic properties on diabetes and other degenerative diseases [8, 9, 70]. Comparatively, the blood glucose reduction potentials of experimental dough meal samples, particularly XYZ, in this present study agreed with the values obtained for dough meals from the blends of plantain, tigernut and defatted soybean flours reported by Oluwajuyitan and Ijarotimi [16].
|
Samples/Parameters |
XYZ |
BCD |
FDT |
QPM |
CER |
|
Moisture |
8.86 ± 0.12a |
8.46 ± 0.13b |
8.61 ± 0.04b |
7.96 ± 0.08c |
8.63 ± 0.16b |
|
Protein |
23.43 ± 0.09b |
27.28 ± 0.04a |
23.20 ± 0.04b |
10.63 ± 0.19d |
19.07 ± 0.39c |
|
Crude fat |
7.47 ± 0.05a |
6.33 ± 0.03c |
7.22 ± 03b |
5 43 ± 0.14d |
5.03 ± 0.25e |
|
Total ash |
4.23 ± 0.01a |
4.14 ± 0,04ab |
4.03 ± 0.05b |
1.53 ± 0.04d |
3.20 ± 0.05c |
|
Crude fibre |
9.47 ± 0.81a |
8.61 ± 0.02b |
8.21 ± 0.05c |
2.24 ± 0.11e |
6.80 ± 0.05d |
|
Carbohydrate |
55.41 ± 0.07d |
53.69 ± 0.04e |
57.34 ± 0.15c |
80.18 ± 0.21a |
65.77 ± 0.25b |
Means of triplicate determinations reported on dry-weight basis except for moisture; Values with the same superscript along rows are not significantly different (p>0.05); XYZ: 76.034(A):16.760(B):7.206(C):5.000(D); BCD: 72.500(A):25.000(B):2.500(C):2.330(D); FDT: 80.524(A):16.420(B):3.056(C):5.000(D); QPM: 100% Quality protein maize; CER: Cerolina flour (A functional flour for diabetic patients)
Table 1: Proximate compositions of Quality Protein Maize, Cerolina and blends of Quality Protein Maize, Soy cake and whole pearl millet flours.
XYZ: 76.034 (QPM): 16.760 (DSF): 7.206 (WMF): 5.000 (CST); BCD: 72.500 (QPM) 25.000 (DSFB): 2.500 (WMF): 2.330 (CST); FDT: 80.524 (QPM): 16.420 (DSF): 3.056 (WMF) 5.000 (CST); QPM: 100% Quality Protein Maize flour; CER: Cerolina; formulated flour for diabetic patients
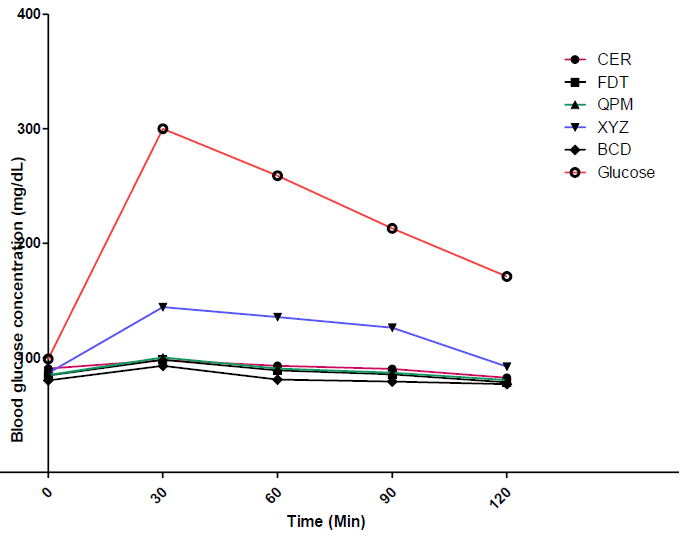
Figure 1: Blood glucose concentration of flour samples.
XYZ: 76.034 (QPM): 16.760 (DSF): 7.206 (WMF): 5.000 (CST); BCD: 72.500 (QPM) 25.000 (DSFB): 2.500 (WMF): 2.330 (CST); FDT: 80.524 (QPM): 16.420 (DSF): 3.056 (WMF) 5.000 (CST); QPM: 100% Quality Protein Maize flour; CER: Cerolina; formulated flour for diabetic patients; Low Glycaemic foods (<55%), Medium Glycaemic foods (55-69%) and high Glycaemic foods (>70%) [24, 26].
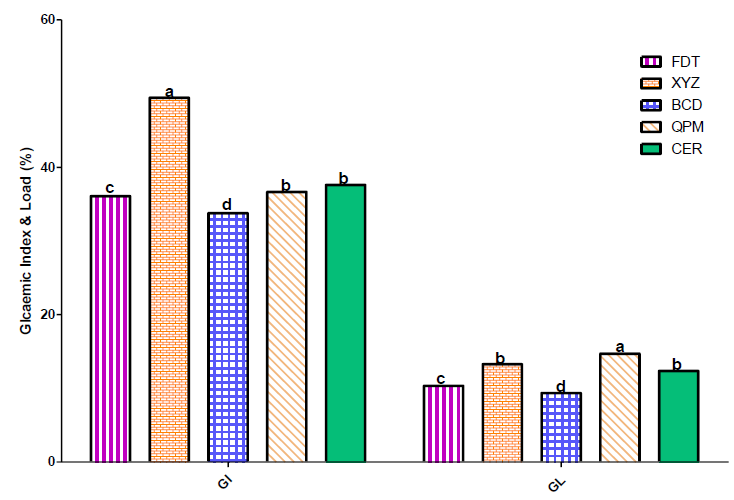
Figure 2: Glycaemic index and load of flour samples using rats.
|
Parameters/Samples |
XYZ |
BCD |
FDT |
QPM |
CER |
STD |
INT |
CNT |
|
PVC (%) |
51.25 ± 0.75ab |
46.35 ± 1.15cd |
48.55 ± 0.75bc |
41.40 ± 5.90e |
43.27 ± 1.37de |
47.95 ± 0.95bc |
49.15 ± 1.35bc |
54.15 ± 2.35a |
|
Hb (g/L) |
18.10 ± 2.30a |
13.40 ± 2.30c |
15.20 ± 0.40abc |
13.67 ± 2.05c |
14.02 ± 0.76bc |
16.92 ± 1.91ab |
15.22 ± 0.40abc |
14.87 ± 0.15bc |
|
WBC (109/L) |
8.75 ± 0.45cd |
8.30 ± 0.50cd |
10.80 ± 0.50ab |
9.55 ± 0.55bc |
11.60 ± 2.10a |
7.35 ± 1.45d |
8.67 ± 0.75cd |
9.05 ± 0.35bcd |
|
RBC (1012/L) |
6.94 ± 0.19a |
4.19 ± 0.14c |
5.69 ± 0.20b |
5.99 ± 0.97ab |
6.24 ± 0.63ab |
5.56 ± 0.76b |
6.12 ± 0.32ab |
6.92 ± 0.26a |
|
MCHC (g/dL) |
32.90 ± 1.70ab |
33.70 ± 0.70a |
31.30 ± 1.30bc |
32.85 ± 0.25ab |
32.87 ± 0.15ab |
32.15 ± 1.15abc |
30.85 ± 0.05c |
27.40 ± 0.90d |
|
MCH (pg) |
19.95 ± 2.15d |
31.43 ± 0.90a |
29.95 ± 1.55a |
22.80 ± 0.30bc |
22.90 ± 1.50bc |
20.45 ± 1.65cd |
24.68 ± 0.79b |
21.57 ± 0.61cd |
|
MCV (fl) |
67.70 ± 3.20e |
89.37 ± 2.15a |
82.00 ± 0.20b |
69.40 ± 1.40de |
74.00 ± 0.30cd |
68.20 ± 7.30e |
80.53 ± 2.05b |
78.53 ± 0.45bc |
|
Neutrophils (%) |
61.15 ± 1.65b |
63.20 ± 0.40b |
63.05 ± 0.05b |
43.75 ± 1.85c |
44.38 ± 2.25c |
39.20 ± 1.90d |
72.33 ± 2.75a |
44.15 ± 0.85c |
|
Lymphocytes (%) |
32.60 ± 2.80e |
33.90 ± 1.10d |
32.40 ± 0.50e |
35.75 ± 0.05c |
25.70 ± 4.80f |
46.77 ± 6.45a |
44.37 ± 0.91a |
37.40 ± 2.45b |
|
Monocytes (%) |
0.037 ± 0.002c |
0.038 ± 0.001c |
0.035 ± 0.004c |
0.044 ± 0.004b |
0.035 ± 0.001c |
0.044 ± 0.004b |
3.185 ± 0226a |
0.002 ± 001d |
|
Eosinophils (%) |
0.067 ± 0.006c |
0.080 ± 0.005c |
0.067 ± 0.004c |
0.092 ± 0.004b |
0.067 ± 0.002c |
0.071 ± 0.002c |
2.180 ± 0.045a |
0.020 ± 0.005d |
|
Basophils (%) |
0.025 ± 0.005 |
0.032 ± 0.025 |
0.020 ± 0.000 |
0.085 ± 0.015 |
0.028 ± 0.015 |
0.032 ± 0.005 |
1.850 ± 0.052 |
0.020 ± 0.040 |
Means of triplicate determinations reported: Means with similar superscripts along rows are not significantly different (p > 0.05); XYZ: 76.034 (QPM): 16.760 (DSF): 7.206 (WMF): 5.000 (CST); BCD: 72.500 (QPM) 25.000 (DSFB): 2.500 (WMF): 2.330 (CST); FDT: 80.524 (QPM): 16.420 (DSF): 3.056 (WMF) 5.000 (CST); QPM: 100% Quality Protein Maize flour; CER: Cerolina; STD: Drug-controlled group; INT: Alloxan-induced but not treated; CNT: Not induced
Table 2: Effects of flour samples on hematological parameters of Alloxan-induced diabetic rats.
|
Parameters/Samples |
XYZ |
BCD |
FDT |
QPM |
CER |
STD |
CNT |
INT |
|
Albumin (g/dL) |
4.35 ± 0.95bc |
4.47 ± 1.15ab |
4.46 ± 1.10ab |
4.19 ± 0.25d |
4.54 ± 0.00a |
4.40 ± 0.95ab |
4.35 ± 0.95bc |
4.20 ± 0.40d |
|
Total protein (g/dL) |
6.13 ± 2.05abc |
5.98 ± 0.60bcd |
6.28 ± 0.70a |
5.80 ± 0.55d |
6.04 ± 0.60bc |
5.95 ± 1.50cd |
6.16 ± 1.15abc |
6.25 ± 1.75ab |
|
ALP (µ/L) |
94.20 ± 2.20de |
92.80 ± 0.80e |
104.20 ± 6.20bc |
111.20 ± 0.80b |
101.20 ± 9.22cde |
102.83 ± 8.50bcd |
74.50 ± 0.90f |
164.25 ± 5.06a |
|
ALT (µ/L) |
55.50 ± 3.50a |
49.00 ± 0.00b |
48.50 ± 1.50b |
48.50 ± 1.50b |
48.00 ± 1.00b |
49.50 ± 0.50b |
40.50 ± 1.50b |
40.50 ± 1.50ab |
|
AST (µ/L) |
43.00 ± 1.00b |
39.50 ± 0.50c |
39.90 ± 0.00c |
41.00 ± 1.00bc |
41.50 ± 2.50bc |
41.50 ± 2.50bc |
40.50 ± 1.50b |
45.50 ± 1.50a |
|
AST/ALT |
0.77 ± 0.02e |
0.81 ± 0.04d |
0.81 ± 0.05d |
0.85 ± 02c |
0.86 ± 0.06c |
0.84 ± 0.01c |
1.00 ± 0.02b |
1.12 ± 0.04a |
|
Creatinine(mg/dL) |
0.75 ± 0.15ab |
1.00 ± 0.10ab |
1.00 ± 0.15ab |
0.60 ± 0.00b |
0.75 ± 0.15ab |
0.90 ± 0.00ab |
1.15 ± 0.25a |
0.85 ± 0.25ab |
|
Urea (mg/dL) |
40.40 ± 0.40c |
41.40 ± 1.40bc |
40.70 ± 2.10c |
44.40 ± 3.60ab |
38.60 ± 0.00c |
38.60 ± 0.00c |
45.40 ± 2.60a |
45.40 ± 2.60a |
|
K (mg/dL) |
4.75 ± 0.15b |
4.60 ± 0.00c |
4.75 ± 0.05b |
4.75 ± 0.05b |
4.90 ± 0.00a |
4.80 ± 0.00ab |
4 85 ± 0.05ab |
4 75 ± 0.05b |
|
Ca(mg/dL) |
10.10 ± 0.30a |
9.80 ± 0.00ab |
9.80 ± 0.60ab |
10.10 ± 0.30a |
10.10 ± 0.30a |
9.90 ± 0.30ab |
9.90 ± 0.10ab |
9.40 ± 0 20b |
|
Chloride(mg/dL) |
98.00 ± 0.00ab |
94.20 ± 3.80bc |
96.40 ± 3.80ab |
96.30 ± 3.70ab |
99.00 ± 1.00a |
98.00 ± 0.00ab |
100.00 ± 0.00a |
91.50 ± 1.10c |
|
Cholesterol (mg/dL) |
216.00 ± 8.00a |
204.00 ± 4.00abc |
205.00 ± 1.00abc |
203.00 ± 3.00bc |
203.33 ± 5.77bc |
214.00 ± 10.00ab |
207.00 ± 11.00abc |
196.00 ± 0.00c |
|
Triglycerides(mg/dL) |
177.00 ± 1.00a |
180.00 ± 2.00a |
179.00 ± 3.00a |
180.00 ± 4.00a |
180.95 ± 6.95a |
180.95 ± 6.95a |
178.00 ± 4.00a |
182.95 ± 4.95a |
|
HDL(mg/dL) |
82.60 ± 3.80a |
83.20 ± 3.20a |
82.00 ± 0.00a |
80.90 ± 1.10a |
84.20 ± 2.20a |
83.20 ± 3.20a |
83.10 ± 1.00a |
81.00 ± 1.00a |
|
LDL(mg/dL) |
94.50 ± 3.70ab |
90.10 ± 0.70b |
95.30 ± 2.90a |
94.50 ± 3.70ab |
95.30 ± 2.90a |
91.60 ± 0.80ab |
83.10 ± 1.00a |
81.00 ± 1.00a |
Means of Triplicate determinations reported; Means with same superscripts along rows are not significantly different (P > 0.05); XYZ: 76.034 (QPM): 16.760 (DSF): 7.206 (WMF): 5.000 (CST); BCD: 72.500 (QPM) 25.000 (DSFB): 2.500 (WMF): 2.330 (CST); FDT: 80.524 (QPM): 16.420 (DSF): 3.056 (WMF) 5.000 (CST); QPM: 100% Quality Protein Maize flour; CER: Cerolina; STD: Drug-controlled group; INT: Alloxan-induced but not treated; CNT: Not induced
Table 3: Effects of flour samples on biochemical properties of Alloxan-induced diabetic rats.
XYZ: 76.034 (QPM): 16.760 (DSF): 7.206 (WMF): 5.000 (CST); BCD: 72.500 (QPM) 25.000 (DSFB): 2.500 (WMF): 2.330 (CST); FDT: 80.524 (QPM): 16.420 (DSF): 3.056 (WMF) 5.000 (CST); QPM: 100% Quality Protein Maize flour; CER: Cerolina; STD: Drug-controlled group; INT: Alloxan-induced but not treated
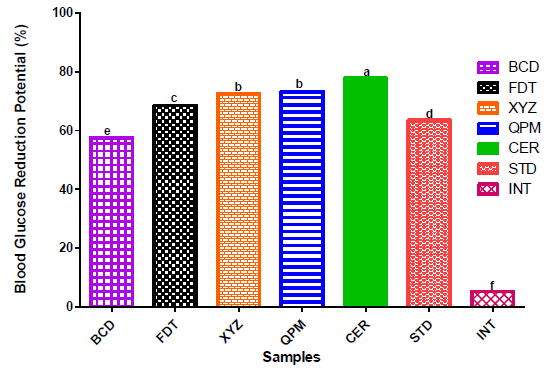
Figure 3: Percentage blood glucose reduction of Alloxan-induced diabetic rats fed with flour samples.
4. Conclusion
This study showed that experimental flour samples from the blends of quality protein maize, soy cake and whole pearl millet flours contain appreciable amount of protein and fibre contents with low glycaemic index and glycaemic load. The dough meal flour samples, particularly XYZ, had high potentials for blood glucose reduction in diabetes-induced rats without any adverse side effects on the growth patterns, haematological and biochemical status of the rats fed on these experimental flour samples. Hence, dough meal prepared from this flour sample, which contains 76.03% quality protein maize, 16.76% soy cake, 7.21% whole pearl millet and 5.00% cassava starch, may be suitable as a functional food for the management and control of diabetes mellitus. This information may be useful for food industries and processors engaged in functional food production. Future study will focus on clinical trials on diabetic patients to determine the effectiveness of the formulated flour blends in the management of diabetes.
References
- Wild S, Roglig GG, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27 (2004): 1047-1053.
- Shaw J, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Research and Clinical Practice 87 (2010): 4-14.
- Pereira MA, Jacobs DR, Pins JJ, et al. Effect of whole grains and insulin sensitivity in overweight hyperinsulinemic adults. American Journal of Clinical Nutrition 75 (2002): 848-855.
- Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999-2004. Journal of American Medical Association 295 (2006): 1549-1555.
- Osuafor TO, Ele PU. The pattern of admissions in the medical wards of Nnamdi Azikiwe University Teaching Hospital Nnewi. Oriental Journal of Medicine 16 (2004): 11-15.
- Odenigbo CU, Oguejiofor OC. Pattern of medical admissions at the Federal Medical Centre, Asaba-a two year review. Nigerian Journal Clinical Practice 12 (2009): 395-397.
- Prasad S, Krishnadas M, McConkey K, et al. The tangled causes of population decline in two harvested species: a comment on Ticktin. Journal of Applied Ecology 51 (2014): 555-559.
- Famakin O, Fatoyinbo A, Ijarotimi OS, et al. Assessment of nutritional quality, glycaemic index, anti-diabetic and sensory properties of plantain (Musa paradisiaca-based functional dough meals. Journal of Food Science and Technology 53 (2016): 865-3875.
- Igwe CU, Ojiako AO, Emejulu AA, et al. Phytochemical Analysis of plants traditionally used in malaria treatment in southeastern Nigeria. Journal of Research in Biochemistry 1 (2012): 015-022.
- Montonen J, Knekt P, Jarvinen R, et al. Whole-grain and fibre intake and the incidence of type 2 diabetes. American Journal of Clinical Nutrition 77 (2003): 622-629.
- Lairon D, Arnault N, Bertrais S, et al. Dietary fibre intake and risk factors for cardiovascular disease in French adults. American Journal of Clinical Nutrition 82 (2005): 1185-1194.
- Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. Journal of American Medical Association 288 (2002): 2569-2578.
- Zhang CX, Ho SC, Chen YM, et al. Greater vegetable and fruit intake is associated with a lower risk of breast cancer among Chinese women. International Journal of Cancer 12 (2014): 181-188.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach position statement of the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetes Care 35 (2012): 1364-1379.
- Agius L. Lessons from glucokinase activitors: the problem of declining efficacy. Expert Opinion Therapeutic Patents 24 (2014): 1155-1159.
- Oluwajuyitan TD, Ijarotimi OS. Nutritional, antioxidant, glycaemic index and Anti-hyperglycaemic properties of improved traditional plantain-based (Musa AAB) dough meal enriched with tigernut (Cyperus esculentus) and defatted soybean (Glycine max) flour for diabetic patients. Heliyon 5 (2019): 1-27.
- Malomo O, Ogunmoyela OAB, Oluwajoba SO, et al. The development of whole wheat dough meal enriched with soybeans. International Research Journal of Biochemistry and Bioinformatics 1 (2011): 266-274.
- Malomo O, Ogunmoyela OAB, Adekoyeni OO, et al. Rheological and Functional Properties of Soy-Poundo Yam Flour. International Journal of Food Science and Nutrition Engineering 2 (2012): 101-107.
- Abioye VF, Ade-Omowaye BIO, Babarinde GO, et al. Chemical, physico-chemical and sensory properties of soy-plantain flour. African Journal of Food Science 5 (2011): 176-180.
- Olaoye OA, Ubbor SC, Okoro VO, et al. Performance of Malted Maize Flour as Composite of Wheat in the Production of Cake. American Journal of Agricultural Science 2 (2015): 126-132.
- Ijarotimi OS, Owoeye OR. Study on energy-nutrient density, functional and organoleptic properties of complementary foods from indigenous plant based food materials. Journal of Advances in Food Science and Technology 4 (2017): 73-83.
- FAO/WHO Protein Quality Evaluation. Report of Joint FAO/WHO Consultation, Bethesda MD Rome (1990).
- Association of Official Analytical Chemists. 16th Edn. Washington DC. USA (2015).
- Wolever TMS, Jenkins DJA, Jenkins AL, et al. The Glycaemic index: methodology and clinical implications. American Journal of Clinical Nutrition 54 (1991): 846-854.
- Dona AC, Guilhem P, Robert GG, et al. Digestion of starch: in vivo and in vitro kinetic models used to characterize oligosaccharide or glucose release. Carbohydrate and Polymer 80 (2010): 599-617.
- Salmeron J, Manson JE, Stampfer MJ, et al. Dietary fibre, glycaemic load, and risk of non-insulin dependent diabetes mellitus in women. Journal American Medical Association 277 (1997): 472-477.
- Abu H, Zulfiker FAR, Mahbubur R, et al. Antidiabetic and Antioxidant Effect of Scoparia dulcis in Alloxan induced Albino Mice. International Journal of Pharmaceutical Technology and Research 2 (2010): 2527-2534.
- Ramdas P, Balakrishnan S. Extract of Adenanthera pavonina L. seed reduces development of diabetic nephropathy in streptozotocin-induced diabetic rats. Avicenna Journal of Phytomedical 2 (2012): 233-242.
- Parks BW, Nam E, Org E, et al. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metabolism 17 (2013): 56-62.
- Meiton DA. Reversal of Type -1 diabetes in mice. New England Journal of Medicine 355 (2006): 89-90.
- Shittu OK, Musa F, Gbadamosi DF. Trypanocidal activity and haematological changes in T. brucei infected rats treated with methanolic leaf extract of thymus vulgaris. International Journal Applied Biological Research 5 (2013): 109-114.
- Dacie JV, Lewis SM. Practical Haematology. 8th Edn, Edinburgh, England. Churchill Livingstone (2002).
- Jasper R, Locatelli OG, Pilati C, et al. Evaluation of biochemical, haematological and oxidative parameters in mice exposed to the herbicide glyphosate- Roundup. Interdisciplinary Toxicology 5 (2012): 133-140.
- Adeneye AA, Ajagbonna OP, Adeleke TI, et al. Preliminary toxicity and phytochemical studies of the stem bark aqueous extract of Musanga cecropioides in rats. Journal of Ethnopharmacology 105 (2006): 374-379.
- Hauwa SB, Baba G, Yusuf H. Hematological and Histological changes induced on the Selected Organs of (Wistar) Rats by leaf extracts from herbs of ethno medicinal application. Nigerian Journal of Chemical Research 18 (2013): 48-57.
- Statistical Packages for the Social Sciences, Version 23.0 IBM Corp., Armonk, NY, USA (2015).
- Elleuch M, Bedigian D, Roiseux O, et al. Dietary fibre and fibre-rich by-products of food processing: characterisation, technological functionality and commercial applications. Rev. Food Chemistry 124 (2011): 411-421.
- Awolu OO, Omoba OS, Olawoye O, et al. Optimization of production and quality evaluation of maize-based snack supplemented with soybean and tiger-nut(Cyperus esculenta) flour. Food Science and Nutrition 5 (2017): 3-13.
- Akinjayeju O, Fagbemi TN, Ijarotimi OS, et al. Optimization and evaluation of some physico-chemical and nutritional properties of cereal-based soya-fortified flours for functional dough meal. Journal of Advances in Food Science and Technology 6 (2019): 40-59.
- Akubor PI. Evaluation of physico-chemical and sensory properties of soybean-sweet potato supplementary foods. Journal of Chemical Society of Nigeria 33 (2008): 15-22.
- Aletor O. Soyabean meal versus soyabean protein isolate: A comparative study of the nutritive and functional attributes. Journal of Food, Agriculture and Environment 8 (2010): 34-38.
- Awolu OO, Oluwaferanmi PM, Fafowora OI, et al. Optimization of the extrusion process for the production of ready-to-eat snack from rice, cassava and kersting’s groundnut composite flours. LWT Food Science and Technology 64 (2015a): 18-24.
- Akinjayeju O, Adekoya MT. Ingredient optimization by response surface methodology and nutritional evaluation of bread from wheat, millet and soy meal flour blends. International Journal of Food Science and Nutrition 3 (2018): 176-184.
- Stuvoll M, Jarvien HY, Mitrakou A, et al. Use the oral glucose tolerance test to assess insulin release and insulin sensitivity, Diabetes Care 23 (2000): 295-301.
- Anitha M, Sakthidevi G, Muthukumarasamy S, et al. Effect of Cynoglossum zeylanicum (Vehl ex Hornem) Thunb. Ex Lehm on Oral Glucose Tolerance in rats. Journal of Applied Pharmaceutical Science 2 (2012): 75-78.
- Okafor HK, Ebuehi OAT. Defatted Soy Flour Supplementation of Wheat Bread Confers Oxidative, Renal, Hepatic and Cardiovascular Protective Effects in Wistar Rats. International Journal of Biochemistry Research and Review 10 (2016): 1-14.
- Akinjayeju O. Human and Applied Nutrition, 2nd Ed. Lagos, Nigeria: Concept Publications (2015): 159, 160.
- Ijarotimi OS, Fagbemi TN, Osundahunsi OF. Evaluation of nutrient composition, glyceamic index and anti-diabetic potentials of multi-plant based functional foods in rats. Sky Journal of Food Science 4 (2015): 078-090.
- Warren IJ, Burnette M, South CS, et al. Psychopathy in women: Structural modeling and comorbidity. International Journal of Law and Psychiatry 26 (2003): 223-243.
- McMillan-Price J, Petocz P, Atkinson F. Comparison of 4 diets of varying glycaemic load on weight loss and cardiovascular risk reduction in overweight and obese young adults. Archives of Internal Medicine 166 (2006): 1466-1475.
- Ochiai Y, Baba A, Hiramatsu M, et al. Blood biochemistry and hematological changes in rats after administration of a mixture of three anesthetic agents. Journal of Veterinary Medical Science 80 (2018): 387-394.
- Diana NC. Appendix: therapeutic drug monitoring and laboratory reference ranges. In Eds.: Stephen JM, Maxine AP. Current Medical Diagnosis and Treatment, 46th Edn. N.Y., USA: McGraw Hill (2007): 1766-1775.
- Osundahunsi OF. Functional properties of extruded soybean with plantain flour blends. Journal of Food, Agriculture and Environment 4 (2006): 57-60.
- Ijarotimi OS, Keshinro OO. Protein quality, hematological properties and nutritional status of albino rats fed complementary foods with fermented popcorn, African locust bean, and bambara groundnut flour blends. Nutrition Research and Practice (2012): 381-388.
- Sridhar P, Padmaja YP, Shajina H, et al. Hematinic and antioxidant potential of aqueous extract of Sesamum indicum seeds against phenylhydrazine-induced hemolytic anemia in albino rats. National Journal of Physiology and Pharmaceutical Pharmacology 8 (2018): 1092-1096.
- Gometi SA, Ogugua VN, Odo CE, et al. Effects of some anti-diabetic plants on the hepatic marker enzymes of diabetic rats. African Journal of Biotechnology 13 (2014): 90-99.
- Gilani GS, Cockell KA, Sepehr E. Effects of anti-nutritional factors on protein digestibility and amino acid availability in foods. Journal of American Oil and Analytical Chemists International 88 (2005): 967-987.
- Daradka HM. Evaluation of Hematological and Biochemical Activity of Ethanolic Extract of Zygophyllum simplex Linn. in Wistar Rats. Pakistan Journal of Biological Sciences 19 (2016): 179-184.
- El-Demerdash FM, Youse MI, Yousef NIA, et al. Biochemical study on the hypoglycaemic effects of onion and garlic in alloxan-induced diabetic rats. Food and Chemical Toxicology 43 (2005): 57-63.
- Thapa BR, Anuj W. Liver Function Tests and their Interpretation. Indian Journal of Pediatrics 74 (2007): 663-671.
- Sanjiv C. The liver book. A comprehensive guide to diagnosis, treatment and recovery. Atria Jimcafe Company, USA (2002).
- Mansour HA, Al-Sayeda AN, Yousef MI, et al. Biochemical study on the effects of some Egyptian herbs in alloxan-induced diabetic rats. Toxicology 170 (2002): 221-228.
- Gowda S, Desai PB, Hull VV, et al. A review on laboratory liver function tests. Pan African Medical Journal 3 (2009): 17-23.
- Giannini E, Risso D, Botta F, et al. Validity and Clinical Utility of the Aspartate Aminotransferase-Alanine Aminotransferase Ratio in Assessing Disease Severity and Prognosis in Patients with Hepatitis C Virus-Related Chronic Liver Disease. Archives of Internal Medicine 163 (2003): 218-224.
- Aniagu S, Nwinyi CF, Akumka DD, et al. Toxicity studies in rats fed nature cure bitters. African Journal of Biotechnology 4 (2005): 72-78.
- Aliyu R, Adebayo AH, Gasting D, et al. The effects of ethanolic leaf extract of Commiphora Africana (Burseraceae) on rat liver and kidney functions. Journal of Pharmacology and Toxicology 2 (2007): 373-379.
- Lobo DN. Fluid, Electrolytes and Nutrition: Physiological and Clinical Aspects. Proceedings of Nutrition Society 63 (2004): 453-466.
- Anderson JW, Baird P, Davis Jr RH, et al. Health benefits of dietary fibre. Nutrition Reviews 67 (2009): 188-205.
- Gupta V, Nagar R. Effect of cooking, fermentation, dehulling and utensils on antioxidants present in pearl millet rabadi-a traditional fermented food. Journal of Food Science and Technology 47 (2012): 73-76.
- Friedman M, Brandon DL. Nutritional and health benefit of soy proteins. Journal of Agriculture and Food Chemistry 49 (2001): 1069-1086.


 Impact Factor: * 3.8
Impact Factor: * 3.8 Acceptance Rate: 77.96%
Acceptance Rate: 77.96%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 