Percutaneous irreversible Electroporation in Locally Advanced Pancreatic Cancer: A Review of Current Literature
Serena Carriero1, Carolina Lanza1, Giuseppe Pellegrino1*, Caterina Sattin1, Mariachiara Basile1, Maria Teresa Contaldo1, Pierpaolo Biondetti2, Salvatore Alessio Angileri3, Filippo Piacentino4, Massimo Venturini4, Giuseppe Guzzardi5, Anna Maria Ierardi3, Gianpaolo Carrafiello6
1Postgraduate School of Radiodiagnostics, University of Milan, Milan, Italy
2Università Degli Studi di Milano, Via Festa del Perdono 7 – 20122, Milan, Italy
3Department of Diagnostic and Interventional Radiology, Foundation IRCCS Cà Granda – Ospedale Maggiore Policlinico, Via Francesco Sforza 35 – 20122, Milan, Italy
4Diagnostic and Interventional Radiology Department, Circolo Hospital, ASST-Sette Laghi, 21100 Varese, Italy
5Department of Radiology, Unit of Interventional Radiology, Ospedale Maggiore della Carità, Corso Giuseppe Mazzini 18 – 28100, Novara, Italy
6Università Degli Studi di Milano, Via Festa del Perdono 7 – 20122, Milan, Italy
*Corresponding Author: Giuseppe Pellegrino, Postgraduate School of Radiodiagnostics, University of Milan, Milan, Italy
Received: 17 June 2023; Accepted: 11 July 2023; Published: 28 July 2023
Article Information
Citation: Serena Carriero, Carolina Lanza, Giuseppe Pellegrino, Caterina Sattin, Mariachiara Basile, Maria Teresa Contaldo, Pierpaolo Biondetti, Salvatore Alessio Angileri, Filippo Piacentino, Massimo Venturini, Giuseppe Guzzardi, Anna Maria Ierardi, Gianpaolo Carrafiello. Percutaneous irreversible Electroporation in Locally Advanced Pancreatic Cancer: A Review of Current Literature. Journal of Cancer Science and Clinical Therapeutics. 7 (2023): 146-157.
View / Download Pdf Share at FacebookAbstract
Pancreatic cancer (PC) is a highly lethal disease with a 5-year survival rate of 5-6%. To date, the only potentially curative option for PC remains surgical resection with microscopically negative margins. Given the poor survival rate of locally advanced PC (LAPC) patients, several studies explored the combination of conventional therapies with ablation therapies, showing promising results. Several studies showed that thermal ablation in PC, due to its anatomical localization, can induce heat-related damage to bile ducts, adjacent vessels, and gastrointestinal structures. Irreversible Electroporation (IRE) is a locoregional nonthermal ablative technique that induces cellular death by creating nanopores avoiding the aforementioned complications. Our review aims to provide an overview of the technique and highlight its current standpoint in the treatment of LAPC.
Keywords
Irreversible Electroporation; Electroporation; IRE; local Ablation; Thermal Ablation; Ablation; Pancreatic Cancer; Locally Advanced Pancreatic Cancer; Immunotherapy
Irreversible Electroporation articles; Electroporation articles; IRE articles; local Ablation articles; Thermal Ablation articles; Ablation articles; Pancreatic Cancer articles; Locally Advanced Pancreatic Cancer articles; Immunotherapy articles
Irreversible Electroporation articles Irreversible Electroporation Research articles Irreversible Electroporation review articles Irreversible Electroporation PubMed articles Irreversible Electroporation PubMed Central articles Irreversible Electroporation 2023 articles Irreversible Electroporation 2024 articles Irreversible Electroporation Scopus articles Irreversible Electroporation impact factor journals Irreversible Electroporation Scopus journals Irreversible Electroporation PubMed journals Irreversible Electroporation medical journals Irreversible Electroporation free journals Irreversible Electroporation best journals Irreversible Electroporation top journals Irreversible Electroporation free medical journals Irreversible Electroporation famous journals Irreversible Electroporation Google Scholar indexed journals Electroporation articles Electroporation Research articles Electroporation review articles Electroporation PubMed articles Electroporation PubMed Central articles Electroporation 2023 articles Electroporation 2024 articles Electroporation Scopus articles Electroporation impact factor journals Electroporation Scopus journals Electroporation PubMed journals Electroporation medical journals Electroporation free journals Electroporation best journals Electroporation top journals Electroporation free medical journals Electroporation famous journals Electroporation Google Scholar indexed journals IRE articles IRE Research articles IRE review articles IRE PubMed articles IRE PubMed Central articles IRE 2023 articles IRE 2024 articles IRE Scopus articles IRE impact factor journals IRE Scopus journals IRE PubMed journals IRE medical journals IRE free journals IRE best journals IRE top journals IRE free medical journals IRE famous journals IRE Google Scholar indexed journals local Ablation articles local Ablation Research articles local Ablation review articles local Ablation PubMed articles local Ablation PubMed Central articles local Ablation 2023 articles local Ablation 2024 articles local Ablation Scopus articles local Ablation impact factor journals local Ablation Scopus journals local Ablation PubMed journals local Ablation medical journals local Ablation free journals local Ablation best journals local Ablation top journals local Ablation free medical journals local Ablation famous journals local Ablation Google Scholar indexed journals Thermal Ablation articles Thermal Ablation Research articles Thermal Ablation review articles Thermal Ablation PubMed articles Thermal Ablation PubMed Central articles Thermal Ablation 2023 articles Thermal Ablation 2024 articles Thermal Ablation Scopus articles Thermal Ablation impact factor journals Thermal Ablation Scopus journals Thermal Ablation PubMed journals Thermal Ablation medical journals Thermal Ablation free journals Thermal Ablation best journals Thermal Ablation top journals Thermal Ablation free medical journals Thermal Ablation famous journals Thermal Ablation Google Scholar indexed journals Ablation articles Ablation Research articles Ablation review articles Ablation PubMed articles Ablation PubMed Central articles Ablation 2023 articles Ablation 2024 articles Ablation Scopus articles Ablation impact factor journals Ablation Scopus journals Ablation PubMed journals Ablation medical journals Ablation free journals Ablation best journals Ablation top journals Ablation free medical journals Ablation famous journals Ablation Google Scholar indexed journals Pancreatic Cancer articles Pancreatic Cancer Research articles Pancreatic Cancer review articles Pancreatic Cancer PubMed articles Pancreatic Cancer PubMed Central articles Pancreatic Cancer 2023 articles Pancreatic Cancer 2024 articles Pancreatic Cancer Scopus articles Pancreatic Cancer impact factor journals Pancreatic Cancer Scopus journals Pancreatic Cancer PubMed journals Pancreatic Cancer medical journals Pancreatic Cancer free journals Pancreatic Cancer best journals Pancreatic Cancer top journals Pancreatic Cancer free medical journals Pancreatic Cancer famous journals Pancreatic Cancer Google Scholar indexed journals Locally Advanced Pancreatic Cancer articles Locally Advanced Pancreatic Cancer Research articles Locally Advanced Pancreatic Cancer review articles Locally Advanced Pancreatic Cancer PubMed articles Locally Advanced Pancreatic Cancer PubMed Central articles Locally Advanced Pancreatic Cancer 2023 articles Locally Advanced Pancreatic Cancer 2024 articles Locally Advanced Pancreatic Cancer Scopus articles Locally Advanced Pancreatic Cancer impact factor journals Locally Advanced Pancreatic Cancer Scopus journals Locally Advanced Pancreatic Cancer PubMed journals Locally Advanced Pancreatic Cancer medical journals Locally Advanced Pancreatic Cancer free journals Locally Advanced Pancreatic Cancer best journals Locally Advanced Pancreatic Cancer top journals Locally Advanced Pancreatic Cancer free medical journals Locally Advanced Pancreatic Cancer famous journals Locally Advanced Pancreatic Cancer Google Scholar indexed journals Immunotherapy articles Immunotherapy Research articles Immunotherapy review articles Immunotherapy PubMed articles Immunotherapy PubMed Central articles Immunotherapy 2023 articles Immunotherapy 2024 articles Immunotherapy Scopus articles Immunotherapy impact factor journals Immunotherapy Scopus journals Immunotherapy PubMed journals Immunotherapy medical journals Immunotherapy free journals Immunotherapy best journals Immunotherapy top journals Immunotherapy free medical journals Immunotherapy famous journals Immunotherapy Google Scholar indexed journals FOLFIRINOX articles FOLFIRINOX Research articles FOLFIRINOX review articles FOLFIRINOX PubMed articles FOLFIRINOX PubMed Central articles FOLFIRINOX 2023 articles FOLFIRINOX 2024 articles FOLFIRINOX Scopus articles FOLFIRINOX impact factor journals FOLFIRINOX Scopus journals FOLFIRINOX PubMed journals FOLFIRINOX medical journals FOLFIRINOX free journals FOLFIRINOX best journals FOLFIRINOX top journals FOLFIRINOX free medical journals FOLFIRINOX famous journals FOLFIRINOX Google Scholar indexed journals
Article Details
1. Introduction
Pancreatic cancer (PC) is a highly lethal disease with a 5-year survival rate of 5-6%, being the third leading cause of death from cancer in both males and females in the USA [1]. To date, the only potentially curative option for PC remains surgical resection with microscopically negative margins, but due to a diagnostic delay attributable to a typical onset of the symptoms in later stages of the disease, only 15% of patients present with resectable disease [2, 3]. For this reason, the majority of patients present with unresectable disease: locally advanced PC (LAPC) in 30% of cases and metastatic PC (MPC) in 50% of cases [4]. According to the American Joint Committee on Cancer (AJCC) guidelines, a locally advanced tumor cannot be completely resected because of the invasion of nearby structures and/ or present with distant metastases [5]. MD Anderson Cancer Centre gives more precise indications about the resectability of pancreatic tumors: a locally advanced, hence unresectable, PC is defined by encasement of superior mesenteric artery greater than 180°, encasement and no technical reconstructive options of celiac axis or hepatic artery, and occlusion and no technical reconstructive options of superior mesenteric vein or portal vein, with no signs of distant metastases [6, 7]. Previously, chemotherapy with gemcitabine with or without radiation therapy has been the standard of care for LAPC with overall survival (OS) of 9-11 months [8]. More recently, due to the implementation of treatments including the association of nab-paclitaxel or FOLFIRINOX (5-fluorouracil, leucovorin, irinotecan, and oxaliplatin) with gemcitabine as neoadjuvant setting, the OS increased to 6-13 month [9]. The goal of these therapies is to obtain the surgical eligibility of patients, even though only a small minority of patients fall within the limits of PC resectability criteria after the aforementioned therapies. Given the poor survival rate of LAPC patients, several studies explored the combination of conventional therapies with ablation therapies, showing promising results [7, 10]. The most commonly employed Thermal Ablation (TA) techniques are radiofrequency ablation (RFA) and microwave ablation (MWA) [11-14]. Nevertheless, despite their reported efficacy, several researches showed that TA can induce heat-related damage to bile ducts, adjacent vessels, and gastrointestinal structures. Irreversible Electroporation (IRE) is locoregional nonthermal ablative technique that induces cellular death by creating nanopores [15]. The very first report of its deployment dates 2009, in a study theorizing the possibility of developing an IRE system that could be both safe and effective in the treatment of human pancreatic malignancies [16], successively tested on a swine model with a good safety profile [17].
In consideration of the potential opportunities given by this technique, IRE has then been widely tested on human PC, mainly in the context of LAPC treatment, with the objective to achieve a greater overall survival (OS) and progression-free survival (PFS) compared to the conventional treatments, to improve quality of life (QoL), and relieve symptoms related to the advanced stage of the disease [10, 18, 19].
Our review aims to provide an overview of percutaneous IRE technique and highlight its current standpoint in the treatment of LAPC.
2. Percutaneous Irreversible Electroporation Technique and Procedure
Irreversible Electroporation is a non-thermal ablative technique based on high-voltage electrical pulses (HEVPs) of up to 3000 V delivered in 70-80 microseconds and applied between needle electrodes inserted within the tumor.
This ablative technique creates multiple microscopic holes in cellular membranes inducing the irreversible permeabilization that leads to programmed cell death [20]. IRE-induced cellular apoptosis of pancreatic pathologic tissue takes place at temperatures inferior to 50 C°: this allows the preservation of the underlying matrix, vessels, and biliary ducts included in the ablation area, avoiding the typical heat-sink effect and without causing coagulation necrosis [13]. To perform an IRE procedure, general anesthesia with a complete neuromuscular block, to reduce contractions of muscles induced by HEVPs, is required. During the procedure, the operator inserts two to six needles (depending on the size and shape of the tumor) within the target lesion. To assure maximum efficacy, it is important to insert each needle parallel to the others with a distance of no less than 1 cm and not more than 2.5 cm. Most recent studies treated tumors whose diameter was up to 6.5 cm [21], but according to several research, the ideal interval, both in terms of prolonged OS and safety, for an IRE procedure ranges between 3-4cm. In fact, Fang et al. reported a survival advantage in patients undergoing IRE with a median OS of 16.2 and 9.9 months for tumors that are ≤ 3 mm and > 3 cm, respectively [22]. Narayanan et al. [23] demonstrated that among different variables, such as age, CA 19-9 values or number of lines of chemotherapy, a tumor size ≤ 3 cm was the only factor significantly associated with better overall survival. In percutaneoud approach, needle insertion can be either under ultrasound (US) or computed tomography (CT) guidance; given the thinness of the needles (22G), in experienced hands trans hepatic or trans gastric approaches are also possible [24]. A case of LAPC treated with IRE using a percutaneous trans-gastric approach is shown in Figures 1, 2, and 3. Specific parameters, such as tumor size, location, and body structure, as well as operators’ expertise, are factors to be considered to choose the best approach. The advantages of a percutaneous approach include shorter procedure time and lower invasivity, hence reducing complication rates and procedural costs. Percutaneous IRE is an “off-label” procedure for stage III PC. The inclusion criteria are different from center to center, and mainly comprise a confirmed diagnosis of a LAPC with a maximum diameter of 4 cm, adequate performance status (mainly evaluated with ECOG), and liver, renal, blood functionalities and with an anesthesiologist’s consult to assess the safety of general anesthesia [25]. Contraindications to the procedure can be tumor-related (metastatic disease, diameter > 5 cm) or patient-related (cardiac diseases, poor liver or renal function, low-performance status). Epilepsy, atrial fibrillation, or other forms of cardiac conditions also represent contraindications to the procedure, because pulses emitted during the procedure could result in de-synchronizations of either brain or cardiac electric waves [26].
Abbreviations –CT: computed tomography.
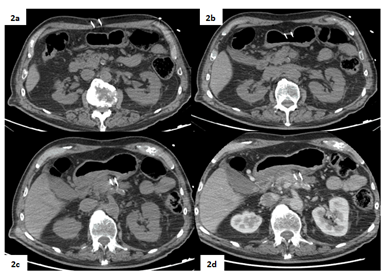
Figure 1: Pre-procedural contrast-enhanced CT (arterial phase) in axial (1a) and coronal planes (1b) shows a lesion in the pancreatic body measuring 31x26mm.
Abbreviations –CT: computed tomography.
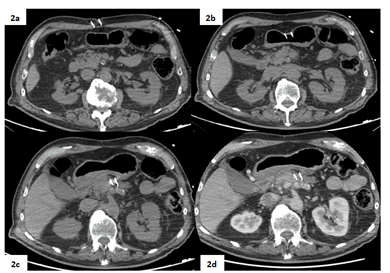
Figure 2: Peri-procedural non-enhanced (2a, 2b, 2c) and contrast-enhanced arterial phase CT scans (2d) acquired during the procedure show the correct positioning of the two needles in the surroundings of the target lesion via trans-gastric approach.
Abbreviations – CECT: contrast-enhanced computed tomography.
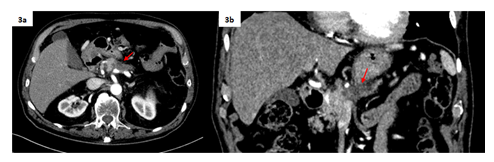
Figure 3: CE-CT (arterial phase) acquired 1 month after the procedure in axial (3a) and coronal planes (3b). The final result is a dimensional reduction of the treated pancreatic lesion (22x19 mm vs 31x26 mm).
3. IRE Treatment for LAPC
Multiple studies have been conducted on the efficacy of local treatments such as IRE, which can be used in combination with induction therapy to obtain down staging and surgical eligibility of LAPC. According to current literature, the clinical outcomes of IRE treatments strongly depend on the timing of the procedure. Studies reported that upfront IRE therapies (performed before chemotherapy cycles) yielded modest increases in median OS, whereas when executed after a systemic treatment granted better results both in terms of clinical efficacy and safety [27, 28]. These results may suggest that modifications to the tumoral microenvironment operated by chemotherapy could catalyze the efficacy of locoregional treatments. Tumor biology and performance status are important factors for the selection of patients who would benefit from neoadjuvant therapy and this synergistic effect. The LAP-PIE feasibility trial is the first UK-based randomized controlled trial of pancreas IRE in patients with LAPC, enrolling 50 patients in whom LAPC remained localized and unresectable after FOLFIRINOX (3-6 cycles). Eligible patients with LAPC who have undergone first-line 5-FluoroUracil, Leucovorin, Irinotecan, and Oxaliplatin chemotherapy were randomized to receive either a single session of IRE followed by (if indicated) further chemotherapy or chemotherapy alone (standard of care). The study will investigate whether IRE improves survival, and health-related QoL [29]. However, the potential increased survival reported in this trial should be carefully evaluated since it may be due to patient selection for IRE rather than the effect of IRE (selection bias). Even in surgery, Oba et al with the SLING trial demonstrated that administration of NAT (neoadjuvant treatment) and CA 19-9 levels are two of the eight prognostic factors of overall survival (OS) in the preoperative setting. Concerning timing, the highest OS rates were observed with IRE after induction chemotherapy, up to 27 months, using FOLFIRINOX in 1st-line treatment, and gemcitabine as a 2nd-line treatment. Some have indicated that ≥8 months of NAT is associated with better prognosis and resectability. Efficacy increases with six or more cycles, but further studies are needed to identify the most appropriate number of cycles of therapy [30].
The phase II PANFIRE study that aimed to compare the efficacy of combining NAT with IRE vs NAT with conversional surgery. The study retrospectively enrolled a total of 140 patients with either LAPC or local recurrence of a previously treated PC, who underwent CT from August 2015 to March 2020. 31 patients underwent chemotherapy, 4 chemotherapy + resection, 64 chemotherapy + IRE, 44 of which then underwent NAT. The median survival was 16.9 months. In the chemotherapy-only group, the mean survival was 8.9 months, lower than in the groups in which chemotherapy was combined with IRE (24 months) or resection (25.3 months), with values even closer with the addition of NAT after IRE. Obviously, characteristics such as tumor size (better outcomes when <4cm) and blood values of CA19.9 were relevant factors to the primary outcome of the trial which was the achievement of a target median OS, exceeded in both patients with locally advanced pancreatic cancer and with local recurrence (respectively 17 and 16 months of OS) [31]. Contrasting results were instead obtained from the IMPALA study, a prospectic cohort study which compared surgery with IRE enrolling 132 patients with LAPC from Semptember 2013 to March 2015, who received 3 months of CT (FOLFIRONOX or gemcitabine) followed by surgery or IRE if non-progressive, IRE-elegible tumors based on RECIST 1.1 criteria. The results showed promising survival rates after resection but no apparent benefit of IRE, despite considerable morbidity [32].
Concluding, preliminary studies emphasize the role of IRE in increasing OS in the treatment of LAPC. However, the survival gains can be confounded by the results of improved systemic therapies and by selection bias, hence further RCTs are needed. IRE can be considered an alternative to surgical resection in LAPC that remains unresectable after induction therapy (75%), often due to persistent local invasion [33].
4. Clinical Outcomes of IRE in the Treatment of LAPC
Woeste et al. reported the outcomes of 187 patients treated with an open IRE approach, showing an OS of 30.7 months and a PFS of 22.4 with a complication rate of 16%. This study reports the results of IRE treatments in LAPC and shows that an accurate patient selection can result in survival times of over 2 years [34]. Veldhuisen et al. and Ruarus et al. report only considered a CT-guided percutaneous approach with an OS respectively of 17.2 months and 17 months [8, 35, 36]. Numerous independent predictive factors were found to influence patients' survival: according to Woeste et al., abnormal CA19-9 values before IRE, and chemotherapy duration ≤ 5 months were predictors of a worse survival rate; on the other hand, age ≤ 61 years and no prior radiotherapy predicted an improvement of OS [34]. Among the most representative included studies (Table 1) the median overall survival (OS) of all patients was 22.7 months (range: 13 –31 months), and the median progression-free survival (PFS) was 11.2 months (range: 7 – 23 months) and the median recurrence-free survival (RFS) was 8 months range. 2.7-12.4).
Two studies reported prognostic factors associated with OS among patients undergoing IRE. Narayanan et al. reported that tumor size was the only factor associated with OS; patients with tumors ≤ 3 cm had a survival advantage [37]. Scheffer et al. reported that early local progression following IRE was the only predictor of worse OS [38]. The most relevant and achievable goal in the management of LAPC appears to be good palliation of symptoms [39]. Results of Phase I/II PANFIRE study [40] showed that, up to 6 months after IRE treatment, overall pain perception and QoL were not affected. After 6 months, several items worsened but they reported that this might reflect disease progression rather than the effect of IRE, also adding that IRE may be a useful adjuvant to slow disease progression and to pre-preserve QoL. M. Lin et al. [41] studied QoL after IRE treatment and NK cell immunotherapy, using the KPS as an index for QoL posttreatment. Results analysis showed QoL markedly improved.
5. Safety and Complications of IRE in the Treatment of LAPC
Safety was assessed based on the onset of complications, graded according to the Clavien-Dindo Classification of Surgical Complications (0-IV), in which severe complications are graded as III/IV [42]. The most frequent complications of IRE in the treatment of LAPC are acute pancreatitis, portal or mesenteric thrombosis, pancreatic fistula, perforations of the gastro-enteric tracts (duodenal or transverse colon), hemorrhages (superior mesenteric artery), vascular lesions (aneurysm and pseudoaneurysm), ascites, lymphatic fistula, delayed gastric emptying/bowel passage, biliary complications, pancreatic leak, chyle leak [43]. These are believed to be directly IRE-related because they are uncommon events in agreement to the literature [43]. Among patients undergoing percutaneous IRE, morbidity was 24.3% and no periprocedural mortality was reported among patients undergoing percutaneous IRE [44]. The percutaneous approach is minimally invasive and has lower complication rates. Patients with stable LAPC and poor performance status should likely be considered for this approach [45]. The largest study of percutaneous IRE, by Leen et al., includes 75 patients, only six (8%) experienced severe toxicities, and no patients died during the first three months.
The rates of IRE’s complications detected in different studies (Table 2) go from 8% to 100% [46, 47], while the percentage of severe complications [48] goes from 0% to 45% [38, 46, 49-55]. The variability in the reported complication rates across studies may be due to the heterogeneity in tumor size, in median of 2.8-4.5 cm, location and treatment protocols [56]. Further studies about its potentialities may increase general awareness about this technique, inducing more interventional radiologists to learn and start practicing this procedure more and more.
|
Authors |
Date |
LAPC n. |
OS (months) |
pfs/RFS (months) |
|
Woeste [57] |
2022 |
187 |
22.4 |
16.1 |
|
Rudno-Rudzin Ska [58] |
2021 |
9 |
45 |
- |
|
Heger [59] |
2021 |
14 |
28 |
7 |
|
He [60] |
2021 |
64 |
26 |
12 |
|
Kwon [47[ |
2021 |
12 |
13.5 |
8.6 |
|
Ruarus [61] |
2020 |
50 |
17 |
10 |
|
Veldhuisen [49] |
2020 |
52 |
17.2 |
9.9 |
|
Hep [49] |
2020 |
32 |
24 |
7.1 |
|
Holland [62] |
2019 |
152 |
30.7 |
22.8 |
|
Flak [63] |
2019 |
33 |
18 |
- |
|
Mansson [64] |
2019 |
24 |
13 |
- |
|
Leen [65] |
2018 |
75 |
27 |
15 |
|
Huang [66] |
2018 |
70 |
22 |
15.4 |
|
Sugimoto [55] |
2018 |
8 |
24 |
- |
|
Scheffer [38] |
2017 |
15 |
16 |
12 |
|
Vogel [52] |
2017 |
25 |
17 |
- |
|
Narayanan [37] |
2016 |
50 |
27 |
- |
|
Mansson [67] |
2016 |
24 |
17.9 |
2.7 |
|
Lambert [53] |
2016 |
21 |
10 |
- |
Abbreviations –LAPC= Local Advanced Carcinoma Pancreas; R= recurrence; OS overall survival; PFS= progression-free survival; CT= chemotherapy; RT= radiotherapy; N: number of patients.
Table 1: Clinical studies reporting results of IRE in treatment of LAPC.
|
Authors |
Date
|
Method of |
All Complications (%) |
Severe Complications (Clavien-Dindo ≥ III) (%) |
Mortality |
|
Heger [59] |
2021 |
Percutaneous |
71.4 |
14 |
0 |
|
Kwon [47] |
2021 |
Percutaneous |
100 |
25 |
8 |
|
Ruarus [61] |
2020 |
Percutaneous |
58 |
42 |
4 |
|
Veldhuisen [49] |
2020 |
Percutaneous |
37 |
0 |
0 |
|
He [46] |
2020 |
Percutaneous |
8 |
0 |
0 |
|
Holland [62] |
2019 |
Percutaneous |
18 |
13 |
2 |
|
Flak [63] |
2019 |
Percutaneous |
33 |
21 |
5 |
|
Mansson [64] |
2019 |
Percutaneous |
46 |
13 |
4 |
|
Leen [65] |
2018 |
Percutaneous |
25 |
8 |
0 |
|
Sugimoto [55] |
2018 |
Percutaneous |
75 |
45 |
0 |
|
Ierardi [50] |
2018 |
Percutaneous |
20 |
0 |
0 |
|
Zhang [68] |
2017 |
Percutaneous |
19 |
0 |
0 |
|
Scheffer [38] |
2017 |
Percutaneous |
48 |
- |
0 |
|
Vogel [52] |
2017 |
Percutaneous |
53 |
- |
0 |
|
Narayanan [37] |
2016 |
Percutaneous |
62 |
20 |
0 |
|
Mansson [67] |
2016 |
Percutaneous |
46 |
13 |
0 |
|
Lambert [53] |
2016 |
Percutaneous |
24 |
- |
0 |
|
Belfiore [54] |
2015 |
Percutaneous |
10 |
0 |
0 |
Abbreviations –LAPC= Local Advanced Carcinoma Pancreas
Table 2: Incidence and severity of complications reported in clinical studies of IRE treatment of LAPC.
6. Radiological response to IRE
Imaging evaluation in tumor response after ablation has an essential role to define the treatment success, the assessment of procedure-related complications, and for the evaluation of the remaining vital tumor. Radiological findings are associated to the type of treatment, the time of response assessment and to the type of imaging technique [69]. Histological findings after IRE demonstrated a necrotic treated area encapsulated in fibrous tissue, with signs of apoptosis and reduced vital signs [69]. Nowadays MRI and CT are the most used diagnostic tools to assess response to IRE [70]. CT is the standard imaging modality in the follow-up for PC and has an accuracy of 93.5 % for the detection of local recurrence [71, 72]. Several studies evaluated the feasibility of contrast-enhanced MRI (CEMRI) for the characterization of solid pancreatic diseases [73] and the assessment of quantitative parameters associated with tumor perfusion, vessel permeability, and extravascular space composition [73, 74]. CE-MRI with T1-weighted GE is particularly effective in discerning treated from untreated areas after ablation treatment, showing hyperintense enhanced areas versus hypointense unenhanced areas [74, 75]. Vroomen et al. [72] aimed to assess specific imaging characteristics after IRE treatment for LAPC and to quantify tumor and ablation-zone volumes with CECT and CEMRI. They reported that ablation zone volume increased on both modalities in the first 6 weeks, followed by a decrease in volume. Both CEMRI and CECT revealed absent or decreased contrast enhancement and a hyperintense rim surrounding the IRE ablation zone was found in 71% of the patients. Moreover, they found a DWI-b800 hyperintense spot at 6 weeks follow-up that predated recurrence on CT. However, when the treatment zone is small, susceptibility effects may obscure small areas of recurrence or create false positives, hence reducing the potential capability of DWI- b800 to interpret the ablated area. The most used criteria to assess a radiological response to treatments are response evaluation criteria in solid tumors (RECIST) and also the “CHOI criteria”, both related to the change in tumor size and in tumor attenuation [71, 72, 74, 76]. In the evaluation of an IRE treatment, the ablation zone size, both in CECT and in CEMRI, is not a reliable indicator of the true extent of the treated area, despite having a good correlation with the histological ablation zone [77, 78]. The evidence of a reduction in viable cells may not always reflect a change in tumor size [72, 77]. Akinwande et al. performed a prospective review soft tissue ablation registry on patients who underwent IRE for LAPC. They concluded that the ablation zone appeared larger than the original target, without clearly demarcated margins, and the nearby vascular structures were narrowed. This aspect is related to the inclusion of the reactive post-procedural area, characterized by edema and hyperemia in the ablation zone [21, 79].
Edema, hyperemia, and granulation tissue decreased over time and facilitated the visualization of the true ablation zone. When the inflammatory process is resolved, postprocedural CT imaging reveals the true ablation zone size, reporting: smaller “true” ablation zone, compared to the treated area, an increased enhancement of the ablation zone (likely linked to the formation of granulation tissue and fibrosis) and that blood vessel caliber came back to normal or remained stable.
Currently, there is no consensus on the ideal post-treatment interval to measure the ablation zone. Histological studies showed that after IRE treatments, the target area becomes necrotic and encapsulated in fibrous tissue [69]. For this reason, is fundamental the use of imaging criteria, which take into account the viability and not only the size of the lesion to assess tumoral response attempting differentiation of fibrosis from residual tumor [69]. Moreover, a positive response in LAPC after IRE should be associated with a decreased metabolism, without a significant reduction in tumor size [74].
The evaluation of any reduction in metabolic activity is more predictive of tumor response than morphological criteria alone. PET criteria in solid tumors (PERCIST) evaluate both the morphological criteria and the fluorodeoxyglucose uptake reduction [80]. FDG PET can differentiate post-therapy changes from recurrence in PC, it is complementary to morphological imaging with CT; therefore, integrated PET/CT imaging provides optimal images for interpretation [81]. In patients that underwent prior or post- IRE radiation therapy, persistent isolated vessel narrowing must be followed with serial imaging, clinical evaluation, CA19-9 serum tumor markers, and, as above, for equivocal cases, PET/CT may play a role in differentiating postablative changes from recurrence [81, 82]. Actually, in addition to the radiological response, Vroomen et al [72] reported in their follow-up study analysis that local recurrence detected at CECT was accompanied by a significant increase in CA 19.9. It would be useful to correlate dimensional and metabolic changes of target lesions to biochemical data, such as tumoral markers, in order to properly assess the effective response to IRE treatment [77]. Response to IRE treatment is a multifactorial results of the anatomical localization of the mass, mechanism of action of given therapeutic strategy, morphological and functional criteria used for each imaging modality [83].
7. Immunotherapy and IRE
Pancreatic carcinoma tumoral microenvironment (TME) has a desmoplastic stroma with a crucial role in the foundation of a highly immunodepressant setting that potentially makes immunotherapeutic treatments less effective [84]. PC has a “cold” immune status, and its TME has a dense extracellular matrix that acts as a physical, rigid barrier resulting in elevated tumor pressure, with reduced vascularization and impaired diffusion of immunotherapeutic agents. Additionally, “cold” immune status is due to downregulation by tumor cells of antigen-presenting pathways as MHC-I, the upregulation of suppressive regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSC), and the restriction of dendritic cell (DC) maturation. All these factors increase apoptotic resistance of tumor cells. Moreover, Tregs of TME limit antitumor-T cells' effectiveness by over-expressing inhibitory receptors (e.g., CTLA-4) and expressing great quantities of PD-L1 and PD-L2, ligands to the inhibitory receptor PD-1 [85]. On the other hand, IRE’s action triggers immune responses, with a direct effect on both innate and adaptive immunity. IRE-induced apoptosis is associated with a release of antigens that result in the secretion of potent proinflammatory cytokines, hence activating the immune system against tumor cells. IRE has a pivotal role because can switch the prior PC “cold” immune status to “hot”, favoring a pro-inflammatory and antitumorigenic microenvironment [86]. Several different immunotherapeutic strategies have been tried along with IRE to enhance the activity of the immune system against PC.
He et al evaluated 85 patients divided into IRE group (70) and IRE + Toripalimab group (15). The IRE plus Toripalimab group showed longer OS (44.33 months versus 23.37 months) and PFS (27.5 months versus 10.6 months) compared with IRE group. Authors concluded that the combined therapy might improve the OS of patients with LAPC (Table 1) [87]. Lin et al. investigated the safety and clinical efficacy in III/IV PC treated with IRE and allogeneic natural killer (NK) cell immunotherapy, evaluating PFS and OS, authors concluded that combination therapy increased median PFS and median OS in stage III PC and extended the median OS of stage IV PC [88]. IRE was also evaluated in combination with DC transfer (DC vaccine) [89]. Promising results have been observed, with only common side effects like fatigue and/or flu-like symptoms, and the median OS was 7.7 months (Table 1) [90]. The combination of IRE + DC vaccine may cause immunogenic cell death and relieve of immunosuppressive components in PC microenvironment: this combination therapy exerted a synergistic effect, enhancing the activity of the immune system.
Another relevant pathway in PC is TGF-β, which has a contrasting role as both tumor suppressor and promoter [91]. Expression of TGF-β type II receptor correlates with reduced survival in patients with PC. Using glutathione-responsive degradable mesoporous silica nanoparticles loaded with SB525334, an inhibitor of TGF-β receptor, Peng et al. demonstrated that local inhibition of TGF-β within the tumor microenvironment promotes neutrophil addressing to an antitumor phenotype, enhances PC response to combined IRE and PD1 therapy, and induces long-term antitumor memory [91]. Nowadays the study of tumor immunophenotype has an important role and may offer opportunities to prolong the immune response following IRE [92]. O’Neill et al using mass cytometry studied the differences in lymphocyte populations in patients who underwent IRE and in patients who did not. They showed that patients without evidence of recurrence had a robust early immune response to IRE with the establishment of significantly higher levels of CD4 and CD8 central memory populations as well as enhanced early NK response [92].
A 2020 clinical trial investigated the antitumor efficacy of IRE plus allogeneic γδ T cells enrolling 62 LAPC patients, the OS in patients with combination therapy was higher than in the other group (Table 3) [93]. These trials with encouraging results are related to a small minority of patients with selected biomarkers, but the majority of trials with promising preclinical data have failed. Immunotherapy and targeted therapy did not yield practice-changing results in PC, probably because pancreatic ductal adenocarcinoma (PDAC) TME and pancreatic carcinoma tumor immune microenvironment are peculiar compared to most tumors, as is the genomic landscape that accompanies this disease. More research efforts are crucial to better select patients that could benefit from immunotherapy and to develop efficient TME modification mechanisms that could make the tumor more immunosensitive [94].
A 2022 review by Ullman et al. [95], summarizes the mechanisms of immunosuppression within the PDAC tumor microenvironment and provides an up-to-date review of completed and ongoing clinical trials using various immunotherapy strategies. While the COMBAT trial offered promising results [96], other studies evaluating CXCR4 inhibition have resulted in poorer treatment responses. [97]. Despite the conduction of several interesting preclinical studies, the translation into clinical practice has proved to be challenging, due to the existence of a complex TME that protects the tumor against a cytotoxic immune response. The intricate pathways of immune evasion will likely require a combination approach to improve efficacy. In conclusion, the alterations of the tumor environment from IRE offer plenty of hope for IRE + immunotherapy, but most studies have not demonstrated a convincing response yet. There is no definite treatment pathway to date the treatment superior to the others, but the combination of IRE with anti-PD-L1 is more studied and employed than the other regimens, albeit in need of further studies. Fortunately, many ongoing clinical trials are evaluating combination immunotherapies, which at the minimum, will be able to shed light on mechanisms of immune evasion to educate future trials [95].
|
Authors |
Ref. |
Date |
n. |
Diagnosis |
Treatment |
OS (month) |
PFS(month) |
|
He |
[57] |
2021 |
85 |
LAPC |
70 IRE |
33.37 |
10.6 |
|
15 IRE + TOR |
44.33 |
27.5 |
|||||
|
Lin |
[70] |
2020 |
62 |
LAPC |
32 IRE |
11 |
|
|
30 IRE + gdT |
14.5 |
||||||
|
Lin |
[58] |
2017 |
67 |
35 STAGE III |
16 IRE |
12.2 |
7.9 |
|
19 IRE + NK |
13.6 |
9.1 |
|||||
|
32 STAGE IV |
14 IRE |
9.1 |
|||||
|
18 IRE + NK |
10.2 |
||||||
|
Mehrota |
[59] |
2017 |
12 |
LAPC |
DCs |
7.7 |
Abbreviations – OS: overall survival; PFS: progression free survival; QoL: Quality of Life; IRE: Irreversible electroporation; LAPC: locally advanced pancreatic cancer patients.
Table 3: Clinical studies comparing OS, PFS, or QoL after IRE combined with different immunotherapic strategies in LAPC.
8. Conclusion
The encouraging results reported by these studies suggest that IRE, especially when used in combination with pre-operative chemotherapy, could increase OS and PFS values in a LAPC setting.
Moreover, its potential use in combination with chemotherapy and immunotherapy is a field of great interest, but despite its potential promise, much about IRE remains unknown, and more prospective randomized controlled trials are necessary.
Author Contributions
All authors contributed to the design and implementation of the research and to the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Institutional Review Board statement was not needed for this review article.
Informed Consent Statement
Informed consent statement was not needed for this review article.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
All authors declare that they have no conflict of interest and that they have nothing to disclose.
References
- An Update on Cancer Deaths in the United States | CDC (2022).
- Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network 19 (2021): 439-457.
- Wong JC, Raman S. Surgical resectability of pancreatic adenocarcinoma: CTA. Abdom Imaging 35 (2010): 471-480.
- Huguet F, Mukherjee S, Javle M. Locally advanced pancreatic cancer: the role of definitive chemoradiotherapy. Clin Oncol (R Coll Radiol) 26 (2014): 560-568.
- Cong L, Liu Q, Zhang R, et al. Tumor size classification of the 8th edition of TNM staging system is superior to that of the 7th edition in predicting the survival outcome of pancreatic cancer patients after radical resection and adjuvant chemotherapy. Sci Rep 8 (2018).
- Mizrahi JD, Surana R, Valle JW, et al. Pancreatic cancer. The Lancet 395 (2020): 2008-2020.
- Timmer FEF, Geboers B, Nieuwenhuizen S, et al. Locally Advanced Pancreatic Cancer: Percutaneous Management Using Ablation, Brachytherapy, Intra-arterial Chemotherapy, and Intra-tumoral Immunotherapy. Curr Oncol Rep 23 (2021).
- Ruarus AH, Vroomen LGPH, Geboers B, et al. Percutaneous Irreversible Electroporation in Locally Advanced and Recurrent Pancreatic Cancer (PANFIRE-2): A Multicenter, Prospective, Single-Arm, Phase II Study. Radiology 294 (2020): 212-220.
- Porcelli L, Quatrale AE, Mantuano P, et al. Optimize radiochemotherapy in pancreatic cancer: PARP inhibitors a new therapeutic opportunity. Mol Oncol 7 (2013): 308-322.
- Rai ZL, Feakins R, Pallett LJ, et al. Irreversible Electroporation (IRE) in Locally Advanced Pancreatic Cancer: A Review of Current Clinical Outcomes, Mechanism of Action and Opportunities for Synergistic Therapy. J Clin Med 10 (2021): 1609.
- Ruarus A, Vroomen L, Puijk R, et al. Locally Advanced Pancreatic Cancer: A Review of Local Ablative Therapies. Cancers (Basel) 10 (2018): 16.
- Carrafiello G, Ierardi AM, Fontana F, et al. Microwave Ablation of Pancreatic Head Cancer: Safety and Efficacy. Journal of Vascular and Interventional Radiology 24 (2013): 1513-1520.
- Microwave ablation in locally advanced pancreatic carcinoma--a new look-PubMed (2022).
- Carrafiello G, Ierardi AM, Fontana F, et al. Microwave ablation of pancreatic head cancer: Safety and efficacy. Journal of Vascular and Interventional Radiology 24 (2013): 1513-1520.
- Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality--clinical implications. Technol Cancer Res Treat 6 (2007): 37-48.
- Arena CB, Rylander MN, Davalos Rv. Theoretical study for the treatment of pancreatic cancer using electric pulses. Annu Int Conf IEEE Eng Med Biol Soc 2009 (2009): 5997-6000.
- Bower M, Sherwood L, Li Y, et al. Irreversible electroporation of the pancreas: definitive local therapy without systemic effects. J Surg Oncol 104 (2011): 22-28.
- Flak RV, Malmberg MM, Stender MT, et al. Irreversible electroporation of pancreatic cancer – Effect on quality of life and pain perception. Pancreatology 21 (2021): 1059-1063.
- Lienden V, Vries JJJ De. Percutaneous Irreversible Electroporation in Locally Advanced and Recurrent Pancreatic Cancer (PANFIRE-2): Radiology (2020).
- Geboers B, Scheffer HJ, Graybill PM, et al. High-Voltage Electrical Pulses in Oncology: Irreversible Electroporation, Electrochemotherapy, Gene Electrotransfer, Electrofusion, and Electroimmunotherapy. Radiology 295 (2020): 254-272.
- Rai ZL, Feakins R, Pallett LJ, et al. Irreversible Electroporation (IRE) in Locally Advanced Pancreatic Cancer: A Review of Current Clinical Outcomes, Mechanism of Action and Opportunities for Synergistic Therapy. J Clin Med 10 (2021):1609.
- Fang C, Kibriya N, Heaton ND, et al. Safety and efficacy of irreversible electroporation treatment in hepatobiliary and pancreatic tumours: a single-centre experience. Clin Radiol 76 (2021): 599-606.
- Narayanan G, Hosein PJ, Beulaygue IC, et al. Percutaneous Image-Guided Irreversible Electroporation for the Treatment of Unresectable, Locally Advanced Pancreatic Adenocarcinoma. Journal of Vascular and Interventional Radiology 28 (2017): 342-348.
- Moris D, Machairas N, Tsilimigras DI, et al. Systematic Review of Surgical and Percutaneous Irreversible Electroporation in the Treatment of Locally Advanced Pancreatic Cancer. Ann Surg Oncol 26 (2019): 1657-1668.
- Scheffer HJ, Vroomen LGPH, de Jong MC, et al. Ablation of Locally Advanced Pancreatic Cancer with Percutaneous Irreversible Electroporation: Results of the Phase I/II PANFIRE Study. Radiology 282 (2017): 585-597.
- Narayanan G. Irreversible Electroporation (2015).
- He C, Wang J, Zhang Y, et al. Irreversible electroporation after induction chemotherapy versus chemotherapy alone for patients with locally advanced pancreatic cancer: A propensity score matching analysis. Pancreatology 20 (2020): 477-484.
- Lafranceschina S, Brunetti O, Delvecchio A, et al. Systematic Review of Irreversible Electroporation Role in Management of Locally Advanced Pancreatic Cancer. Cancers (Basel) 11 (2019): 1718.
- Rai ZL, Ranieri V, Palmer DH, et al. Treatment of unrespectable locally advanced pancreatic cancer with percutaneous irreversible electroporation (IRE) following initial systemic chemotherapy (LAP-PIE) trial: study protocol for a feasibility randomised controlled trial. BMJ Open 12 (2022): 50166.
- Chen Z, Lv Y, Li H, et al. Meta-analysis of FOLFIRINOX-based neoadjuvant therapy for locally advanced pancreatic cancer. Medicine 100 (2021): e24068.
- Ruarus AH, Vroomen LGPH, Geboers B, et al. Percutaneous irreversible electroporation in locally advanced and recurrent pancreatic cancer (PANFIRE-2): A multicenter, prospective, single-arm, phase II study. Radiology 294 (2020): 212-220.
- Vogel JA, Rombouts SJ, de Rooij T, et al. Induction Chemotherapy Followed by Resection or Irreversible Electroporation in Locally Advanced Pancreatic Cancer (IMPALA): A Prospective Cohort Study. Ann Surg Oncol 24 (2017): 2734-2743.
- Heger U, Hackert T. Can local ablative techniques replace surgery for locally advanced pancreatic cancer? J Gastrointest Oncol 12 (2021): 2536-2546.
- Woeste MR, Wilson KD, Kruse EJ, et al. Optimizing Patient Selection for Irreversible Electroporation of Locally Advanced Pancreatic Cancer: Analyses of Survival. Front Oncol 11 (2022): 1-12.
- van Veldhuisen E, Vroomen LG, Ruarus AH, et al. Value of CT-Guided Percutaneous Irreversible Electroporation Added to FOLFIRINOX Chemotherapy in Locally Advanced Pancreatic Cancer: A Post Hoc Comparison. J Vasc Interv Radiol 31 (2020): 1600-1608.
- van Veldhuisen E, Vroomen LG, Ruarus AH, et al. Value of CT-Guided Percutaneous Irreversible Electroporation Added to FOLFIRINOX Chemotherapy in Locally Advanced Pancreatic Cancer: A Post Hoc Comparison. Journal of Vascular and Interventional Radiology 31 (2020): 1600-1608.
- Narayanan G, Hosein PJ, Beulaygue IC, et al. Percutaneous Image-Guided Irreversible Electroporation for the Treatment of Unresectable, Locally Advanced Pancreatic Adenocarcinoma. Journal of Vascular and Interventional Radiology 28 (2017):
- Scheffer HJ, Vroomen LGPH, de Jong MC, et al. Ablation of locally advanced pancreatic cancer with percutaneous irreversible electroporation: Results of the phase I/II PANFIRE study. Radiology 282 (2017).
- Flak RV, Malmberg MM, Stender MT, et al. Irreversible electroporation of pancreatic cancer-Effect on quality of life and pain perception. Pancreatology 21 (2021): 1059-1063.
- Scheffer HJ, Vroomen LGPH, de Jong MC, et al. Ablation of locally advanced pancreatic cancer with percutaneous irreversible electroporation: Results of the phase I/II PANFIRE study. Radiology 282 (2017): 585-597.
- Lin M, Liang S, Wang X, et al. Short-term clinical efficacy of percutaneous irreversible electroporation combined with allogeneic natural killer cell for treating metastatic pancreatic cancer. Immunol Lett 186 (2017): 20-27.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240 (2004).
- He C, Sun S, Huang X, et al. Survival Comparison of Neoadjuvant Chemotherapy Followed by Irreversible Electroporation Versus Conversional Resection for Locally Advanced Pancreatic Cancer. Front Oncol 10 (2021): 1-11.
- Timmer FEF, Geboers B, Ruarus AH, et al. Irreversible Electroporation for Locally Advanced Pancreatic Cancer. Tech Vasc Interv Radiol 23 (2020).
- Moris D, Machairas N, Tsilimigras DI, et al. Systematic Review of Surgical and Percutaneous Irreversible Electroporation in the Treatment of Locally Advanced Pancreatic Cancer. Ann Surg Oncol 26 (2019).
- He C, Huang X, Zhang Y, et al. Comparison of Survival Between Irreversible Electroporation Followed by Chemotherapy and Chemotherapy Alone for Locally Advanced Pancreatic Cancer. Front Oncol 10 (2020).
- Kwon JH, Chung MJ, Park JY, et al. Initial experience of irreversible electroporation for locally advanced pancreatic cancer in a Korean population. Acta radiol 62 (2021).
- Mahnken AH, Boullosa Seoane E, Cannavale A, et al. CIRSE Clinical Practice Manual. Cardiovasc Intervent Radiol 44 (2021): 1323-1353.
- van Veldhuisen E, Vroomen LG, Ruarus AH, et al. Value of CT-Guided Percutaneous Irreversible Electroporation Added to FOLFIRINOX Chemotherapy in Locally Advanced Pancreatic Cancer: A Post Hoc Comparison. Journal of Vascular and Interventional Radiology 31 (2020).
- Ierardi AM, Biondetti P, Coppola A, et al. Percutaneous microwave thermosphere ablation of pancreatic tumours. Gland Surg 7 (2018).
- Zhang LN, Wan WB, Wang YX, et al. Evaluation of elastic stiffness in healing Achilles tendon after surgical repair of a tendon rupture using in vivo ultrasound shear wave elastography. Medical Science Monitor 22 (2016).
- Vogel JA, Rombouts SJ, de Rooij T, et al. Induction Chemotherapy Followed by Resection or Irreversible Electroporation in Locally Advanced Pancreatic Cancer (IMPALA): A Prospective Cohort Study. Ann Surg Oncol 24 (2017).
- Lambert L, Horejs J, Krska Z, et al. Treatment of locally advanced pancreatic cancer by percutaneous and intraoperative irreversible electroporation: General hospital cancer center experience. Neoplasma 63 (2016).
- Belfiore MP, Ronza FM, Romano F, et al. Percutaneous CT-guided irreversible electroporation followed by chemotherapy as a novel neoadjuvant protocol in locally advanced pancreatic cancer: Our preliminary experience. International Journal of Surgery 21 (2015).
- Sugimoto K, Moriyasu F, Tsuchiya T, et al. Irreversible electroporation for nonthermal tumor ablation in patients with locally advanced pancreatic cancer: Initial clinical experience in japan. Internal Medicine 57 (2018).
- Rai ZL, Feakins R, Pallett LJ, et al. Irreversible electroporation (Ire) in locally advanced pancreatic cancer: A review of current clinical outcomes, mechanism of action and opportunities for synergistic therapy. J Clin Med 10 (2021).
- Woeste MR, Wilson KD, Kruse EJ, et al. Optimizing Patient Selection for Irreversible Electroporation of Locally Advanced Pancreatic Cancer: Analyses of Survival. Front Oncol 11 (2022).
- Rudno-Rudzinska J, Kielan W, Guzinski M, et al. New therapeutic strategy: Personalization of pancreatic cancer treatment-irreversible electroporation (IRE), electrochemotherapy (ECT) and calcium electroporation (CaEP)-A pilot preclinical study. Surg Oncol 38 (2021).
- Heger U, Mack C, Tjaden C, et al. Open irreversible electroporation for isolated local recurrence of pancreatic ductal adenocarcinoma after primary surgery. Pancreatology 21 (2021).
- He C, Sun S, Huang X, et al. Survival Comparison of Neoadjuvant Chemotherapy Followed by Irreversible Electroporation Versus Conversional Resection for Locally Advanced Pancreatic Cancer. Front Oncol 10 (2021).
- Ruarus AH, Vroomen LGPH, Geboers B, et al. Percutaneous irreversible electroporation in locally advanced and recurrent pancreatic cancer (PANFIRE-2): A multicenter, prospective, single-arm, phase II study. Radiology 294 (2020).
- Holland MM, Bhutiani N, Kruse EJ, et al. A prospective, multi-institution assessment of irreversible electroporation for treatment of locally advanced pancreatic adenocarcinoma: initial outcomes from the AHPBA pancreatic registry. HPB 21 (2019).
- Flak RV, Stender MT, Jensen TM, et al. Treatment of locally advanced pancreatic cancer with irreversible electroporation–a Danish single center study of safety and feasibility. Scand J Gastroenterol 54 (2019).
- Månsson C, Brahmstaedt R, Nygren P, et al. Percutaneous irreversible electroporation as first-line treatment of locally advanced pancreatic cancer. Anticancer Res 39 (2019).
- Leen E, Picard J, Stebbing J, et al. Percutaneous irreversible electroporation with systemic treatment for locally advanced pancreatic adenocarcinoma. J Gastrointest Oncol 9 (2018).
- Huang KW, Yang PC, Pua U, et al. The efficacy of combination of induction chemotherapy and irreversible electroporation ablation for patients with locally advanced pancreatic adenocarcinoma. J Surg Oncol 118 (2018).
- Månsson C, Brahmstaedt R, Nilsson A, et al. Percutaneous irreversible electroporation for treatment of locally advanced pancreatic cancer following chemotherapy or radiochemotherapy. European Journal of Surgical Oncology 42 (2016).
- Zhang Y, Shi J, Zeng J, et al. Percutaneous irreversible electroporation for ablation of locally advanced pancreatic cancer experience from a Chinese institution. Pancreas 46 (2017).
- Granata V, Fusco R, Salati S, et al. A Systematic Review about Imaging and Histopathological Findings for Detecting and Evaluating Electroporation Based Treatments Response. International Journal of Environmental Research and Public Health 18 (2021): 5592.
- Granata V, Fusco R, Petrillo A, et al. Local ablation of pancreatic tumors: State of the art and future perspectives. World J Gastroenterol 27 (2021): 3413-3428.
- Tirkes T, Hollar MA, Tann M, et al. Response criteria in oncologic imaging: Review of traditional and new criteria. Radiographics 33 (2013).
- Vroomen LGPH, Scheffer HJ, Melenhorst MCAM, et al. MR and CT imaging characteristics and ablation zone volumetry of locally advanced pancreatic cancer treated with irreversible electroporation. Eur Radiol 27 (2017): 2521-2531.
- Granata V, Fusco R, Sansone M, et al. Magnetic resonance imaging in the assessment of pancreatic cancer with quantitative parameter extraction by means of dynamic contrast-enhanced magnetic resonance imaging, diffusion kurtosis imaging and intravoxel incoherent motion diffusion-weighted imaging. Therap Adv Gastroenterol 13 (2020).
- Granata V, Fusco R, Salati S, et al. A Systematic Review about Imaging and Histopathological Findings for Detecting and Evaluating Electroporation Based Treatments Response. Int J Environ Res Public Health 18 (2021).
- Granata V, Grassi R, Fusco R, et al. Assessment of Ablation Therapy in Pancreatic Cancer: The Radiologist’s Challenge. Front Oncol 10 (2020).
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45 (2009).
- Rai ZL, Feakins R, Pallett LJ, et al. Irreversible electroporation (Ire) in locally advanced pancreatic cancer: A review of current clinical outcomes, mechanism of action and opportunities for synergistic therapy. J Clin Med 10 (2021).
- Vroomen LGPH, Scheffer HJ, Melenhorst MCAM, et al. MR and CT imaging characteristics and ablation zone volumetry of locally advanced pancreatic cancer treated with irreversible electroporation. Eur Radiol 27 (2017): 2521-2531.
- Akinwande O, Ahmad SS, van Meter T, et al. CT Findings of Patients Treated with Irreversible Electroporation for Locally Advanced Pancreatic Cancer. J Oncol 2015 (2015).
- Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. Journal of Nuclear Medicine 50 (2009).
- Delbeke D, Martin WH. Update of PET and PET/CT for hepatobiliary and pancreatic malignancies. HPB (Oxford) 7 (2005): 166.
- Akinwande O, Ahmad SS, van Meter T, et al. CT Findings of Patients Treated with Irreversible Electroporation for Locally Advanced Pancreatic Cancer. J Oncol 2015 (2015): 1-8.
- García-Figueiras R, Padhani AR, Baleato-González S. Therapy Monitoring with Functional and Molecular MR Imaging. Magn Reson Imaging Clin N Am 24 (2016): 261-288.
- Rai ZL, Feakins R, Pallett LJ, et al. Irreversible electroporation (Ire) in locally advanced pancreatic cancer: A review of current clinical outcomes, mechanism of action and opportunities for synergistic therapy. J Clin Med 10 (2021).
- Garnier J, Turrini O, Chretien A-S, et al. Local Ablative Therapy Associated with Immunotherapy in Locally Advanced Pancreatic Cancer: A Solution to Overcome the Double Trouble?-A Comprehensive Review. J Clin Med 11 (2022): 1948.
- Imran KM, Nagai-Singer MA, Brock RM, et al. Exploration of Novel Pathways Underlying Irreversible Electroporation Induced Anti-Tumor Immunity in Pancreatic Cancer. Front Oncol 12 (2022).
- He C, Sun S, Zhang Y, et al. Irreversible electroporation plus anti-pd-1 antibody versus irreversible electroporation alone for patients with locally advanced pancreatic cancer. J Inflamm Res 14 (2021): 4795-4807.
- Lin M, Liang S, Wang X, et al. Percutaneous irreversible electroporation combined with allogeneic natural killer cell immunotherapy for patients with unresectable (Stage III/IV) pancreatic cancer: A promising treatment. J Cancer Res Clin Oncol 143 (2017): 2607-2618.
- Tian G, Guan J, Chu Y, et al. Immunomodulatory Effect of Irreversible Electroporation Alone and Its Cooperating With Immunotherapy in Pancreatic Cancer. Front Oncol 11 (2021).
- Mehrotra S, Britten CD, Chin S, et al. Vaccination with poly(IC: LC) and peptide-pulsed autologous dendritic cells in patients with pancreatic cancer. J Hematol Oncol 10 (2017).
- Peng H, Shen J, Long X, et al. Local Release of TGF-β Inhibitor Modulates Tumor-Associated Neutrophils and Enhances Pancreatic Cancer Response to Combined Irreversible Electroporation and Immunotherapy. Advanced Science 9 (2022).
- O CH, Tan M, Yan J, et al. Perioperative systemic immunophenotype following irreversible electroporation (IRE) predicts recurrence (2022).
- Lin M, Zhang X, Liang S, et al. Irreversible electroporation plus allogenic Vγ9Vδ2 T a cell enhances antitumor effect for locally advanced pancreatic cancer patients. Signal Transduct Target Ther 5 (2020).
- di Federico A, Mosca M, Pagani R, et al. Immunotherapy in Pancreatic Cancer: Why Do We Keep Failing? A Focus on Tumor Immune Microenvironment, Predictive Biomarkers and Treatment Outcomes. Cancers (Basel) 14 (2022).
- Ullman NA, Burchard PR, Dunne RF, et al SPECIAL SERIES: PRECISION MEDICINE AND IMMUNOTHERAPY IN GI MALIGNANCIES review articles Immunologic Strategies in Pancreatic Cancer: Making Cold Tumors Hot (2022).
- Bockorny B, Semenisty V, Macarulla T, et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat Med 26 (2020): 878-885.
- O’Hara MH, Messersmith W, Kindler H, et al. Safety and Pharmacokinetics of CXCR4 Peptide Antagonist, LY2510924, in Combination with Durvalumab in Advanced Refractory Solid Tumors. J Pancreat Cancer 6 (2020): 21-31.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 74.74%
Acceptance Rate: 74.74%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 