Prognostic Role of Tumor Size Reduction >50% after Neoadjuvant Chemotherapy for Breast Cancer
Milo Giani1, Ketty Tavella2, Irene Renda1, Enrico Tartarotti1, Jacopo Nori3, Ermanno Vanzi3, Simonetta Bianchi4, Tommaso Susini1*
1Breast Unit, Gynecology Section, Department of Health Sciences, University of Florence, Florence, Italy
2Medical Oncology Unit, Azienda Ospedaliero-Universitaria Careggi, Florence, Italy
3Diagnostic Senology Unit, Azienda Ospedaliero-Universitaria Careggi, Florence, Italy
4Pathology Unit, Department of Health Sciences, University of Florence, Florence, Italy
*Corresponding Author: Tommaso Susini, Breast Unit, Gynecology Section, Department of Health Sciences, University of Florence, Florence, Italy.
Received: 20 August 2022; Accepted: 07 September 2022; Published: 06 October 2022
Article Information
Citation: Milo Giani, Ketty Tavella, Irene Renda, Enrico Tartarotti, Jacopo Nori, Ermanno Vanzi, Simonetta Bianchi, Tommaso Susini. Prognostic Role of Tumor Size Reduction >50% after Neoadjuvant Chemotherapy for Breast Cancer 6 (2022): 349-357.
View / Download Pdf Share at FacebookAbstract
Purpose: We evaluated the efficacy of neoadjuvant chemotherapy in reducing locally advanced and early breast cancers size, improving breastconserving surgery rates and its long-term outcomes. Our first aim was to test whether patients achieving a partial pathological response of good quality after neoadjuvant chemotherapy (tumor shrinkage >50% from the original clinical-instrumental size to the size evaluated by the pathologist on the surgical specimen) had better disease-free and overall survival rates than those with a tumor size reduction <50%.
Patients and Methods: We analyzed 64 patients initially candidate to mastectomy, treated with neoadjuvant chemotherapy and subsequent surgery at our institution.
Results: We observed tumor size reduction in 95% of the cases resulting in downstaging in 67.2% of the patients. Women with tumor size reduction >50% after NACT had better 10-years disease-free survival and overall survival rates than women with reduction <50% (p=0.002 and p<0.05, respectively). In a multivariate analysis, tumor size reduction >50% (HR=4.29, p=0.004) was an independent predictor of disease-free survival, whereas significance was not reached concerning overall survival. Treatment with neoadjuvant chemotherapy allowed to half the rate of mastectomy, as breast-conserving surgery was used in 50% of the cases. Overall, we had recurrences in 37.5% patients. We found no significant increase in local or distant recurrences after breast conserving surgery, as compared with mastectomy.
Conclusions: Our data suggest that a tumor size reduction >50% after neoadjuvant chemotherapy may represent a prognostic factor for low risk of recurrence. The use of breast-conserving surgery was not associated with significantly higher risk of loca
Keywords
<p>Breast Conserving Surgery; Down-Staging; Long-Term Outcome; Mastectomy; Tumor Shrinkage</p>
Article Details
1. Introduction
Preoperative chemotherapy was introduced for treatment of patients with locally advanced breast cancers (LABC) since the late 70's - early 80's [1,2]. Large randomized and non-randomized trials have shown that women treated with neoadjuvant chemotherapy (NACT) and subsequent surgery have the same disease-free survival (DFS) and overall survival (OS) rates than women treated with surgery and subsequent chemotherapy [3-5]. Tumor down-staging achieved with NACT allows to convert inoperable disease into operable one and to perform breast-conserving surgery (BCS) in some patients for whom mastectomy is initially the only surgical option. Therefore, NACT has become the standard of treatment for LABC and inflammatory (inoperable) breast cancers [6-10]. Recently, primary chemotherapy has become an option to be considered also in early (operable) breast cancer patients who at first would have been candidate to a mastectomy, or that, because of the molecular type of their disease (eg triple negative breast cancer, HER-2 positive cancers, ouvert axillary metastases) would receive chemotherapy anyway at some point in their treatment course [11,12]. In addition, NACT has the potential to treat immediately any micrometastatic disease and also permits an early evaluation of the effectiveness of the systemic treatment. Indeed, NACT has allowed to reduce the rate of mastectomy in favor of BCS. This may increase the risk of loco-regional recurrence because of the difficulty in assessing tumor margins after the administration of preoperative chemotherapy. Several studies investigated the oncologic safety of BCS after neoadjuvant chemotherapy and, despite initial discordant results, it has now been clarified that BCS after NACT, is not associated with a significantly increased risk of loco-regional recurrences [13-18]. The degree of clinical and pathological response to NACT is of paramount importance to allow breast-conserving surgery. In addition, several studies reported a better DFS and OS rates for the patients who achieved a pathologic complete response (pCR) after NACT. However, only a relatively small proportion of patients (3-26%) achieve a pCR in both breast and axillary lymph nodes [3-6,19-22]. Most of the patients obtain a partial pathologic response (pPR) to NACT and this group includes patients with very different prognosis. Previous studies showed that the residual cancer burden after primary chemotherapy is an important prognostic factor and that a near-pCR may predict long term survival [23-25]. These studies used the Nottingham Prognostic Index (NPI) and other derived classifications (MNPI and MBGI) to define different group of responders. However, these indexes are generated using relatively complex formulae and are not straightforward in the clinical practice. In a previous study, we analyzed the outcome of patients with locally advanced and inflammatory breast cancers who received NACT [26]. The aim of the current study was to update the experience of our center in the use of NACT for treatment of locally advanced and early breast cancer, excluding patients with inflammatory carcinoma. Our main purpose was to test whether also achieving a pPR of good quality after NACT evaluated in a simple manner (tumor shrinkage >50% from the original clinical-instrumental size, to the size evaluated by the pathologist on the surgical specimen), may have a prognostic significance.
2. Material and Methods
2.1 Study Population
An observational, retrospective, single institution study was conducted. We retrieved data of all the patients with early or locally advanced breast cancer who underwent NACT, between January 2000 and September 2020, at our Breast Unit, Gynecology section, Department of Health Science, Careggi Hospital, University of Florence. Overall, we identified 73 patients treated with NACT. After the exclusions of 9 patients with inflammatory breast cancer, 64 patients were included in the study. All patients had biopsy proven invasive breast cancer. Clinical T (cT) and clinical N (cN) stages were evaluated by physical examination, breast and axillary ultrasound, mammography and MRI before the start of NACT, according to the system set by the American Joint Committee on Cancer (AJCC) [27]. The same examinations were performed after NACT to assess tumor response in terms of tumor shrinkage. Hormone receptor status was assessed by immunohistochemistry (IHC) and HER2 status was first evaluated by IHC and if necessary, by fluorescence in situ hybridization (FISH). These same molecular characteristics were determined on both core-biopsy material and then after surgery on the surgical specimen.
2.2 Treatment
Primary chemotherapy consisted of intravenously administered anthracycline (7.8%) or combined anthracycline-taxane (92.2%) [28-30]. Trastuzumab was administered to patients with c-erB2 (HER2)-overexpressing tumors in 11 patients (17.2%) [31-33]. BCS or mastectomy (with or without immediate breast reconstruction) was performed according to clinical response, tumor biological features and in accordance with the patients. All patients treated with BCS underwent subsequent breast irradiation. Patients with clinically or biopsy-proven lymph node involvement prior to NACT, who remained clinically positive after the treatment (ycN1), underwent complete axillary dissection. Patients with clinically or biopsy-proven lymph node involvement prior to NACT, who down-staged from cN1 to ycN0 after NACT, underwent sentinel node biopsy; patients with positive sentinel node underwent complete axillary dissection, whereas those with negative sentinel node underwent ultrasound-based follow-up of the axilla [34-36].
2.3 Response to NACT
A complete pathological response (pCR) was defined as no evidence of residual invasive tumor in the surgical specimen, in both the breast and the axillary lymph nodes (ypT0/Tis, ypN0). Partial pathologic response (pPR) was considered when invasive tumor was still present in the surgical specimen, although reduced in size. We defined as stable disease when no change in tumor size was observed. No case of disease progression during NACT was registered. We divided the study population into two groups according to the degree of response to NACT: reduction of original size <50% versus >50%. The ratio of reduction was calculated by dividing the size of the largest residual tumor foci in the surgical specimen (in mm), as measured by the pathologist, by the size of the original tumor (clinical and/or instrumental) (in mm).
2.4 Follow-up and Clinical Outcome
During the first 5 years after NACT and surgical treatment, patients underwent physical examination and tumor markers evaluation (Ca-15.3, CEA) every six months and mammography, breast and axillary ultrasound every year. Abdominal ultrasound and bone scintigraphy or total body CT scans were also performed every year in case of LABC. The end points analyzed were DFS and OS rates. DFS was measured from the date of surgical treatment to the date of onset of the first loco-regional or systemic recurrence. OS was measured from the date of surgical treatment to the date of the last follow-up or death from any cause. Loco-regional recurrence (LR) was defined as recurrent disease in the ipsilateral breast or chest wall, in the ipsilateral axillary, supraclavicular, subclavian, or internal mammary lymph nodes. Recurrence at any other site was considered as distant metastasis (DM).
2.5 Statistical Analysis
Distribution of patients’ according to clinical-pathologic features were evaluated by Fisher’s Exact Test or Chi-Square Test, as appropriate. The disease-free and overall survival rates were calculated by the Kaplan-Meier method and differences in survival estimates were evaluated by the Log-Rank Test. Univariate Cox proportional hazards regression analysis was used to evaluate the effect of each prognostic factor on disease-free survival and overall survival. We used a multivariate Cox proportional hazards regression analysis, with forward selection of variables, to assess the independence of each prognostic variable. A p-value <0.05 was considered statistically significant. Data analysis was performed using IBM SPSS Statistics, version 27.0.
3. Results
Data from 64 patients who received NACT for locally advanced or early breast cancer were analyzed. The median age at diagnosis was 48 years (range: 21-74 years). All patients were treated with anthracycline (7.8%) or combined anthracycline-taxane (92.2%) NACT. Neoadjuvant Trastuzumab was given to most of the patients with HER2 positive tumors (17.2%). We observed a tumor size reduction in 61 out of 64 patients (95.3%), with average tumor shrinkage of 53% from the original size and a downstaging in 43 out of 64 (67.2%) cases. A tumor size reduction >50% was obtained in 33 cases (51.6%). Patients’ clinical and pathological features distribution according to degree of response to NACT is reported in Table 1. The distribution of patients by tumor stage (TNM classification), before and after NACT, is shown in Table 2.
|
All N° (%) |
< 50% N° (%) |
> 50% N° (%) |
P value |
||
|
Total |
64 (100) |
31 (48.4) |
33 (51.6) |
||
|
Mean age (years) |
48 |
47.6 |
48.4 |
||
|
Surgery: |
|||||
|
BCS |
32 (50.0) |
13 (40.6) |
19 (59.4) |
ns |
|
|
Mastectomy |
32 (50.0) |
18 (56.2) |
14 (43.8) |
||
|
Histologic type: |
|||||
|
Ductal |
38 (59.4) |
14 (36.8) |
24 (63.2) |
0.055 |
|
|
Lobular |
20 (31.2) |
14 (70.0) |
6 (30.0) |
||
|
Other |
6 (9.4) |
3 (50.0) |
3 (50.0) |
||
|
Histologic grade: |
|||||
|
G1 |
10 (15.6) |
8 (80.0) |
2 (20.0) |
0.019 |
|
|
G2 |
23 (35.9) |
13 (56.6) |
10 (43.4) |
||
|
G3 |
31 (48.5) |
10 (32.2) |
21 (67.8) |
||
|
Hormone receptors: |
|||||
|
ER+ and/or PR+ |
49 (76.6) |
23 (46.9) |
26 (53.1) |
ns |
|
|
ER- and PR- |
15 (23.4) |
8 (53.3) |
7 (46.7) |
||
|
HER2 status: |
|||||
|
Positive |
16 (25) |
4 (25.0) |
12 (75.0) |
0.03 |
|
|
Negative |
48 (75) |
27 (56.2) |
21 (43.8) |
||
|
Triple negative: |
|||||
|
Yes |
11 (17.2) |
6 (54.5) |
5 (45.5) |
ns |
|
|
No |
53 (82.8) |
25 (47.2) |
28 (52.8) |
||
|
Tumor Stage (AJCC): |
|||||
|
IIA |
18 (28.1) |
8 (44.4) |
10 (55.6) |
0.11 |
|
|
IIB |
16 (25.0) |
7 (43.7) |
9 (56.3) |
||
|
IIIA |
25 (39.1) |
16 (64.0) |
9 (36.0) |
||
|
IIIB |
3 (4.7) |
0 - |
3 (100.0) |
||
|
IIIC |
2 (3.1) |
0 - |
2 (100.0) |
Table 1: Clinicopathologic features according to response to neoadjuvant chemotherapy.
Abbreviations: ER- Estrogen Receptors; PR- Progesteron Receptors.
We performed BCS in 32 out of 64 patients (50%). Therefore, considering that all the patients initially should have undergone mastectomy, NACT allowed sparing such invasive procedure in half of the cases. Then, we assessed the relationship between tumor histologic type and tumor shrinkage after NACT. Invasive ductal carcinoma was more likely to have a >50% shrinkage than invasive lobular carcinoma or other histologic types, however the statistical significance was only approached (p=0.055, Chi-square test). Hence, patients with invasive ductal carcinoma were more likely to undergo BCS (55.2%) than patients with lobular carcinoma (50%). In addition, concerning grade of differentiation, patients with poorly differentiated tumors (G3) were more likely to have >50% tumor shrinkage (p=0.019, Chi-square test).
|
Pre-chemo stage |
Post-chemo stage |
||||
|
(cT) |
(ypT) |
||||
|
n° |
% |
n° |
% |
||
|
ypT0 |
8 |
12.5 |
|||
|
cT1 |
5 |
7.8 |
ypT1 |
31 |
48.4 |
|
cT2 |
35 |
54.7 |
ypT2 |
22 |
34.4 |
|
cT3 |
22 |
34.4 |
ypT3 |
3 |
4.7 |
|
cT4 |
2 |
3.1 |
ypT4 |
- |
- |
|
Total |
64 |
100 |
Total |
64 |
100 |
Table 2: Tumor stage (TNM classification) before and after NACT.
Abbreviations: cT- Clinical Tumor Stage; ypT- Pathologic Tumor Stage after NACT
3.1 Tumor Size Reduction after NACT
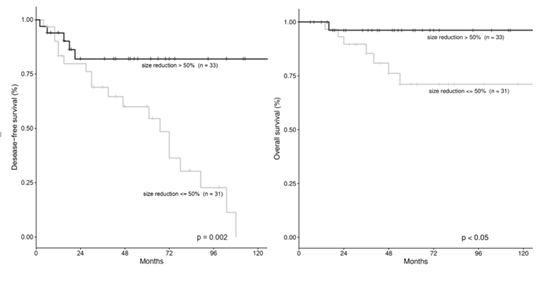
We divided the whole series into two groups according to the extent of tumor shrinkage after NACT. In 31 out of 64 patients (48.4%) the tumor size reduction was <50%, whereas in 33 out of 64 patients (51.6%) tumor size reduction was >50%. Overall, during follow up (median 55.5 months, range 12-181 months) we observed 8 deaths (12.5%) and 24 recurrences (37.5%). Using Kaplan-Meier method, we found that patients with good response to NACT (tumor size reduction >50%) had a significantly better DFS and OS (p=0.002 and p<0.05, respectively) (Figure 1). Furthermore, we analyzed the results of multivariate Cox proportional hazards regression in which tumor size reduction (>50% vs <50%), ypT (T0-1 vs T2 vs T3-4) and ypN (N0-1 vs N2-3) were tested simultaneously to assess the risk ratio for recurrences and for death and the independence of each variable. Tumor size reduction was the strongest independent predictor of recurrence, whereas ypT and ypN were not significant and were not included in the equation. Patients with tumor size reduction <50% had the higher risk of recurrence (HR=4.29, p=0.004).
We had 5 recurrences (15.1%) and one death (3.0%) in the group with tumor reduction >50%, in comparison with 19 recurrences (61.3%) and 7 deaths (22.6%) in the group with tumor reduction <50% (p=0.002 and p<0.05, respectively, Fisher’s Exact test). The site of recurrence was locoregional (LR) in 4 cases and distant metastasis (DM) in one case in the former group, versus 9 LR and 10 DM, in the latter group (difference not significant, Fisher’s Exact test). Recurrences in the group with tumor reduction >50% occurred after an average of 12.4 months and were concentrated in the first 24 months after surgery, whereas recurrences in the <50% tumor size reduction group tended to occurr continuosly during follow up, even many years after surgery (on average after 46.8 months). Concerning the type of surgery, the BCS was used more frequently in the >50% size reduction group (59.4% vs 40.6%) and conversely, mastectomy was more frequently used in the <50% tumor reduction group (56.2% vs 43.8%), however the difference was not statistically significant (p=0.2, Fisher’s Exact test).
3.2 Neoadjuvant Trastuzumab
Trastuzumab was administered in addition to preoperative chemotherapy to patients with HER2-positive breast cancer in 11 patients (17.2%). The average tumor shrinkage of the patients treated with NACT and Trastuzumab was 83% (vs 46.6% average tumor shrinkage of patients treated with NACT without Trastuzumab). Most of the patients who received Trastuzumab obtained a very good response (>50% tumor size reduction) to NACT (10 out of 11 cases: 90.9%) (p=0.006, Fisher’s Exact test). Among the patients who received Trastuzumab, there were no LR and deaths. Only one patient had a DM (the one who obtained a reduction <50%).
3.3 Pathological Complete Response
Overall, we had 5 patients (7.8%) with pCR after NACT. Three of these patients (60%) received also Trastuzumab. In the pCR group, one patient had a LR after 6 months, subsequently developed a DM and died after 16 months. The other 4 patients had no recurrences and are free from disease.
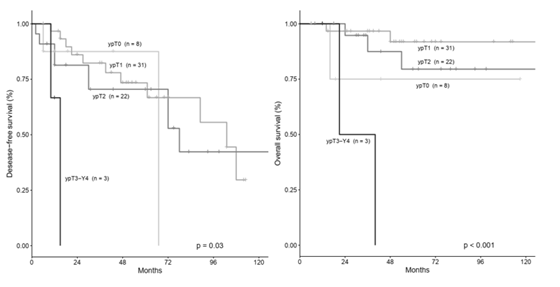
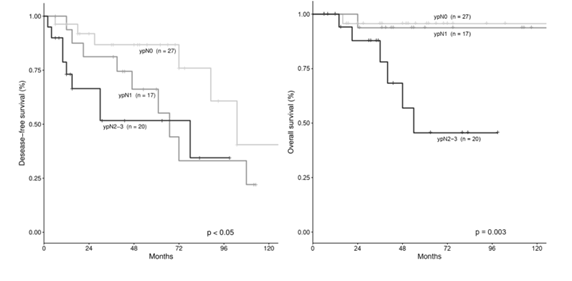
3.4 Survival According to Pathological Tumor Stage after NACT
DFS and OS were evaluated according to tumor stage after NACT using K-M method and multivariate Cox proportional hazards regression. Using K-M method, patients with less advanced tumor stage after NACT (ypT0, ypT1 or ypT2) had better DFS (p=0.03) and OS (p<0.001) rates than patients with more advanced stages after NACT (ypT3-ypT4) (Figure 2). Similarly, patients with less involved lymph nodes after NACT (ypN0 or ypN1) had better DFS (p<0.05) and OS (p=0.003) rates compared to patients with more advanced lymph node involvement (ypN2 or ypN3) (Figure 3). By multivariate analysis in which tumor size reduction >50%, ypT (ypT0-1 vs ypT2 vs ypT3-4) and ypN (ypN0-1 vs ypN2-3) were tested simultaneously, we observed that ypT stage was the strongest independent predictor for OS, whereas the other variables were not included in the equation. As compared with patients with ypT0-1, those with ypT3-4 showed the higest risk to die of disease (HR=20.2), followed by patients with ypT2 (HR=1.7)( p=0.006).
3.5 Recurrences and Deaths According to type of Surgery
We than analyzed recurrences and deaths according to surgery: BCS or mastectomy. We observed 6 LR, 3 DM, 2 deaths in the BCS group and 7 LRR, 8 DM and 6 deaths in the mastectomy group. None of the patients who relapsed locally after BCS died of the disease, as all of them underwent salvage mastectomy. In fact, the 2 patients who died after BCS were among those who developed distant metastasis. Overall, the incidence of relapse after mastectomy was not significantly different from that observed after BCS (p=0.3, Fisher’s exact test).
4. Discussion
It is known that NACT allows to reduce the rate of mastectomy for treatment of LABC and that patients who obtain a pCR after NACT have better outcomes than patients with a minor response [37]. The prognostic role of a partial response of good quality, defined as a tumor size reduction >50%, was not previously investigated, except in a previous study from our group in which were included also inflammatory breast cancers [26]. We retrospectively analyzed data of 64 patients who underwent NACT for locally advanced and early breast cancer at our institution. Overall, we obtained a pPR of good quality in more than half of the patients (51.6%). In patients with HER2-positive tumor, when Trastuzumab was associated to NACT, a significantly higher average tumor size reduction was obtained (83.0% vs 46.6%), in accordance with literature [31-32,37-38]. Conversely, lobular histologic type and well differentiated (G1) tumors were features associated with a poor response to NACT. Indeed, our results confirmed the efficacy of NACT in reducing the mastectomy rate. In fact, in our series NACT allowed to spare half of the mastectomy procedures, in agreement with previous studies [3,39]. Concerning pattern of recurrences and death in relation to type of surgery, we did not find significant differences, especially in the occurrence of LR. This result suggests that the choice of a BCS procedure after NACT is safe and does not expose women to an increased risk of LR. Hence, there was a trend toward a higher incidence of DM and deaths among patients in the mastectomy group, however the difference was not significant. This result is possibly due to the more aggressive nature of the tumors that ultimately required a mastectomy. The most relevant finding of the current study was that tumor size reduction after NACT, was the strongest independent predictor of recurrence by multivariate analysis. Hence, patients with tumor size reduction <50% showed a 4.29 higher risk of relapse (p=0.004). However, tumor size reduction (<50% vs >50%) was not an independent predictor for overall survival. Nonetheless, tumor size reduction >50% after NACT was associated with significantly better DFS and OS rates than those in patients with a reduction <50% (p=0.002 and p<0.05, respectively). Obviously, the results of the current study must be interpreted with caution, because of the retrospective design and the relatively small number of patients. So, we don’t draw any definitive conclusion. However, our results may suggest that not only a pCR, but also a partial response of good quality can be of prognostic significance. The cut-off >50% of size reduction was previously used to discriminate between partial responders and non-responders and was based only on clinical examination [12]. However, it is known that in many instances patients who obtain a complete response on clinical examination, eventually have residual disease in the surgical specimen that prevent them to be classified as pCR. Nonetheless, this type of response (clinically complete and pPR) denotes a good chemo-sensitivity of the tumor, which may translate into a more favorable outcome after surgery. Previous studies highlighted the role of near-pCR and residual tumor burden as prognostic factors after NACT [23-25]. However, the definition of these categories of response was complex and based on several parameters. For this reason, we decided to test the meaning of a partial response of good quality, based on a simple method, comparing the initial tumor diameter with that evaluated by the pathologist on the surgical specimen. Our results seem to support the role of a partial response of good quality (size reduction >50%) as a potential prognostic factor after NACT. Another interesting outcome of our study concerns the timing of recurrence onset according to response to NACT. Indeed, we observed that the few patients who relapsed after a good response to NACT tended to do it early after surgery, whereas among patients with limited response after NACT (size reduction <50%) recurrences occurred at any time, even many years after the operation. The reason of this difference is unclear, but one could hypothesize that tumors that respond less to NACT (lobular histologic type, well differentiated tumors) are often of hormone-responsive type and may display a more indolent behavior. This may also translate in occurrence of late recurrences, e.g. after discontinuation of the 5-year adjuvant hormonal treatment. Another interesting finding was that other indicators of response to NACT, such as ypT stage and ypN stage, confirmed their role of prognostic indicators in our series. In particular, ypT stage was the strongest independent predictor of death of disease, with ypT3-4 patients showing a very high risk to die of disease as compared with ypT0-1 (HR=20.2, p=0.006). Patients with less advanced stage of disease after NACT (ypT0, ypT1 or ypT2) showed better DFS (p=0.03) and OS (p<0.001) rates than patients with more advanced tumor stage after NACT using K-M method (Figure 2). Similarly, we found that a greater response to NACT in the lymph nodes (ypN0-ypN1) was significantly associated with better DFS and OS (p<0.05 and p=0.003, respectively) than that of patients with poor response (ypN2-ypN3) (Figure 3). This result may indirectly support the idea that patients with negative axillary lymph node after NACT on clinical and ultrasound examination (excluding N2-N3 patients before NACT) may undergo sentinel lymph node biopsy, instead of complete axillary dissection, thus, preventing its associated side effects. This approach is supported also by a previous trial [34], which confirmed the accuracy of axillary ultrasound after NACT to select patients for sentinel lymph node biopsy or axillary dissection, as appropriate.
5. Conclusion
A tumor size reduction >50% after NACT was an independent predictor of low risk of recurrence in patients with locally advanced or early breast cancer. So, not only pCR but also a pPR of good quality may be a useful prognostic indicator in patients treated with NACT. The achievement of a >50% tumor size reduction represents an easily identifiable target, more frequently hit in clinical practice than pCR, that may significantly aid the clinicians in the management of these patients. The pattern of relapse after mastectomy was not significantly different from that observed after BCS, bringing further evidence that a conservative approach after NACT is feasible. Further studies on larger series are required to confirm the outcome of the current study.
Compliance with Ethical Standards
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments. All patients at the time of surgery signed a written informed consent to the use of their data for clinical purposes. The study was approved by the local ethics committee (Azienda Ospedaliera Universitaria Careggi, Florence, Italy).
Disclosure
All authors contributed to drafting or revising the article and gave final approval of the version to be published. All authors report no conflicts of interest in this work.
References
- Rubens RD, Sexton S, Tong D, et al. Combined chemotherapy and radiotherapy for locally advanced breast cancer. Eur J Cancer 16 (1980): 351-356.
- Schick P, Goodstein J, Moor J, et al. Preoperative chemotherapy followed by mastectomy for locally advanced breast cancer. J Surg Oncol 22 (1983): 278-282.
- Bonadonna G, Valgussa P, Brambilla C, et al. Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol 16 (1998): 93-100.
- Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16 (1998): 2672-2685.
- Wolmark N, Wang J, Mamounas E, et al. Preoperative Chemotherapy in Patients wWith Operable Breast Cancer: Nine-Year Results From National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 30 (2001): 96-102.
- Rastogi P, Anderson SJ, Bear HD, et al. Preoperative Chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 26 (2008): 778-785.
- Gralow JR, Burstein HL, Wood W, et al. Preoperative Therapy in Invasive Breast Cancer: Pathologic Assessment and Systemic Therapy Issues in Operable Disease. J Clin Oncol 26 (2008): 814-819.
- Monneur A, Goncalves A, Gilabert M, et al. Similar response profile to neoadjuvant chemotherapy, but different survival, in inflammatory versus locally advanced breast cancers. Oncotarget 8 (2017): 66019-66032.
- Baldini E, Gardin G, Evangelista G, et al. Long-Term Results of Combined-Modality Therapy for Inflammatory Breast Carcinoma. Clin Breast Cancer 5 (2004): 358-363.
- Jaiyesimi A, Buzdar AU, Hortobagyi G. Inflammatory breast cancer: a review. J Clin Oncol 10 (1992): 1014-1024.
- Van der Hage JA, Van de Velde CJ, Julien JP, et al. Preoperative Chemotherapy in Primary Operable Breast Cancer: Results From the European Organization for Research and Treatment of Cancer Trial 10902. J Clin Oncol 19 (2001): 4224-4237.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 19 (2018): 27-39.
- Mauri D, Pavlidis N, Ioannidis JPA. Neoadjuvant versus Adjuvant Systemic Treatment in Breast Cancer: A Meta-Analysis. J Natl Cancer Inst 97 (2005): 188-194.
- Fitzal F, Riedl O, Mittlbock M, et al. Oncologic safety of breast conserving surgery after tumour downsizing by neoadjuvant therapy: a retrospective single center cohort study. Breast Cancer Res Treat 127 (2011): 121-128.
- Levy A, Borget I, Bahri M, et al. Loco-regional Control After Neo-adjuvant Chemotherapy and Conservative Treatment for Locally Advanced Breast Cancer Patients. Breast J 20 (2014): 381-387.
- Mieog JSD, Van der Hage JA, Van de Velde CJH. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database System Rev 2007 (2007): CD005002.
- Simons JM, Jacobs JG, Roijers JP, et al. Disease-free and overall survival after neoadjuvant chemotherapy in breast cancer: breast-conserving surgery compared to mastectomy in a large single-centre cohort study. Breast Cancer Res Treat 185 (2021): 441-451.
- Teshome M, Kuerer HM. Breast conserving surgery and locoregional control after neoadjuvant chemotherapy. Eur J Surg Oncol 43 (2017): 865-874.
- Gajdos C, Tartter PI, Estabrook A, Gistrak MA, Jaffer S, Bleiweiss IJet al. Relationship of clinical and pathologic response to neoadjuvant chemotherapy and outcome of locally advanced breast cancer. J Surg Oncol 80 (2002): 4-11.
- Kurosumi M. Significance of histopathological evaluation in primary therapy for breast cancer--recent trends in primary modality with pathological complete response (pCR) as endpoint. Breast Cancer 11 (2004): 139-147.
- Symmans WF, Peintinger F, Hatzis C, et al. Measurement of Residual Breast Cancer Burden to Predict Survival After Neoadjuvant Chemotherapy. J Clin Oncol 25 (2007): 4414-4422.
- Sataloff DM, Mason BA, Prestipino AJ, et al. Pathologic response to induction chemotherapy in locally advanced carcinoma of the breast: a determinant of outcome. J Am Coll Surg 180 (1995): 297-306.
- Abdel-Fatah TM, Ball G, Lee AHS, et al. Notthingam Clinico-Pathological Response Index (NPRI) after Neoadjuvant Chemotherapy (Neo-ACT) Accurately Predicts Clinical Outcome in Locally Advanced Breast Cancer. Clin Cancer Res 21 (2015): 1052-1062.
- Chollet P, Amat S, Belembaogo E, et al. Is Notthingham prognostic index useful after induction chemotherapy in operable breast cancer?. Br J Cancer 89 (2003): 1185-1191.
- Abrial SC, Penault-Llorca F, Delva R, et al. High prognostic significance of residual disease after neoadjuvant chemotherapy: a retrospective study in 710 patients with operable breast cancer. Breast Cancer Res Treat 94 (2005): 255-263.
- Giani M, Renda I, Vallario A, et al. Long-term Results after Neoadjuvant Chemotherapy for Breast Cancer: A Single-center Experience. Anticancer Res 40 (2020): 1079-1085.
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann Surg Oncol 17 (2010): 1471-1474.
- Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after Doxorubicin Plus Cyclophosphamide As Adjuvant Chemotherapy for Node-Positive Breast Cancer: Results From NSABP B-28. J Clin Oncol 23 (2005): 3686-3696.
- Sparano JA, Wang M, Martino S, et al. Weekly Paclitaxel in the Adjuvant Treatment of Breast Cancer. N Engl J Med 358 (2008): 1663-1671.
- Bines J, Earl H, Buzaid AC, et al. Anthracyclines and taxanes in the neo/adjuvant treatment of breast cancer: does the sequence matter?. Ann Oncol 25 (2014): 1079-1085.
- Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 375 (2010): 377-384.
- Hamy AS, Belin L, Bonsang-Kitzis H, et al. Pathological complete response and prognosis after neoadjuvant chemotherapy for HER2-positive breast cancers before and after trastuzumab era: results from a real-life cohort. Br J Cancer 114 (2016): 44-52.
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Breast Cancer. N Engl J Med 353 (2005): 1659-1672.
- Mocellin S, Goldin E, Marchet A, et al. Sentinel node biopsy performance after neoadjuvant chemotherapy in locally advanced breast cancer: A systematic review and meta-analysis. Int J Cancer 138 (2016): 472-480.
- Classe JM, Loaec C, Gimbergues P, et al. Sentinel lymph node biopsy without axillary lymphadenectomy after neoadjuvant chemotherapy is accurate and safe for selected patients: the GANEA 2 study. Breast Cancer Res Treat 173 (2019): 343-352.
- Zetterund L, Celebioglu F, Hatschek T, et al. Long-term prognosis in breast cancer is associated with residual disease after neoadjuvant systemic therapy but not with initial nodal status. Br J Surg 108 (2021): 583-589.
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384 (2014): 164-172.
- Samiei S, Simons JM, Engelen SM, et al. Axillary Pathological Complete Response After Neoadjuvant Systemic Therapy by Breast Cancer Subtype in Patients With Initially Clinically Node-Positive Disease: A Systematic Review and Meta-analysis. JAMA Surg 156 (2021): e210891.
- Mieog JSD, Van der Hage JA, Van de Velde CJH. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg 94 (2007): 1189-1200.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 74.74%
Acceptance Rate: 74.74%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 