Real World Experience with Osteosarcoma from a Tertiary Cancer Centre in India
Pankaj Goyal1, Srujana Joga1, Rupal Tripathi2, Arpit Jain1, Manish Sharma1, Varun Goel1, Vineet Talwar1, Ullas Batra1, Sunil Pasricha3, Dinesh Chandra Doval1*
1Department of Medical Oncology, Rajiv Gandhi Cancer Institute and Research Centre, Delhi, India
2Department of Research, Rajiv Gandhi Cancer Institute and Research Centre, Delhi, India
3Department of Pathology, Rajiv Gandhi Cancer Institute and Research Centre, Delhi, India
*Corresponding Author: Dinesh Chandra Doval, Chair, Department of Medical Oncology, Rajiv Gandhi Cancer Institute and Research Centre, Sector-5, Rohini, Delhi, India.
Received: 27 March 2023; Accepted: 17 April 2023; Published: 28 April 2023
Article Information
Citation: Pankaj Goyal, Srujana Joga, Rupal Tripathi, Arpit Jain, Manish Sharma, Varun Goel, Vineet Talwar, Ullas Batra, Sunil Pasricha, Dinesh Chandra Doval. Real World Experience with Osteosarcoma from a Tertiary Cancer Centre in India. Journal of Cancer Science and Clinical Therapeutics. 7 (2023): 93-99.
View / Download Pdf Share at FacebookAbstract
Background: Osteosarcoma represents the commonest category of bone tumors in the children and young adults and stability in its incidence rates have been observed throughout the world. The present study evaluated the varied profile of Indian patients with osteosarcoma with a special emphasis on the survival patterns in a tertiary cancer care centre in India.
Methods: A retrospective review of all patients diagnosed with osteosarcoma during the year 2000 to 2020 was included in the study. Details of their demographic, treatment and survival profile were collected from the electronic medical records of the patients.
Results: Among 112 patients, male gender (75.9%), disease in extremity sites (89.3%), conventional histology (95.5%), serum alphos >120U/L (75.9%) and non-metastatic disease at presentation (66.1%) and IAP regime (57.1%) were more commonly reported. The OS of the patients was 51% at 20 years. Statistical associations were observed with respect to age (p-value 0.019), site (p-value 0.017), grade (p-value 0.019), metastatic disease (p-value 0.006) and site of metastasis (p-value <0.0001). The PFS of the patients was 48% at 20 years and correlations were observed with respect to age (p-value 0.021), site (p-value 0.002), grade (p-value 0.016), metastatic disease (p-value 0.001), site of metastasis (p-value <0.0001) and HUVOS grade (p-value 0.026).
Conclusion: With a static pattern in survival, there is an imperative need to characterize the genetic, epigenetic and immunologic basis of the disease in order to look for newer targets, inhibitors and therapies.
Keywords
<p>Real World Experience; Osteosarcoma; Tertiary Cancer Centre</p>
Article Details
1. Introduction
Osteosarcoma derived from bone forming mesenchymal cells, represents the commonest category of bone tumors in the children and young adults [1]. Stability in its incidence rates have been observed throughout the world along with a decline in the mortality rates. This has been largely possible with the advent of multi agent chemotherapy [2, 3]. The most widely reported prognostic factors for osteosarcoma include tumor size and site, surgical resectability, response to chemotherapy and presence of metastases [4].
Survival outcomes in osteosarcoma depend to a great extent on the choice of therapy. Induction chemotherapy followed by surgical resection for local tumor control and consolidation local control to metastatic sites is the standard treatment regime for these patients [1]. Studies have reported an improvement in the survival outcomes from 17% with surgical resection to 66% with the addition of adjuvant chemotherapy in a multi-institutional randomized trial conducted in the 1980’s. Also, with respect to therapy, a meta-analysis has demonstrated the superiority of three drug regimens to two drug regimens, also highlighting the importance of high dose methotrexate [5]. In a Pediatric Oncology Group study by Harris et al, a five year event free and overall survival of 47% and 53% were observed with the use of induction ifosfamide, resection and adjuvant MAP ifosphamide and improvements in outcomes were observed with unilateral lung metastases and limited number of lung metastases [6]. In terms of radiotherapy, a study by Ozaki et al has shown an improvement in overall survival in the patients without resection for pelvic tumors [7].
The present study was conducted to evaluate the varied profile of Indian patients with osteosarcoma with a special emphasis on the survival patterns in a tertiary cancer care centre in India.
2. Materials and Methods
2.1 Patients
A retrospective review of all patients diagnosed with osteosarcoma during the year 2000 to 2020 was included in the study. Patients presenting with secondary osteosarcoma or relapsed disease at diagnosis were excluded from the analysis. Details of their demographic, treatment and survival profile were collected from the electronic medical records of the patients. The study was conducted as per the Declaration of Helsinki and was given a waiver of the informed consent process.
2.2 Diagnostic and staging evaluation
Baseline nutritional status (body mass index, haemoglobin, albumin) and tumor burden markers (tumor dimensions, Lactate dehydrogenase) was documented. All patients underwent baseline plain radiographs and magnetic resonance imaging (MRI) of the primary disease site prior to initial histopathological evaluation and management.
Besides routine blood investigations (complete blood count, renal function and liver function test), echocardiography, staging work up including computed tomography (NCCT) thorax and bone scintigraphy were performed. Core needle biopsy using standard recommended practices with histopathological confirmation and morphological subtyping was performed.
2.3 Treatment and toxicity
Multimodality treatment including surgery, systemic chemotherapy and/or radiation therapy was adopted for patients with nonmetastatic osteosarcoma. Surgical management protocol ensured the complete safe resection of tumor with functional preservation including limb salvage and adequate surgical margin with enbloc excision of biopsy tract by dedicated orthopedic oncology surgeon. Two standard chemotherapy protocols followed at our centre and evaluated in this study are summarized in Table 1. Granulocyte colony-stimulating factor (G-CSF) products were administered as primary prophylaxis following each cycle of chemotherapy. Planned dose reductions in subsequent cycles were based on occurrence of clinically significant hematological and/or non-hematological toxicities. Toxicities were documented according to Common Toxicity Criteria for Adverse Events (CTCAE v4.0) recommendations. Histological evaluation of extent of tumor necrosis was assessed using Huvos grading system. Chemotherapy treatment was not adopted for good or poor histological responders.
MAP (high-dose methotrexate + cisplatin + doxorubicin)
|
Cycle length: 5 weeks (cycles 1 through 4), 4 weeks (cycle 5 and 6) Duration of therapy: 6 cycles. |
|||
|
Drug |
Dose and route |
Week of treatment |
Given on days |
|
Cisplatin |
120 mg/m² (4 h infusion of 60 mg/m² per day for 2 days) |
Preoperative: Weeks 1 and 6 Postoperative: Weeks 12 and 17 |
Days 1 and 2 |
|
Doxorubicin |
37·5 mg/m² per day on days 1 and 2 as 4-hour infusion |
Preoperative: Weeks 1 and 6 Postoperative: Weeks 12, 17, 22, and 26 |
Days 1 through 2 |
|
Methotrexate |
12 g/m² over 4 h (maximum dose 20 gm) with hyper-hydration, alkalinisation |
Preoperative: Week 4, 5, 9, and 10. Postoperative: Week 15, 16, 20, 21, 24, 25, 28, and 29. |
Day 1 |
|
Leucovorin |
15 mg orally, IV, or IM every six hours Dose adjusted according to serum methotrexate concentrations. |
Preoperative: Week 4, 5, 9, and 10. Postoperative: Week 15, 16, 20, 21, 24, 25, 28, and 29. |
Starting 24–48 h from methotrexate infusion and continuing until methotrexate concentration was less than 0·1 μM. |
|
Surgery |
Week 11 |
||
Ifosfamide, Adriamycin and Cisplatin (IAP)
|
Cycle length: 21 days Duration of therapy: 6 cycles. |
||
|
Drug |
Dose and route |
Given on days |
|
Ifosfamide |
1.8 gm/m2 per day IV |
Days 1 to 5 |
|
Doxorubicin |
25 mg/m2 per day IV |
Days 1, 2 and 3 |
|
Cisplatin |
37·5 mg/m² per day IV |
Day 1 and 2 |
Table 1: Two standard chemotherapy protocols (MAP and IAP) followed at our centre.
2.4 Follow up
Post-treatment follow-up included physical examination, complete blood count, chest imaging and local imaging of the primary site every three months for two years, every four months for year 3, every six months for years 4 and 5, and annually thereafter or as clinically indicated; in line with the recommendations by National Comprehensive Cancer Network (NCCN) and Children’s Oncology Group (COG) guidelines. Tumor surveillance was continued for longer time as late metastases may occur 10 years after diagnosis. Further investigations to monitor the possible cardiac, renal, pulmonary, cognitive, developmental and late side effects of therapies were included in follow up visits. In suspected relapse/metastatic presentation, management was planned after biopsy confirmation of malignancy. Patients who were lost to regular follow up were communicated telephonically.
2.5 Statistical analysis
SPSS version 23 for Windows (SPSS Inc, Chicago IL, USA) was used for the statistical analysis of the data obtained. Pearson chi square or Fisher’s Exact Test, whichever appropriate, was used for the analysis of categorical variables. Kaplan Meier method was used for survival analysis [8]. Overall survival was calculated as the time duration between the date of diagnosis and the last date of follow up whereas progression free survival was calculated on the basis of the time duration between the date of diagnosis and the date of progression, if occurred. The differences in survival among the groups were compared using the Log Rank test. A two sided p-value <0.05 was considered as significant.
3. Results
A total of 112 patients were included in the study. Of these, 85/112 (75.9%) patients were males. The disease was more commonly observed in the extremity sites (89.3%, 100/112). Conventional histology (95.5%, 107/112), serum alphos >120U/L (75.9%, 85/112) and non-metastatic disease at presentation (66.1%, 74/112) were more commonly reported in the patients. MAP regime was given to 48 (42.9%) patients whereas IAP regime was given to 64 (57.1%) patients in the study. Differences in the profile of patients were observed on the basis of age group as shown in Table 2.
|
Characteristics |
Total patients N=112 |
Age group <20 years; N=50 |
Age group 20-40 years ; N=47 |
Age group >40 years; N=15 |
p-value** |
|
Gender Male Female |
85 27 |
40 10 |
36 11 |
9 6 |
0.280 |
|
Site Extremity Axial |
100 12 |
49 1 |
42 5 |
9 6 |
<0.0001 |
|
BMI <18.5 Underweight 18.5-24.9 Healthy >25 Overweight |
34 56 22 |
22 22 6 |
12 26 9 |
0 8 7 |
0.003 |
|
Histology Conventional Extraskeletal |
107 5 |
49 1 |
45 2 |
13 2 |
0.175 |
|
Grade 1-2 3-4 |
24 88 |
8 42 |
11 36 |
5 10 |
0.325 |
|
S. alphos 30-120U/L >120U/L |
27 85 |
8 42 |
13 34 |
6 9 |
0.123 |
|
Stage Non metastatic Metastatic |
86 26 |
42 8 |
34 13 |
10 5 |
0.241 |
|
Site of metastasis No metastasis Lung |
74 38 |
35 15 |
29 18 |
10 5 |
0.688 |
|
Chemoprotocol IAP MAP |
64 48 |
16 34 |
36 11 |
12 3 |
<0.0001 |
|
HUVOS grade 1-2 3-4 Not available* |
43 43 26 |
15 26 - |
24 13 - |
4 4 - |
0.045 |
|
Progression Yes No |
53 59 |
16 34 |
27 20 |
10 5 |
0.012 |
|
Status Alive Dead |
69 43 |
38 12 |
23 24 |
8 7 |
0.018 |
BMI, body mass index; MAP, methorexate-adriamycin-cisplatin; IAP, ifosfamide-adriamycin-cisplatin.
*Not available cases were not considered for p-value calculation.
**p-value <0.05 was considered as significant.
Table 2: Profile of 112 patients with osteosarcoma on the basis of age group.
Patients were also compared on the basis of disease progression (Table 3). Statistical associations were observed with age group (p-value 0.012), site of disease (p-value 0.042), grade of disease (p-value 0.013), stage of disease (p-value 0.003), site of metastasis (p-value <0.0001), HUVOS grade (p-value 0.048) and vital status of the patient (p-value <0.0001).
The OS of the patients was 51% at 20 years. At 5 years, improved OS was seen in patients with age group <20 years (74%), male gender (58%), disease in extremities (62%), conventional histology (59%), grade 1-2 (82%), S alphos 30-120U/L (73%), non-metastatic disease (63%), MAP chemoprotocol (53%) and HUVOS grade 3-4 (73%).
|
Characteristics |
Total patients N=112 |
Yes N=53 |
No N=59 |
p-value** |
|
Age group <20 years 20-40 years >40 years |
50 47 15 |
16 27 10 |
34 20 5 |
0.012 |
|
Gender Male Female |
85 27 |
39 14 |
46 13 |
0.588 |
|
Site Extremity Axial |
100 12 |
44 9 |
56 3 |
0.042 |
|
BMI <18.5 Underweight 18.5-24.9 Healthy >25 Overweight |
34 56 22 |
13 28 12 |
21 28 10 |
0.417 |
|
Histology Conventional Extraskeletal |
107 5 |
50 3 |
57 2 |
0.561 |
|
Grade 1-2 3-4 |
24 88 |
6 47 |
18 41 |
0.013 |
|
S. alphos 30-120U/L >120U/L |
27 85 |
9 44 |
18 41 |
0.095 |
|
Stage Non metastatic Metastatic |
86 26 |
34 19 |
52 7 |
0.003 |
|
Site of metastasis No metastasis Lung |
74 38 |
20 33 |
54 5 |
<0.0001 |
|
HUVOS grade 1-2 3-4 Not available* |
43 43 26 |
22 13 - |
21 30 - |
0.048 |
|
Chemoprotocol IAP MAP |
53 59 |
33 20 |
31 28 |
0.299 |
|
Status Alive Dead |
69 43 |
13 40 |
56 3 |
<0.0001 |
BMI, body mass index; MAP, methorexate-adriamycin-cisplatin; IAP, ifosfamide-adriamycin-cisplatin.
*Not available cases were not considered for p-value calculation.
**p-value <0.05 was considered as significant.
Table 3: Profile of 112 patients with osteosarcoma on the basis of disease progression.
Using the Log Rank test, statistical associations were observed with respect to age (p-value 0.019), site (p-value 0.017), grade (p-value 0.019), metastatic disease (p-value 0.006) and site of metastasis (p-value <0.0001). The PFS of the patients was 48% at 20 years. A similar trend was observed in the case of PFS and statistical correlations were observed with respect to age (p-value 0.021), site (p-value 0.002), grade (p-value 0.016), metastatic disease (p-value 0.001), site of metastasis (p-value <0.0001) and HUVOS grade (p-value 0.026). The OS and PFS status of the patients with respect to the various characteristics is depicted in Table 4.
|
Characteristics |
Total patients N=112 |
OS (%) |
p-value |
PFS (%) |
p-value** |
|
Age group <20 years 20-40 years >40 years |
50 47 15 |
74 41 51 |
0.019 |
65 42 27 |
0.021 |
|
Gender Male Female |
85 27 |
58 52 |
0.367 |
50 47 |
0.302 |
|
Site Extremity Axial |
100 12 |
62 15 |
0.017 |
53 19 |
0.002 |
|
BMI <18.5 Underweight 18.5-24.9 Healthy >25 Overweight |
34 56 22 |
59 54 59 |
0.880 |
55 48 42 |
0.565 |
|
Histology Conventional Extraskeletal |
107 5 |
59 40 |
0.735 |
50 40 |
0.765 |
|
Grade 1-2 3-4 |
24 88 |
82 50 |
0.019 |
78 42 |
0.016 |
|
S. alphos 30-120U/L >120U/L |
27 85 |
73 52 |
0.055 |
70 43 |
0.064 |
|
Stage Non metastatic Metastatic |
86 26 |
63 36 |
0.006 |
57 24 |
0.001 |
|
Site of metastasis No metastasis Lung |
74 38 |
72 29 |
<0.0001 |
70 13 |
<0.0001 |
|
HUVOS grade 1-2 3-4 Not available* |
43 43 26 |
54 73 |
0.066 |
42 68 |
0.026 |
|
Chemoprotocol IAP MAP |
53 59 |
53 62 |
0.307 |
45 54 |
0.314 |
BMI, body mass index; MAP, methorexate-adriamycin-cisplatin; IAP, ifosfamide-adriamycin-cisplatin; OS, overall survival; PFS, progression free survival.
*Not available cases were not considered for p-value calculation.
**p-value <0.05 was considered as significant.
Table 4: Five year survival profile of 112 patients with osteosarcoma.
Comparisons were also made on the basis of the vital status of the patients and statistical correlations were observed with respect to the age group, site of disease, grade, S alphos and the site of metastasis as shown in Figure 1.
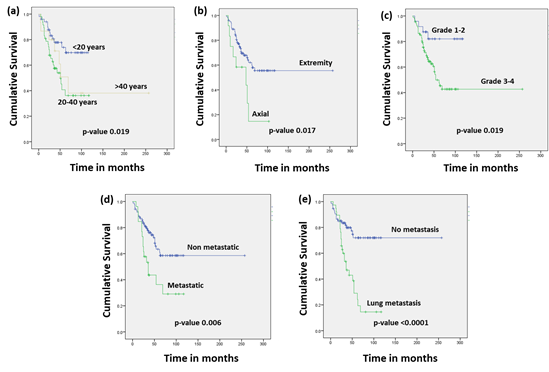
Figure 1: Survival graphs of 112 patients with osteosarcoma on the basis of vital status with respect to (a) age group (b) site of disease (c) histological grade (d) metastatic disease and (e) site of metastasis. p-value <0.05 was considered as significant.
4. Discussion
Osteosarcoma is a mesenchymal malignancy, mainly affecting the children and adolescents. Outcomes of patients with localized, progressive, recurrent or metastatic disease still remains unchanged in the last few decades. Advancements in surgery, newer chemotherapeutic targets, novel immunomodulators, and improved diagnostic facilities have all together reinstated the hopes for improved outcomes in the future which have yet not translated into improvements in progression free/ overall survival.
The present study reflects the data of young, adolescents, middle age and older age group patients with osteosarcoma and profiles the experience of a single tertiary care cancer centre in India.
Overall, in a total of 112 patients, the patients presenting in the age group of <20 years and 20-40 years were nearly equal and much more than the age group >40 years. It has also been highlighted in other studies where it has been shown that osteosarcoma is the most common primary bone tumor in the children and adolescents [1, 9]. It was observed in our study that it occurred most commonly in the males and extremities were the commonest site of disease occurrence which also reciprocates with the published literature [1, 9]. Metastatic disease was observed in 26/112 patients, which highlights the fact that the disease largely goes unnoticed in our population leading to potential delay in its diagnosis. Further, Huvos grade III/IV was observed in 43/86 patients and a statistical correlation was well evident among the different age groups viz a viz <20 years, 20-40 years and >40 years. Differences on the basis of chemotherapy have been observed in trials including MSKCC (65%), INT0133 (48%) and Cooperative German-Austrian-Swiss osteosarcoma study group (COSS) protocol (43%) [10-12].
A large number of clinical trials have been initiated in the European population on pediatric patients with osteosarcoma focusing on multiagent chemotherapy and surgical resection for local control wherever possible. MAP is the routine multiagent chemotherapy schedule in the European and North American populations in the pediatric patients [13, 14]. Although numerous trials have investigated the effect of dose intensification, no substantial benefits of using MAP chemotherapy has been shown [15, 16]. The EURAMOS-1 trial was conducted to test the improvement in outcomes if any, upon the addition of ifosphamide and etoposide to MAP in the postoperative setting in patients with < 90% histologic response to preoperative chemotherapy [16].
Further, studies from different settings has shown comparable results with three-drug regimen without using HDMTX [9, 17-20]. However, the feasibility of testing non-HDMTX triple-drug therapy against HDMTX containing triple-drug therapy which requires meticulous training and monitoring is a matter of grave concern, especially for the clinicians in the developing countries.
In terms of survival, a study by Lee et al assessing the survival trends of adolescents and young adults and comparison with the other age groups, a 5 year OS of 61% was observed in a total of 3017 patients. Further, an inverse correlation with age was reported. Also, a 5% difference in the OS rate among the children and the adolescents and young adults was also observed [9]. In our study, an OS of 51% and PFS of 48% at 20 years were seen. This remains the biggest strength of the study with a large follow up period of patients around 20 years, thus depicting their factual survival rates. Statistical associations were observed with location & extent of disease and age group. Similarly, in our study, correlations were observed with age group, site and metastatic disease in terms of OS (p-value <0.05). An association with these factors was also seen with respect to the PFS (p-value <0.05). In another retrospective study conducted at our institution including all patients up to the age of 18 years with a confirmed diagnosis of osteosarcoma, the 5-year OS and EFS was 55% and 52% respectively [21]. Our results are very similar to the study even though it includes a much varied age group and with a much longer follow up period, thus clearly pointing towards the fact that the survival rates of patients with osteosarcoma have remained largely the same over the years.
The study has certain inherent biases due to its retrospective nature and hence complete data was not available in certain cases. However, the greatest strength of this study is the long follow up period of the patients which truly reflects the survival patterns over a long period of time. Given the fact that the survival of these patients has remained nearly the same over the years, hence, there is an imperative need to characterize the genetic, epigenetic and immunologic basis of the disease in order to look for newer targets, inhibitors and therapies.
References
- Eaton BR, Schwarz R, Vatner R, et al. Osteosarcoma. Pediatr Blood Cancer 68 (2021): e28352.
- Esiashvili N, Goodman M, Marcus RB Jr. Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. J Pediatr Hematol Oncol 30 (2008): 425-430.
- Ward E, DeSantis C, Robbins A, et al. Childhood and adolescent cancer statistics. CA Cancer J Clin 64 (2014): 83-103.
- Ferrari S, Ruggieri P, Cefalo G, et al. Neoadjuvant chemotherapy with methotrexate, cisplatin, and doxorubicin with or without ifosfamide in nonmetastatic osteosarcoma of the extremity: an Italian sarcoma group trial ISG/OS-1. J Clin Oncol 30 (2012): 2112-2118.
- Anninga JK, Gelderblom H, Fiocco M, et al. Chemotherapeutic adjuvant treatment for osteosarcoma: where do we stand? Eur J Cancer 47 (2011): 2431-2445.
- Harris MB, Gieser P, Goorin AM, et al. Treatment of metastatic osteosarcoma at diagnosis: a Pediatric Oncology Group Study. J Clin Oncol 16 (1998): 3641-3648.
- Ozaki T, Flege S, Kevric M, et al. Osteosarcoma of the pelvis: experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol 15 (2003): 334-341.
- Kaplan EL, Meier P. Non Parametric estimation from incomplete observations. J Am Stat Assoc 53 (1958): 457-481.
- Lee JA, Lim J, Jin HY, et al. Osteosarcoma in Adolescents and Young Adults. Cells 10 (2021): 2684.
- Venkatramani R, Murray J, Helman L, et al. Risk-based therapy for localized osteosarcoma. Pediatr Blood Cancer 63 (2016): 412-417.
- Winkler K, Beron G, Delling G, et al. Neoadjuvant chemotherapy of osteosarcoma: results of a randomized cooperative trial (COSS-82) with salvage chemotherapy based on histological tumor response. J Clin Oncol 6 (1988): 329-337.
- Bielack SS, Smeland S, Whelan JS, et al. Methotrexate, Doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: first results of the EURAMOS-1 good response randomized controlled trial. J Clin Oncol 33 (2015): 2279-2287.
- Casali PG, Bielack S, Abecassis N, et al. Bone sarcomas: ESMO-PaedCan-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29 (2018): iv79-iv95.
- Ferrari S, Bielack SS, Smeland S, et al. EURO-B.O.S.S.: a European study on chemotherapy in bone-sarcoma patients aged over 40: outcome in primary high-grade osteosarcoma. Tumori 104 (2018): 30-36.
- Schwartz CL, Wexler LH, Krailo MD, et al. Intensified chemotherapy with dexrazoxane cardioprotection in newly diagnosed nonmetastatic osteosarcoma: a report from the Children's Oncology Group. Pediatr Blood Cancer 63 (2016): 54-61.
- Marina NM, Smeland S, Bielack SS, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol 17 (2016): 1396-1408.
- Belayneh R, Fourman MS, Bhogal S, Weiss KR. Update on Osteosarcoma. Curr Oncol Rep 23 (2021): 71.
- Bajpai J, Chandrasekharan A, Talreja V, et al. Outcomes in non-metastatic treatment naive extremity osteosarcoma patients treated with a novel non-high dose methotrexate-based, dose-dense combination chemotherapy regimen ‘OGS-12’. Eur J Cancer 85 (2017): 49-58.
- Patel SJ, Lynch JW Jr, Johnson T, et al. Dose-intense ifosfamide/doxorubicin/cisplatin based chemotherapy for osteosarcoma in adults. Am J Clin Oncol 25 (2002): 489-495.
- Bajpai J, Chandrasekharan A, Simha V, et al. Outcomes in treatment-naive patients with metastatic extremity osteosarcoma treated with OGS-12, a novel non-high-dose methotrexate-based, dose-dense combination chemotherapy, in a tertiary care cancer center. J Glob Oncol 4 (2018): 1-10.
- Verma P, Jain S, Kapoor G, et al. IAP Chemotherapy Regimen Is a Viable and Cost-effective Option in Children and Adolescents With Osteosarcoma: A Comparative Analysis With MAP Regimen on Toxicity and Survival. J Pediatr Hematol Oncol 43 (2021): e466-e471.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 74.74%
Acceptance Rate: 74.74%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 