Simultaneous Bilateral Bone Bridge Implantation in a Two-Year-Old Child with Atresia of the External Auditory canal: A Case Report
Thomas Mayr1, Astrid Magele1,2, Philipp Schörg1,2, Georg M Sprinzl1,2*
1University Clinic St. Poelten, Department of Otorhinolaryngology, Head & Neck Surgery, St. Poelten, (Lower Austria,) Austria
2Karl Landsteiner Institute of Implantable Hearing Devices, St. Poelten, (Lower Austria,) Austria
*Corresponding author: Georg Mathias Sprinzl, University Clinic St. Poelten, Department of Otorhinolaryngology, Head & Neck Surgery, St. Poelten, (Lower Austria,) Austria.
Received: 06 August 2022; Accepted: 16 August 2022; Published: 22 September 2022
Article Information
Citation: Thomas Mayr, Astrid Magele, Philipp Schörg, Georg M Sprinzl. Simultaneous Bilateral Bone Bridge Implantation in a Two-Year-Old Child with Atresia of the External Auditory canal: A Case Report. Journal of Biotechnology and Biomedicine 5 (2022): 180-186.
DOI: 10.26502/jbb.2642-91280058
View / Download Pdf Share at FacebookAbstract
Introduction: Active transcutaneous bone conduction implants are indicated for mild to moderate conductive or mixed hearing loss (intact inner ear and auditory nerve) for subjects 5 years or older. The aim of the here presented study was to share our results with the Bonebridge in a child aged 2 years at time of surgery. Especially the new generation of the implant being half of the size, a re-evaluation of the given indication for children 5 years or older is highly recommended.
Methods: case presentation Results: At the age of one month bilateral auricle malformation as well as auditory canal atresia was diagnosed. The family history showed no further malformations and an uneventful pregnancy. The infant was provided with a Baha-Softband as well as speech therapy. Diagnostic BERA and MRI showed suitable anatomical structures allowing placement of an implant. The parents were counseled on available treatment options and the fact that the Bonebridge implantation would be off-label use due to the given indication age. Nonetheless, the parents insisted on the Bonebridge. At the age of 28 months the bilateral implantation (BCI 602) was performed within 40 minutes and without any intra- nor post-operative complications.
Conclusion: Based on the safe, easy and eventless implantation, the highly satisfactory results and the beneficial audiological outcomes, the authors propose to lower the implantation age based on available bone tissue allowing device placement. Similar as for Cochlear Implant recipients, a stable and early auditory input to ensure unrestricted speech development should be the major goal.
Keywords
Active transcutaneous bone conduction implant; Bonebridge BCI602; Bilateral atresia; Very young children; Early implantation; Bilateral implantation
Active transcutaneous bone conduction implant articles Active transcutaneous bone conduction implant Research articles Active transcutaneous bone conduction implant review articles Active transcutaneous bone conduction implant PubMed articles Active transcutaneous bone conduction implant PubMed Central articles Active transcutaneous bone conduction implant 2023 articles Active transcutaneous bone conduction implant 2024 articles Active transcutaneous bone conduction implant Scopus articles Active transcutaneous bone conduction implant impact factor journals Active transcutaneous bone conduction implant Scopus journals Active transcutaneous bone conduction implant PubMed journals Active transcutaneous bone conduction implant medical journals Active transcutaneous bone conduction implant free journals Active transcutaneous bone conduction implant best journals Active transcutaneous bone conduction implant top journals Active transcutaneous bone conduction implant free medical journals Active transcutaneous bone conduction implant famous journals Active transcutaneous bone conduction implant Google Scholar indexed journals Bonebridge BCI602 articles Bonebridge BCI602 Research articles Bonebridge BCI602 review articles Bonebridge BCI602 PubMed articles Bonebridge BCI602 PubMed Central articles Bonebridge BCI602 2023 articles Bonebridge BCI602 2024 articles Bonebridge BCI602 Scopus articles Bonebridge BCI602 impact factor journals Bonebridge BCI602 Scopus journals Bonebridge BCI602 PubMed journals Bonebridge BCI602 medical journals Bonebridge BCI602 free journals Bonebridge BCI602 best journals Bonebridge BCI602 top journals Bonebridge BCI602 free medical journals Bonebridge BCI602 famous journals Bonebridge BCI602 Google Scholar indexed journals Bilateral atresia articles Bilateral atresia Research articles Bilateral atresia review articles Bilateral atresia PubMed articles Bilateral atresia PubMed Central articles Bilateral atresia 2023 articles Bilateral atresia 2024 articles Bilateral atresia Scopus articles Bilateral atresia impact factor journals Bilateral atresia Scopus journals Bilateral atresia PubMed journals Bilateral atresia medical journals Bilateral atresia free journals Bilateral atresia best journals Bilateral atresia top journals Bilateral atresia free medical journals Bilateral atresia famous journals Bilateral atresia Google Scholar indexed journals Very young children articles Very young children Research articles Very young children review articles Very young children PubMed articles Very young children PubMed Central articles Very young children 2023 articles Very young children 2024 articles Very young children Scopus articles Very young children impact factor journals Very young children Scopus journals Very young children PubMed journals Very young children medical journals Very young children free journals Very young children best journals Very young children top journals Very young children free medical journals Very young children famous journals Very young children Google Scholar indexed journals Early implantation articles Early implantation Research articles Early implantation review articles Early implantation PubMed articles Early implantation PubMed Central articles Early implantation 2023 articles Early implantation 2024 articles Early implantation Scopus articles Early implantation impact factor journals Early implantation Scopus journals Early implantation PubMed journals Early implantation medical journals Early implantation free journals Early implantation best journals Early implantation top journals Early implantation free medical journals Early implantation famous journals Early implantation Google Scholar indexed journals Bilateral implantation articles Bilateral implantation Research articles Bilateral implantation review articles Bilateral implantation PubMed articles Bilateral implantation PubMed Central articles Bilateral implantation 2023 articles Bilateral implantation 2024 articles Bilateral implantation Scopus articles Bilateral implantation impact factor journals Bilateral implantation Scopus journals Bilateral implantation PubMed journals Bilateral implantation medical journals Bilateral implantation free journals Bilateral implantation best journals Bilateral implantation top journals Bilateral implantation free medical journals Bilateral implantation famous journals Bilateral implantation Google Scholar indexed journals Bonebridge articles Bonebridge Research articles Bonebridge review articles Bonebridge PubMed articles Bonebridge PubMed Central articles Bonebridge 2023 articles Bonebridge 2024 articles Bonebridge Scopus articles Bonebridge impact factor journals Bonebridge Scopus journals Bonebridge PubMed journals Bonebridge medical journals Bonebridge free journals Bonebridge best journals Bonebridge top journals Bonebridge free medical journals Bonebridge famous journals Bonebridge Google Scholar indexed journals Brainstem articles Brainstem Research articles Brainstem review articles Brainstem PubMed articles Brainstem PubMed Central articles Brainstem 2023 articles Brainstem 2024 articles Brainstem Scopus articles Brainstem impact factor journals Brainstem Scopus journals Brainstem PubMed journals Brainstem medical journals Brainstem free journals Brainstem best journals Brainstem top journals Brainstem free medical journals Brainstem famous journals Brainstem Google Scholar indexed journals Diagnostic articles Diagnostic Research articles Diagnostic review articles Diagnostic PubMed articles Diagnostic PubMed Central articles Diagnostic 2023 articles Diagnostic 2024 articles Diagnostic Scopus articles Diagnostic impact factor journals Diagnostic Scopus journals Diagnostic PubMed journals Diagnostic medical journals Diagnostic free journals Diagnostic best journals Diagnostic top journals Diagnostic free medical journals Diagnostic famous journals Diagnostic Google Scholar indexed journals children articles children Research articles children review articles children PubMed articles children PubMed Central articles children 2023 articles children 2024 articles children Scopus articles children impact factor journals children Scopus journals children PubMed journals children medical journals children free journals children best journals children top journals children free medical journals children famous journals children Google Scholar indexed journals
Article Details
1. Introduction
Implants using Bone Conduction (BC) already have a long history in the treatment of conductive hearing loss and recent technical advances have improved the variety of options to treat hearing loss. The Bonebridge (MED-EL, Innsbruck, Austria) is the first available active transcutaneous Bone Conduction Implant (BCI) featuring a sound processor that transmits sound energy transcutaneously to an internal coil. Subsequently the sound is transmitted to the floating mass transducer which transduces the signal into mechanical vibrations conducted to the mastoid bone via the cortical screws.
Currently the Bonebridge has its approval for implantation in adults as well as children aged 5 years or above with conductive or mixed hearing impairment and single-sided deafness being the main indications [1, 2]. Also current long-term data is showing satisfying results concerning safety and performance in children and adults in a 36 months follow-up [3]. With the change in shape of the internal part of the device (BCI 602) released in 2019 the drilling depth was reduced from 8.7 to 4,5 mm, giving new opportunities for different anatomical conditions in which the first generation may have been impossible to use, while having the same audiological and medical criteria [4, 5].
2. Case Presentation
The first admission of our patient occurred at the age of one month due to bilateral auricle malformation as well as auditory canal atresia. In the family history were no further malformations and the pregnancy was uneventful. To bridge the time until operation the infant was provided with a non-surgical bone conduction solution (Baha-Softband) as well as speech therapy. Unfortunately, the patient’s parents were not satisfied with the benefit and furthermore complained about the handling- and wearing difficulties of the toddler regarding keeping the device in place. Another point of concern was the constant stigmata the child had to experience and the fact that it therefore also retreated very much from social interactions with its peers. At 20 months of age a MRI, as well as a CT-Scan and a Brainstem Electric Response Audiometry (BERA) were made for further diagnostic purposes at our hospital. The BERA showed regular inner ear function on both sides (Figure 1), and the MRI revealed no malformations of the cerebellopontine angle, internal auditory meatus, cochlea and vestibular system. At the CT-Scan it was noticeable that beside the bilateral atresia of the outer ear channel the malformation of the left ossicular chain was more pronounced than on the right side coherent with the auricle malformation (Figure 2 a-d). The stapes was regularly developed on both sides. Furthermore, the CT-Scan showed well-pneumatized mastoid bones on both sides. After informing the parents about the different treatment options (Bone Anchored Hearing Devices (BAHA) and passive BCI Sophono) and the fact that the Bonebridge implantation would be off-label use due to the young age, the parents decided in favor of the Bonebridge as the most suitable option. The bilateral implantation (BCI 602) took place at the age of 28 months and was completed without any complications. We performed a retroauricular incision with a mucoperiosteal flap, uncovering the planum mastoideum, drilling the 4,5mm deep implant bed and finally placing the BCI-FMT with three standard screws and one emergency screw for both sides (Figure 3 a, b). Neither sinus nor dura were explored nor compressed (Figure 3 c, d). The surgical time for bilateral implantation took from skin incision to final closing 40 Minutes. The girl was discharged to home care two days after surgery and the parents were advised to keep the incision area clean and dry. There were no issues during the outpatient checkup 13 days after surgery. On the same day the two processors were successfully fitted. Further follow-ups were made after one month, three months and half a year after surgery (Figure 3 e, f). The most recent check-up was carried out 18 months after surgery showing 100% speech understanding at 65dB (Figure 4). The parents reported that she was wearing her audio processors all waking hours and that she was no longer shy and withdrawn from other children. On the contrary, the parents believe, that their daughter appears to be at the same level of language development as her kindergarten peers.
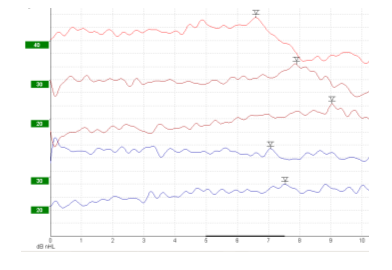
Figure 1: Brainstem Evoked Response Audiometry (BERA) signals. Bone conduction BERA potentials were detected up to 20dB in the presented case.
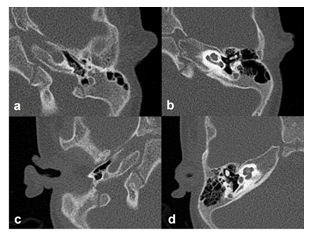
Figure 2: Computed Tomography (CT) scan from left side, axial projection displaying Atresia of the auditory canal (a); left side, axial projection showing regular Inner Ear anatomy (b); right side, axial projection presenting Atresia of the auditory canal (c); right side, axial projection showing regular Inner Ear anatomy (d).
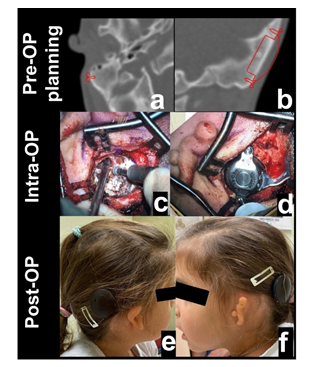
Figure 3: Shows the pre-op planning based on CT-images to ensure enough space for drilling and appropriate screw fixation (left side)(a,b). Intra-op photographs of the left side, showing the drilled implant bed without any sinus nor dura exposure and the final position of the BC-FMT with both screws in place (c,d). Most recent follow-up picture of the patient, showing the daily situs of the implant with the Audio Processor (SAMBA2) in place (left and right patient side)(e,f).
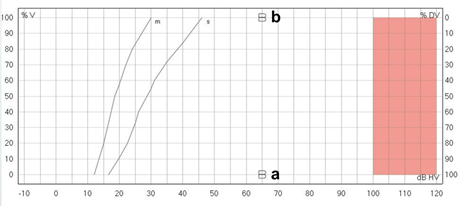
Figure 4: Speech audiogram showing no measurable speech understanding in the unaided condition (a) and 100% speech understanding at 65dB with the Bonebridge (b).
3. Discussion
The Bonebridge as the first active transcutaneous bone conduction implant represents an effective treatment for conductive or mixed hearing impairment, without certain disadvantages of passive Bone-Anchored Hearing Devices (BAHD), especially in children where the skull is not yet sufficiently strong. Moreover, skin reactions, loss of osseointegration and revision surgery are less common when using the Bonebridge device, making it a safe and effective long-term hearing system even in very young patients. Especially the change in shape of the new model (BCI 602) widened the range of possible areas of application, particularly in children concerning the generally thinner bone in the possible surgical areas [1, 4, 6-9]. In literature satisfaction with the audiological outcome, aesthetic perception, and daily comfort of the Bonebridge is high. Especially pediatric patients report good results in communication and daily usage of the audio processor, leading to a noticeable improvement of the child’s speech development and social interactions, this holds also true to our experience with our own patients [4, 6, 10]. Our case shows that beside the age of the patient, especially anatomical circumstances like a well-pneumatized mastoid bone and sufficient bone thickness should support the decision for surgery. Facing that problem, 3D planning methods can be useful preoperatively for the implant decision and optimal anatomical location [11, 12].
4. Conclusion
Hearing Rehabilitation with the Bonebridge shows a significant audiological benefit for a variety of patients with conductive or mixed hearing impairment while showing safe and effective long-term results. Our case displays the successful use of bilateral Bonebridge in a 28 months old pediatric patient with excellent postoperative development and audiological results. Careful patient selection, considering the individual patient-anatomy, preoperative planning and proper surgical techniques are the most important factors for postoperative satisfaction and a low complication rate. Based on the here presented results and high patient satisfaction, the authors propose to lower the present age limit of 5 years or older, to implantation based on available bone tissue allowing device placement. Similar as for Cochlear Implant recipients, were the earliest possible hearing rehabilitations allows the full auditory potential for normal development to be exploited.
Statements
Statement of Ethics
The presented case was part of a study with Institutional Review Board approval from the University Hospital St. Pölten, St. Pölten, Austria (Ethics Committee Decision no: GS1-EK-3/197-2021). The legal guardians of the child signed the informed consent form which was approved by the Ethics Committee.
Conflict of Interest Statement
The authors have no conflict of interest to declare
Funding Sources
No funding was received for this manuscript
Author Contributions
Conception and design (TM, AM GMS); data acquisition (PS); data analysis (PS); interpretation of data for the work (TM, AM GMS); drafting the manuscript (TM); revision of the final manuscript (TM, AM GMS); approval of the final manuscript (ALL authors).
Data Availability Statement
Anonymized patient data is available on request form the corresponding author (GMS).
References
- Sprinzl GM, Wolf-Magele A. The Bonebridge Bone Conduction Hearing Implant: indication criteria, surgery and a systematic review of the literature. Clin Otolaryngol 41 (2016): 131-143.
- Riss D, Arnoldner C, Baumgartner W-D, et al.: Indication criteria and outcomes with the Bonebridge transcutaneous bone-conduction implant: Bonebridge Indication Criteria and Outcomes. The Laryngoscope 124 (2014): 2802-2806.
- Sprinzl G, Lenarz T, Hagen R, et al.: Long-Term, Multicenter Results With the First Transcutaneous Bone Conduction Implant. Otology & Neurotology 42 (2021): 858-866.
- Cywka KB, Skarzynski H, Król B, et al. The Bonebridge BCI 602 Active Transcutaneous Bone Conduction Implant in Children: Objective and Subjective Benefits. JCM 10 (2021): 5916.
- Plontke SK, Götze G, Wenzel C, et al. Implantation of a new active bone conduction hearing device with optimized geometry. HNO 68 (2020): 106-115.
- Kraai T, Brown C, Neeff M, et al. Complications of bone-anchored hearing aids in pediatric patients. International Journal of Pediatric Otorhinolaryngology 75 (2011): 749-753.
- Brkic, Riss, Scheuba, et al. Medical, Technical and Audiological Outcomes of Hearing Rehabilitation with the Bonebridge Transcutaneous Bone-Conduction Implant: A Single-Center Experience. JCM 8 (2019): 1614.
- Zernotti M, Sarasty A. Active Bone Conduction Prosthesis: BonebridgeTM. Int Arch Otorhinolaryngol 19 (2015): 343-348.
- Magele A, Schoerg P, Stanek B, et al. Active transcutaneous bone conduction hearing implants: Systematic review and meta-analysis. PLoS ONE 14 (2019): e0221484.
- Seiwerth I, Fröhlich L, Schilde S, et al. Clinical and functional results after implantation of the bonebridge, a semi-implantable, active transcutaneous bone conduction device, in children and adults. Eur Arch Otorhinolaryngol 279 (2022): 101-113.
- Plontke SK, Radetzki F, Seiwerth I, et al. Individual Computer-Assisted 3D Planning for Surgical Placement of a New Bone Conduction Hearing Device. Otology & Neurotology 35 (2014): 1251-1257.
- Lee MY, Cho Y-S, Han GC, et al. Current Treatments for Congenital Aural Atresia. J Audiol Otol 24 (2020): 161-166.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 