Structural and Rheological Characterisation of Rye-Wheat Dough Fermented using Limosilactobacillus Fermentum
Julia Nutter2, Miriam O Iurlina1, Amelia I Saiz1*
1Laboratorio de Bromatología, Departamento de Química y Bioquímica, Universidad Nacional de Mar del Plata. Dean Funes 3350. Postal Code 7600. Mar del Plata. Argentina
2Universidad de Buenos Aires (UBA). Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). Instituto de Tecnologías y Ciencias de la Ingeniería (INTECIN), Argentina
*Corresponding Author: Amelia Ivone Saiz. Departamento de Química y Bioquímica, Universidad Nacional de Mar del Plata. Dean Funes 3350. Postal Code 7600. Mar del Plata. ARGENTINA.
Received: 10 September 2025; Accepted: 18 September 2025; Published: 03 November 2025
Article Information
Citation: Julia Nutter, Miriam O Iurlina, Amelia I Saiz. Structural and Rheological Characterisation of Rye-Wheat Dough Fermented using Limosilactobacillus Fermentum. Journal of Food Science and Nutrition Research. 8 (2025): 107-114.
DOI: 10.26502/jfsnr.2642-110000181
View / Download Pdf Share at FacebookAbstract
The acidification performance of Limosilactobacillus fermentum was studied in relation to the effect on the microstructure and rheology of sourdough. Scanning electron microscopy revealed a disruptive matrix of gluten without structures such as films or fibers that characterize a cohesive network of gluten. The production of lactic and acetic acids and ethanol was monitored by gaseous chromatography; the values obtained at the end of fermentation, 19 hours, were 5.86 g of lactic acid, 1.00 g of acetic acid, and 2.96 g of ethanol. Acidification profile of L. fermentum promoted the cleavage of disulphide linkages and was responsible for disordered protein structure and therefore a disrupted matrix of gluten. This observation was in line with viscoelastic performance of sourdough. G’ and G’’, determined by dynamic oscillatory assays, shown the same values to control dough and dough inoculate with L. fermentum. This event confirmed the absence of gluten network that account for sourdough elasticity.
Keywords
<p><em>Limosilactobacillus fermentum</em>; Rye-wheat dough; Metabolic products; Microstructure</p>
Article Details
Introduction
Sourdough technology is not so popular in South America; however, fermented dough bread is reaching a place between the consumers due to its particular taste and benefit on health. It is well known that sourdough bread impact consumers health by providing additional health benefits in addition to improve texture and flavour [1]. Cereals provide basic dietary compounds, and some of them are considered healthy due to its functional food attributes [2]. Bread from rye flour increases the fiber content of our diet, and protects us against many diseases such as cancer, cardiovascular and diverticular diseases [3].
Typical sourdough LAB mainly belongs to the genus Lactobacillus and include obligate and facultative heterofermentative and obligate homofermentative species [4]. The metabolites produced by lactobacilli characterise the sourdough and contribute to structural, sensory, and nutritional properties of bread [5]. Changes in pH level, caused by the production of lactic acid, alter the rheological behaviour of dough. Even small chemical and physical changes at the gluten network can lead to significant changes in rheological properties [6,7].
Concerning fermented foods, L. fermentum has repeatedly been reported as one of the dominant microorganisms in sourdough fermentation [4, 8]. The occurrence of L. fermentum is based on factors as metabolic versatility and stress resistance, because lactobacilli that are obligately heterofermentative prevail in these ecosystems due to their ability to use maltose [9].
Gluten is the main functional component of wheat, and it is responsible for the viscoelastic properties of dough. There is a widely held view of gluten structure in which the high molecular weight glutenin subunits (HMW-GS) form an “elastic backbone” consisting largely of head-to-tail polymers with interchain disulphide bonds. This backbone forms a basis for low molecular weight glutenin subunits (LMW-GS) branches linked by disulphide bonds, thus disulphide bonds are essential for glutenin viscoelasticity [10].
While starch and water are the main components of doughs, the physical properties of dough arise from interactions between gluten proteins, particularly the disulphide-bonded glutenin macropolymer (GMP) [11]. The breakdown of gluten proteins in wheat sourdough is among the key features that affect wheat dough rheology and bread quality. Gluten breakdown is favoured by acidification due to the solubility of gluten proteins increases at low pH, and the aspartic proteases of wheat grain operate optimally under acidic conditions [12].
Additionally, the amount of GMP in dough is strongly affected by reducing agents, such as glutathione (GSH) or L-cysteine, that significantly reduce the size of large proteins, which results in softening and weakening of dough [4]. Some heterofermentative LAB can express glutathione reductase that contribute to the GMP hydrolysis, thus, protein hydrolysis influences the final bread quality due to the loss of the gluten cohesive network and viscoelastic properties of dough [13]. The changes on the gluten macropolymer are the basis for accurately controlling the process and extent of polymerization [14]. We speculate that dynamic acid production by LAB may have different effect on GMP, which contributes to the viscoelastic performance of gluten. Therefore, the aim of this work was to evaluate the microstructure and rheological changes of dough regarding the acidification performance . fermentum.
Material and Methods
Sourdough preparation and fermentation
Limosilactobacillus fermentum CRL 220, an obligate heterofermentative strain, was obtained from the culture collection of the Centro de Referencia para Lactobacilos (CERELA, Argentina). The strain was precultured twice on MRS broth at 30ºC for 24 h. The inocula were standardized to a concentration of 1.5 x 108 colony-forming units per mL (CFU/mL) using the Mc Farland turbidity scale. The cells were collected by centrifugation at 2490 g for 10 min, washed twice with sterile 0.15 M NaCl, and resuspended in sterile 0.15 M NaCl.
Sourdough were prepared according to Nutter et al. [28], all doughs were made from rye and wheat flours (1:1), 150 mL tap water, and 3.8 g salt and were inoculated with 10 mL of the standardized cellular suspension. After kneading (240 rpm) with a dough mixer machine (Hobart Model N-50, Ontario, Canada) for 5 min at room temperature, dough fermentation was conducted at 30ºC for 19 h. Uninoculated dough was prepared under the same conditions as a control.
Physicochemical and microbial analyses
For both physicochemical and microbial viability analyses, dough samples were regularly withdrawn at 0, 6, 12, and 19 h of fermentation. Dough acidity was assessed by potentiometry. Briefly, 10 g of sourdough were blended with 90 mL of distilled water and were homogenized for 10 min. The pH values and total titratable acidity (TTA) of the samples were determined using a pH-meter (Hanna instruments HI 9321). TTA was expressed as the amount of 0.1 M NaOH (mL) to reach a final pH of 8.5. In addition, for LAB viability assays, 25 g-sample were withdrawn from each dough and were aseptically homogenized in 225 mL of sterile Butterfield‘s phosphate-buffered dilution water [15]. The total cell number of L. fermentum was determined anaerobically on de Man Rogosa Sharpe agar (MRS, Britania, Argentina) (incubation conditions: 32°C for 72 h).
Extraction procedure for GC analysis
The extraction method was performed as described previously Bervas [16]. Samples were regularly removed from dough at 0, 6, 12, and 19 h for gas chromatography (GC) analysis. Ten grams of each sample were homogenized with 20 mL of cold distilled water using a magnetic stirrer (Semedic, Argentina). One millilitre of 1 M HClO4 solution was added to 2 mL of homogenate. The mixture was centrifuged for 15 min at 2490 g at 15ºC, the supernatant was neutralized (pH 7.0 ± 0.1) with 2 M KOH and the volume was measured. After 30 min of precipitation on ice, the solution was filtered on a 0.45 µm MF-Millipore filter (Merck, Argentina).
Quantitative determination of organic volatile compounds
Calibration curves were performed with external standards added to the samples. Standard solutions and extracts obtained from control dough and dough with added known concentrations of acetic acid, lactic acid, ethanol, acetaldehyde, and diacetyl were analysed by GC. Each sample (2 µL) was injected into a KNK-3000-HRGC gas chromatograph (Kornik, Spain) equipped with a flame ionization detector (H2 flow rate 30 mL/min, airflow rate 300 mL/min) and an Alltech Capillary Column Econo-CapTM ECTM 1000 (15 m x 0.53 mm i.d., 1.2 µm film thickness, USA). Detector temperature and injector port temperature were 280 and 240ºC, respectively. Hydrogen was used as carrier gas at a flow rate of 3 mL/min. The oven temperature was programmed at 75 ºC for 1 min, raised to 180ºC at 6 ºC/min, then increased to 230ºC at 10 ºC/min, and finally held at 230ºC for 5 min [17]. During fermentation, samples were withdrawn from the dough at 0, 6, 12 and 19 h and assessed as mentioned above.
Scanning electron microscopy
Representative portions of doughs fermented for 19 h (uninoculated and inoculated with L. fermentum) were air frozen at −20°C and freeze-dried. The freeze-dried pieces of dough were fractured to expose interior surfaces. The samples were mounted on specimen holders with conductive glue (Leit C) and sputter-coated with gold-palladium. The samples were analysed and photographed in a Jeol JSM 6460 LV scanning electron microscope (Jeol, USA) at an accelerating voltage of 20 kV.
Proteolytic activity of L. fermentum
In order to evaluate L. fermentum proteolytic activity on gluten, the basic medium containing 1 g glucose, 2.5 g yeast extract, and 14 g agar-agar was prepared [18]. In addition, a suspension containing 2.5 g of gluten in 50 ml of water was prepared and homogenized by stirring for 10 minutes. Then, one mL of this suspension was placed in each plate, the medium was poured and L. fermentum was inoculated by spread on surface. The incubation was carried out at 32ºC and the plates were observed daily during a week and the hydrolytic activity was confirmed using trichloroacetic acid (TCA), which allows the visualisation of a clarification halo.
Carbohydrate fermentation profile
The carbohydrate fermentation profile was determined using the commercial API® 50CH test (BioMérieux, Germany). The inoculation and evaluation (using the apilab software version 5.1) of the test kit were carried out according to the manufacturer's instructions.
Dynamic rheological measurements
Tests were performed in a Haake RS600 controlled stress oscillatory rheometer (Haake, Germany) at 25 ± 0.1°C, using a plate-plate sensor system with a 1.5 mm gap between plates. Before starting the oscillatory measurements, all samples were placed between plates and were incubated at room temperature for 15 min to allow dough relaxation. In order to determine the linear viscoelastic range of each sample, deformation sweep tests were performed at a constant frequency (1 Hz). Frequency sweep tests (from 0.005 to 100 Hz) at constant stress (5 Pa) within the linear viscoelastic range were performed at 25°C. Dynamic module G’, G’’ and tan δ (G’’/G’) were obtained as a function of frequency. G’ is the dynamic elastic or storage modulus, related to the material response as a solid, while G’’ is the viscous dynamic or loss modulus, related to the material response as a fluid. Tan δ is related to the overall viscoelastic response: low values of this parameter indicate a more elastic sample. Assays were performed in triplicate and data were analysed using the Oscillation 2.0 Haake software (Haake, Germany).
Statistical analysis
All data presented represent the mean values from three replicate experiments ± standard deviation (SD) and were performed with SPSS statistic 15.1 for Windows using ANOVA General Linear Models followed by a Tukey’s posthoc test, and p<0.05 was considered significant.
Results
Acidification potential of L. fermentum
This experiment was conducted in order to evaluate the acidification properties (kinetics of pH and TTA) of the freeze-dried LAB starter, L. fermentum CRL 220.
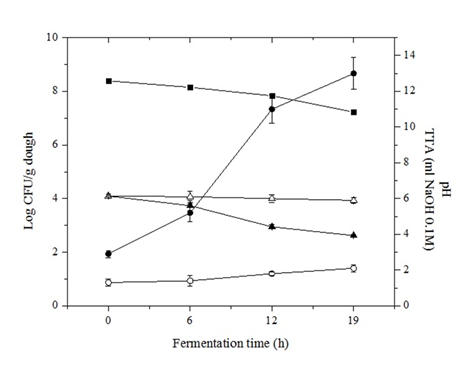
The effect of the LAB starter on sourdough pH values was monitored over the 19 h fermentation period and it was compared with uninoculated dough prepared under the same conditions (Figure 1). As expected, the uninoculated flour-water-salt mixture presented no acidification, the pH value remained nearly constant (6.1). During the same period, the endogenous LAB microflora of the flour, initially 100 CFU/g dough, reached 1.2 x 103 CFU/g dough leading to a slight pH decrease at the end of the incubation period. When dough was prepared using L. fermentum as starter, the average pH-values were 6.1 ± 0.1 at the beginning of fermentation and then decreased to pH 4.5 ± 0.1 at 12 h, to finally reach 3.93 ± 0.5 at the end of fermentation (19 h). This decrease in pH values was in accordance with an increase in L. fermentum population growth, 2 x 109 CFU/g dough after 19 h of incubation.
The average TTA values during the fermentation processes were 5.2 ± 0.5 after 6 h, 11 ± 0.8 at 12 h, and 13 ml ± 0.9 of NaOH at the end of fermentation. After a period of 6 h of fermentation, L. fermentum showed a marked increase on TTA values measured. This can, for instance, be ascribed to their maltose fermentation ability via maltose phosphorylase activity and the phosphogluconate pathway, energetically more advantageous than common heterofermentation [19].
Carbohydrate fermentation and Proteolytic activity
To assess the ability of the selected strain to metabolise different carbohydrates, L. fermentum was evaluated for their carbohydrate fermentation properties with the commercial API® CH50 test. The strain showed the ability to metabolize all sugars present in dough (maltose, sacarose, glucose, and fructose). Rye and especially wheat flours contain low amounts of soluble carbohydrates, the total concentration of maltose, sucrose, glucose and fructose varies from 1.55 to 1.85% (w/w), depending on the balance between starch hydrolysis by the flour enzymes and/or microbial enzymes [20]. In addition, L. fermentum did not exert proteolytic activity on gluten proteins (subsection 2.6).
Metabolite production
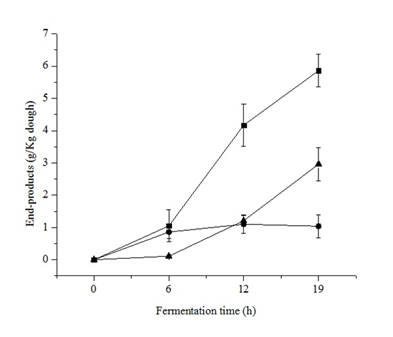
The production of lactic acid, acetic acid, and ethanol was monitored on sourdough at 0, 6, 12, and 19 h of fermentation (Figure 2) and it was compared to uninoculated dough prepared under the same conditions. In the control dough, neither organic acids nor ethanol production was observed, thus confirming that the effect of indigenous LAB from the flours should be negligible.
During the first 6 h of fermentation, when the pH value of the system was 6.14 (0 h) - 5.60 (6 h), acetic acid was the only product detected (Figure 2). This dominance occurs when fructose is utilized as electron acceptor rendering the higher energetic yield [21]. Rye flour contains an important fraction of fructosans; thus, it could be expected a higher fructose content than that obtained in wheat dough. In fact, the availability of fructose could promote the acetate pathway of the heterofermentative LAB since it is a suitable electron acceptor [22]. After 6 h of fermentation, when the pH values decreased (Figure 1), the concentration of both ethanol and lactic acid was progressively increased. At the end of the process (19 h), ethanol and lactic acid reached a maximum concentration, 2.96 and 5.84 g/kg of dough, respectively. Maltose metabolism promotes the production of lactic acid and ethanol. At high concentrations of maltose and under stress conditions, such as low pH, maltose phosphorylase is constitutively expressed in L. sanfranciscensis, L. reuteri, L. fermentum, and L. pontis which enables maltose hydrolysis without the expenditure of ATP [23].
Microstructure by SEM
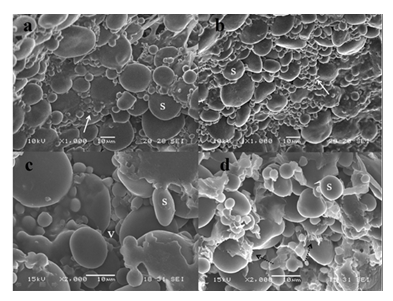
The structure of dough is comprised of several elements, which included a protein matrix, small starch granules strung together, large individual starch granules, and dispersed gas vacuoles trapped in the structure. Changes in microstructure of uninoculated dough and dough inoculated with L. fermentum are shown in Figure 3. Scanning electron micrographs of the samples correspond to a fermentation period of 19 h.
Figures 3a and 3b show a typical micrograph obtained from control rye-wheat dough. Microstructure was determined by numerous starch granules, occurring in two different sizes, embedded in the gluten matrix. These results were in accordance to Aponte et al. [24]. Gluten macropolymer did not display any kind of organized structure, such as film or fibrils, therefore neither enzymatic activity coming from the flour nor the microorganisms activity produced hydrolysis of the gluten matrix.
The cross-section of dough fermented by L. fermentum (Figures 3c and 3d) showed a porous structure with relatively large vacuoles corresponding to CO2 bubbles produced during heterolactic fermentation by this strain. The microstructure was dominated by starch granules glued together and the gluten macropolymer became into a depolymerised matrix with short and disrupted strands.
Figure 3: SEM micrographs of uninoculated dough at x 1000 magnification (a and b) and dough inoculated with L. fermentum at 19 h of fermentation at x 2000 magnification (c and d). S: starch granules, V: CO2 vacuoles. Arrows indicate starch granules glued by gluten. Dotted arrows indicate short and disrupted gluten strands.
Rheological studies
Rheological analysis, especially in the linear viscoelastic region, has been used to follow up on the structure and properties of dough and to study the functions of dough ingredients [25,26]. This assay simultaneously measures the viscous and elastic character of dough expressed in storage and loss module, G’ and G’’, and loss tangent tan δ.
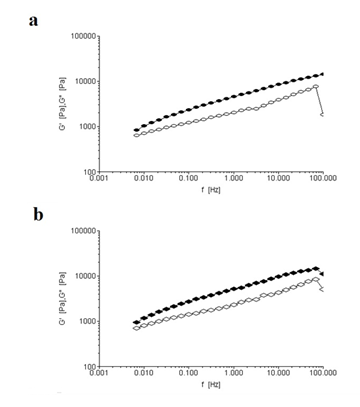
The viscoelasticity of the gluten network depends on intermolecular interactions. Under mechanical action, gradual destruction of intermolecular interactions causes the non-linear rheological behaviour above a critical strain value. For the level of strength applied, 5 Pa, the values obtained were within the linear viscoelastic region. Figures 4a and 4b show the mechanical spectrum for fermented and control dough. It is particularly important to keep in mind that the temperature, the water added, the mixing time and the rest period are the factors determining the rheological properties of the dough system and they remained constant during the kneading and fermentation stages.
Dough samples exhibited viscoelastic behaviour, since the elastic modulus (G’) exceeded the viscous modulus (G’’) throughout the frequency range (0.1-100 Hz). Both doughs showed the same trends for G’ and G’’ over the range of frequencies measured, therefore only the data collected at 1 Hz were compared (Table 1). There were no significant differences in G’ and G’’ values between L. fermentum inoculated dough and control dough. Concerning tan δ values, this parameter is considered as an indicator of the structural organization or molecular interactions in the material: highly structured materials generally present low tan δ values. Uninoculated and L. fermentum inoculated dough exhibited the same tan δ value, 0.45, therefore the glutenin polymers showed the same degree of cross-linking in both control and fermented doughs. Thus, L. fermentum did not improve the elasticity in relation to control dough.
|
G´ |
G´´ |
|G*| |
Tg δ |
|
|
Control |
4521 |
2016 |
4950 |
0.45 |
|
L. fermetum |
5172 |
2312 |
5666 |
0.45 |
Table 1: Values of G', G'', |G*|, and loss tangent (tan δ) collected at 1 Hz of frecuency from the mechanical spectrum for fermented dough and control dough.
Discussion
Microbial growth, the concentration of organic acids, pH, TTA, proteolytic activity, fermentation profile of sugars, changes in microstructure, and elastic and viscous modulus in sourdough were determined using Limosilactobacillus fermentum as fermenting microorganism.
Results presented by different authors about the effect produced by acids on sourdough elasticity are usually opposite [26, 27]. In this regard, acidification kinetics should be considered because the fermentation profile of different LAB strains accounts for the differences observed in GMP organization [28] and therefore modifies the viscoelastic behaviour and functional properties.
It is widely known that a limited extent of proteolysis during sourdough fermentation improves the bread flavour without adverse effects on its texture and volume [29]. The largest polymers, termed GMP, make the greatest contribution to dough properties and their amount in wheat flour is strongly correlated with dough strength and loaf volume [30].
Delcour et al. [31] analysed the role of different glutenin subunits in doughs and they observed that HMW-GS play a primary role in the formation of a network. Additionally, using immune-electron microscopy, they determined that HMW-GS formed linear chains and networks, while LMW-GS formed clusters and aggregates, consistent with LMW-GS forming branches from the linear HMW-GS chains [32]. In this regard, the aggregates between gluten and starch granules observed in control dough (Figures 3a and 3b) are in accordance with the presence of LMW-GS. The pH values of control dough slightly varied during the fermentation period and remained constant at around 6.14. Cereal proteases have optimum activity around pH 3.7 [33], therefore, their activity is negligible at pH 6. The endogenous LAB population of flours, around 1 x 103 CFU/g, is not enough to decrease the pH values and promote enzymatic activity, thus LMW-GS are able for cross-linking. The micrographs showed a protein-starch matrix that interacts strongly, and the rheological data could be taken as a reference of dough with low elasticity. The presence of flexible linear chains and networks formed by HMW-GS are a condition for promoting elasticity or strength properties of dough [10]. The fermentation process affects the organization of the gluten macropolymer, and some degree of hydrolysis is necessary for making fibres of gluten with crosslinked arrangements that lead to a network [28].
Thiele et al. [29] reported that the hydrolysis of glutenins is mainly related to the pH drop during the fermentation process that, in turn, promotes the activation of endogenous flour enzymes, rather than specific LAB proteinases. In fact, L. fermentum did not show proteolytic activity on gluten proteins. The pH values of fermented dough decreased from 6 to 4.5 during the first 6 h, then remained at 4.5 until 12 h of fermentation, to finally achieve a value of 3.93 at the end of fermentation. According to this behaviour, the activity of endogenous wheat enzymes was not promoted until the end of the fermentation process. Cereal aspartic proteinases are the most active proteinase group in sourdough, with an optimum pH comprised between 3.5 and 4 [33]. Therefore, we would expect a limited extent of proteolysis during sourdough fermentation that improve the bread flavour without adverse effects on texture and volume.
The relative amount of acids produced during LAB fermentation contributes to make the gluten network and it is relevant to explain the microstructure and rheologycal results. L. fermentum produced 5.86 g of lactic acid and 1 g of acetic acid; In this regard, L. fermentum has been proposed as a suitable microorganism for sourdough bakery processes due to its ability to produce acetic acid, which is responsible for pleasant organoleptic features as well as for antimicrobial and antimould effect. To whole rye sourdough bread, optimal fermentation quotient (FQ, lactic acid/acetic acid) is comprised between 1.5-4.0 [22]. The FQ obtained from 50-50% rye-wheat flour dough was 3.8, a value that is thought as proper regarding sourdough aroma profile and structure. In addition, sourdough acidic conditions promote gluten depolymerisation. In fact, repulsive effects, occurred as the result of an increase proton concentration, inhibit further aggregation between protein chains and enhance the interaction with water molecules. As the levels of hydration increase, plasticization of the system allows the formation of hydrogen-bonded structures between the chains and water through glutamine residues, resulting in the formation of film-like regions [26].
According to rheological data (Table1) sourdough elasticity was not improved in relation to control dough. This event is in line with excessive depolymerisation of gluten when dough was inoculated with L. fermentum, which was confirmed by SEM micrograph (Figure 3b). In this regard, Raman spectroscopy studies carried out by our group on gluten proteins from L. fermentum sourdough [22], showed 30% decrease in disulphide SS intensity band in comparison to control dough, which corresponded to SS bond cleavage and gluten depolymerisation. Lancelot et al. [34] demonstrated that when the SS disulphide linkages were reduced by adding SH blocking reagent resistance to extension was lower and the quality of resulting dough was poor.
Heterofermentative lactobacilli can express glutathione reductase during growth in sourdough. The reduction of GSSG to GSH maintains high GSH levels during fermentation and contributes to deepening gluten depolymerisation [30]. The optimum activity of GSH reductase occurs at pH 5 and its activity decreases when pH lowers [35]. Therefore, heterofermentative microorganisms as L. fermentum producing moderate acidification promote the activity of glutathione reductase
Conclusions
The acidification profile of each microorganism throughout fermentation stage may influence the activity of endogenous enzymes like proteases and glutathione reductase, deepens the repulsive effects between functional groups of proteins, and, in turn, promote interactions between molecules of water and amino acids. Therefore, fermentation performances of LAB can modify the gluten macropolymer in different ways. The results of L. fermentum in relation to the viscoelastic properties were attributed to the excessive breakdown of cross-linking points between HMW-GS. The acidic conditions during the fermentation favoured the cleavage of disulphide bounds by catalytic activity of hydrolytic enzymes, which promoted a disruptive matrix of gluten. A disordered protein structure is responsible for low elasticity and poor quality of dough.
Acknowledgments
This work was founded by National University of Mar del Plata for the research project Effect of honeys on cellular communication and LAB activity in foods. Part II” (15/EB47 EXA 889/18) and by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
References
- Minervini F, Lattanzi A, Dinardo FR, et al. Wheat endophytic lactobacilli drive the microbial and biochemical features of sourdoughs. Food Microbiology 70 (2018): 162-171.
- Wang Z, Wang L. Review article Impact of sourdough fermentation on nutrient transformations in cereal-based foods: Mechanisms, practical applications, and health implications Grain & Oil Science and Technology 7 (2024): 124-132
- Koj K, Pejcz Rye Dietary Fiber Components upon the Influence of Fermentation Inoculated with Probiotic Microorganisms. Molecules 28 (2023): 1910.
- Gänzle MG, Zheng J. Lifestyle of sourdough lactobacilli. Do they matter for microbial ecology and bread quality? International Journal of Food Microbiology 302 (2019): 15-23.
- Sakandar HA, Hussain R, Kubow S, et al. Sourdough bread: A contemporary cereal fermented product. Journal of Food Processing and Preservation (2019).
- Zhang Y, Hong T,Yu W, et al. Structural, thermal and rheological properties of gluten dough: Comparative changes by dextran, weak acidification and their combination. Food Chemistry 330 (2020):127154.
- Kłosok K, Welc R, Fornal E, et al, Effects of Physical and Chemical Factors on the Structure of Gluten, Gliadins and Glutenins as Studied with Spectroscopic Methods. Molecules 26 (2021):508.
- De Vuyst L, Van Kerrebroeck S, Leroy F. Microbial Ecology and Process Technology of Sourdough Fermentation. Advances in Applied Microbiology 100 (2017): 49-160.
- Lancetti R, Sciarini1 L, Pérez GT, et al. Technological Performance and Selection of Lactic Acid Bacteria Isolated from Argentinian Grains as Starters for Wheat Sourdough. Current Microbiology 78 (2021): 255-264.
- Li Y, Fu J, Shen Q, et al. High-Molecular-Weight Glutenin Subunits: Genetics, Structures, and Relation to End Use Qualities. International Journal of Molecular. Sciences 22 (2021):184.
- Feng Y, Zhang H, Wang J, et al. Dynamic Changes in Glutenin Macropolymer during Different Dough Mixing and Resting Processes. Molecules 26 (2021):541.
- Gerez CL, Dallagnol A, Rollán G, et al. A combination of two lactic acid bacteria improves the hydrolysis of gliadin during wheat dough fermentation. Food Microbiology 32 (2012): 427-430.
- Pico J, Bernal J, Gomez M. Wheat bread aroma compounds in crumb and crust: A review. Food Research International 75 (2015):10566.
- Han C, Ma M, Li M, et al. Further interpretation of the underlying causes of the strengthening effect of alkali on gluten and noodle quality: Studies on gluten, gliadin, and glutenin. Food Hydrocolloids 103 (2020):
- Butterfield CT. A selection of dilution water for bacteriological examination. Journal of Applied Bacteriology 23 (1932): 355-68.
- Bervas E. Mise au point de levains bacteriens pour la panification. Ph. D. thesis, Université Blaise Pascal, Clermont-Ferrand, France (1991).
- Yang MH, Choong YM. A rapid gas chromatographic method for direct determination of short-chain (C2-C12) volatile organic acids in foods. Food Chemistry 75 (2001): 101-108.
- Perez Borla O, Davidovich L, Roura S. Isolation and Characterization of proteolytic microorganisms from fresh and fermented cabbage. LWT-Food Science and Technology 43 (2010): 298-301.
- Gänzle Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Current Opinion in Food Science 2 (2015): 106−117.
- Gobbetti M. The sourdough microflora: Interactions of lactic acid bacteria and yeasts. Trends in Food Science and Technology 9 (1998): 267-274.
- Gänzle MG, Vermeulen N, Vogel R. Carbohydrate, peptide and lipid metabolism of lactic acid bacteria in sourdough. Food Microbiology 24 (2007): 128-138.
- Corsetti A, Settanni L. Lactobacilli in Sourdough Fermentation. Food Research International 40 (2007): 539-558.
- Stolz P, Hammes W, Vogel R. Maltose-phosphorylase and hexokinase activity in Lactobacilli from traditionally prepared sourdoughs. Advances in Food Science 18 (1996): 1-6.
- Aponte M, Boscaino F, Sorrentino A, et al. Effects of fermentation and rye flour on microstructure and volatile compounds of chestnut flour based sourdoughs. LWT-Food Science and Technology 58 (2014): 387-395.
- Miller K, Hoseney R. Dynamic Rheological Properties of Wheat Starch-Gluten Doughs Cereal Chemistry 76 (1999): 105-109.
- Clarke C, Schober T, Dockery P, et al. Wheat Sourdough Fermentation: Effects of time and acidification on fundamental rheological properties. Cereal Chemistry 81 (2004): 409-417.
- Maher Galal A, Varriano-Marston E, Johnson JA. Rheological dough properties as affected by organic acids and salt. Cereal Chemistry 55 (1978): 683-691.
- Nutter J, Saiz AI, Iurlina MO. Microstructural and conformational changes of gluten proteins in wheat-rye sourdough. Journal of Cereal Science 87 (2019): 91-97.
- Thiele C, Gänzle M, Vogel R. Contribution of sourdough lactobacilli, yeast, and cereal enzymes to the generation of amino acids in dough relevant for bread flavour. Cereal Chemistry 79 (2002): 45-51.
- Vermeulen N, Kretzer J, Machalitza H, et al. Influence of redox-reactions catalyzed by homo- and heterofermentative lactobacilli on gluten in wheat sourdoughs. Journal of Cereal Science 43 (2006): 137-143.
- Delcour JA, Joye I J, Pareyt B, et al. Wheat gluten functionality as a quality determinant in cereal-based food products. Annual Review of Food Science and Technology 3 (2012): 469-92.
- Lindsay M, Skerritt J. The glutenin macropolymer of wheat flour doughs: structure-function perspectives. Trends in Food Science & Technology 10 (1999): 247-253.
- Gänzle MG, Loponen J, Gobbetti M. Proteolysis in sourdough fermentations: mechanisms and potential for improved bread quality. Trends in Food Science and Technology 19 (2008): 513-521.
- Lancelot E, Fontaine J, Grua-Priol J, et al. Study of structural changes of gluten proteins during bread dough mixing by Raman spectroscopy. Food Chemistry 358 (2021): 129916.
- Kaid N, Rakotozafy L, Potus J, et al. Studies on the Glutathione-dehydroascorbate oxidoreductase (EC 1.8.5.1) from wheat flour. Cereal Chemistry 74 (1997): 605-611.


 Impact Factor: * 3.8
Impact Factor: * 3.8 Acceptance Rate: 77.96%
Acceptance Rate: 77.96%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 