The Association of Programmed Death-Ligand 1 Expression with Clinicopathological Features, Lymph Node Metastasis and Survival Prognosis in Patients with Colorectal Carcinoma
Weifang Shao1#, Yanhua Xu1#, Suzhen Lin1*, Junshun Gao2, Junli Gao2, Hong Wang2*
1Medical Laboratory Department, ChangXing People’s Hospital, 66 Taihu Middle Road, ChangXing County, Hangzhou City, Zhejiang Province, P.R. China
2Hangzhou Cosmos Wisdom Mass Spectrometry Center of Zhejiang University Medical School, 198 Qidi Road, Xiaoshan District, Hangzhou City, Zhejiang Province, P.R. China
#These authors contributed equally to this work
*Corresponding Authors: Suzhen Lin, Medical Laboratory Department, ChangXing People’s Hospital, 66 Taihu Middle Road, ChangXing County, Hangzhou City, Zhejiang Province, P.R. China
Hong Wang, Hangzhou Cosmos Wisdom Mass Spectrometry Center of Zhejiang University Medical School, 198 Qidi Road, Xiaoshan District, Hangzhou City, Zhejiang Province, P.R. China
Received: 02 July 2022; Accepted: 12 July 2022; Published: 21 July 2022
Article Information
Citation: Weifang Shao, Yanhua Xu, Suzhen Lin, Junshun Gao, Junli Gao, Hong Wang. The Association of Programmed Death-Ligand 1 Expression with Clinicopathological Features, Lymph Node Metastasis and Survival Prognosis in Patients with Colorectal Carcinoma. Journal of Cancer Science and Clinical Therapeutics 6 (2022): 262-268.
View / Download Pdf Share at FacebookAbstract
Colorectal carcinoma (CRC) is one of the most frequently encountered neoplasms with high morbidity and mortality. Activation of the programmed death protein 1/ programmed death ligand 1 (PD-1/PD-L1) pathway results in tumor immune evasion by suppressing the activity of T cells. The correlation of PD-L1 with clinicopathological features, lymph node metastasis and prognosis is less clear. The aim of present work was to study the relationship between PD-L1 and clinicopathological features and prognosis of CRC patients. Three hundred and eighty-six patients were included in this study. Serum PD-L1 was measured by ELISA, and PD-L1 on tumor cells was evaluated by immunohistochemistry. Pretreatment levels of PD-L1 were significantly elevated in CRC patient sera compared to healthy donors (P<0.001). The mean value of PD-L1 in healthy donors, CRC with non-lymph node metastasis, and CRC with lymph node metastasis were 229.22±54.7pg/mL, 400.77±66.3pg/ mL, and 414.29±59.1pg/mL, respectively. The positive rate of PD-L1 in metastatic lymph node was higher than in primary tumor (P<0.001). PD-L1 negative patients had higher five-year survival rate than PD-L1 positive patients (68.57% vs 46.98%, P=0.012). The univariate analysis indicated that tumor differentiation, lymph node metastasis, and PD-L1 were correlated with five-year survival rate of CRC patients (all P < 0.018). Multivariate analysis showed that lymph node metastasis and PD-L1 were independent prognostic factors (all P < 0.008). Our study demonstrates that PD-L1 negative patients have better five-year survival rate, and PD-L1 and lymph node metastasis are independent prognostic factors in CRC patients.
Keywords
<p>Colorectal cancer; Serum PD-L1; Tissue PD-L1; Lymph node metastasis; Prognosis</p>
Article Details
1. Introduction
Colorectal carcinoma (CRC) is a major health concern, being the third most commonly diagnosed malignancy and the second leading cause of mortality among cancer-related deaths worldwide [1]. The incidence and mortality of CRC are trending to ascend each year, and over 2.2 million new cases and 1.1 million patient deaths are expected by 2030 [2]. Despite the improvement in overall survival (OS) due to new medications and therapies in CRC, local regional recurrence and distant metastasis remain the major causes of treatment failure. About 25% of CRC patients display metastasis at diagnosis, and 50% of those treated will develop metastasis during their lifetime [3]. It is known that cancer immune suppression and immune escape play the crucial roles in tumor progression, recurrence and metastasis. Among these processes, the activation of the programmed death protein 1/programmed death ligand 1 (PD-1/PD-L1) signaling pathway was identified as the key mechanism of tumor immune evasion through the inhibition of T-cell proliferation and suppression of the CD8 cytotoxic immune response [4].
PD-L1 is mainly expressed on the surface of activated T cells. PD-1, the receptor of PD-L1, is absent in resting T cells and is induced in activated T cells. PD-1 interacts with PD-L1 on the surface of T cells to form a negative feedback pathway that suppresses the activity of T cells by impairing the immune surveillance function of T cells through the phosphorylation of tyrosine kinase and phosphoinositide-3 kinase to inhibit downstream signal transduction pathway, T cell proliferation, cytokine secretion and cytotoxicity [5]. Over expression of PD-L1 across a spectrum of different tumor cells has been co-opted as a high efficient immunoediting approach to evade immune attack and facilitate cancer growth [6]. Immune checkpoint blockade as the oncological immune therapy with antibodies against PD-1/PD-L1 pathways has shown clinical efficacy in several types of cancers, including lung, melanoma, renal cell carcinoma, esophageal , gastric, liver and CRC [7-12]. The expression level of PD-L1 on tumor cells is linked to the response to anti-PD-1 blockade and plays an important role in evaluating the prognosis of targeted immunotherapy [13-16]. PD-1/PD-L1 inhibitors represent a major step in the management of CRC, although several questions remain in routine clinical practice. PD-L1 immunohistochemistry (IHC) expression on cancer cells has been used to the patients with high likelihood to respond to immunotherapy, with some reported studies showing inconsistency results as to whether PD-L1 level indicates a better or worse prognosis [17-21]. Soluble PD-L1 (sPD-L1) was detected in the serum or plasma from patients with various cancer, and the elevated sPD-L1 is usually an indicative of poor prognosis of cancer patients [22-27]. However, it is still less clear on the clinicopathologic and prognostic characteristics of CRC with PD-L1 expression either on tumor cells or in serum/plasma. In addition, the correlation between the PD-L1 levels and overall survival of CRC patients needs to be further investigated.
In the present study, we aimed to analyze the serum levels of PD-L1 and expression of PD-L1 on tumor cells in patients with CRC, and investigate the associations between PD-L1 level and clinicopathological features, lymph node metastasis, and prognosis of CRC patients. In addition, the status of lymph node metastasis and PD-L1 expression as the independent factors of prediction and prognosis for immunotherapy were further addressed.
2. Materials and Methods
2.1 Clinical sample collection
This study was approved by the institutional review board of the ChangXing People Hospital, ChangXing, Zhejiang, PR. China. To conduct research, permission of the Committee on Bioethics of 2019ZLK-072 dated July 30, 2016, was obtained. All patients gave informed consent to participate in the study. The inclusion criteria were: (1) pathologically diagnosed CRC; and (2) curative surgical resection as the initial treatment. The exclusion criteria were: (1) previous or concurrent cancer; (2) non primary metastatic CRC; (3) patients with heart, liver and kidney insufficiency and serious complications; and (4) lack of tumor tissue samples.
The control group was represented by 400 healthy individuals matched for gender and age with the study groups. All donors of the control group underwent a test of feces for occult blood and fibrocolonoscopy screening. Therefor, the control group did not include patients with colorectal cancer, polyposis, or other defects of the colon or rectum. The case group consisted of 400 patients diagnosed with colorectal carcinoma who underwent surgical resection of primary or metastatic tumors and clinical molecular testing between the years of 2010 and 2015. All patients underwent a general clinical examination at the outpatient stage. The clinical diagnosis was established according to International Classification of diseases, ICD 10. The TNM classification developed by the International Cancer Union was used to classify the stage of cancer and the degree of differentiation. We excluded 14 patients who received radiotherapy or chemoradiotherapy before surgery. A total of 386 patients were included in this study including CRC without lymph node metastasis (n=194) and CRC with lymph node metastasis (n=192). Clinicopathological characteristics including age, gender, tumor size, grades of differentiation, and stage of cancer were obtained from the electronic medical record (Table 1).
2.2 Enzyme-linked immunosorbent assay, ELISA
Blood sampling was carried out in the morning, on an empty stomach, before any treatment. The levels of PD-L1 in serum were analyzed using Human B7H1/PD-L1 ELISA Kit (ELH-B7H1, RayBiotech, Norcross, GA, USA). All experiments were conducted according to the manufacturer’s instructions. In brief, add 100 µL of standard or twofold-diluted serum sample to each well, and incubated 2.5 h at RT with gentle shaking. Discard the solution and wash 4 times by filling each well with Wash Buffer (300 µL) using a multi-channel Pipette. After the last wash, remove any remaining Wash Buffer by aspirating. Invert the plate and blot it against clean paper towels. Add 100 µL of prepared biotin antibody to each well and incubated 1 h at RT with gentle shaking. Discard the solution, and repeat the wash step. Then, add 100 µL of prepared Streptavidin solution was added to each well and incubated 45min at RT with gentle shaking. Discard the solution, and repeat the wash step followed by adding 100 µL of TMB One-Step Substrate Reagent to each well and incubating for 30min in the dark at RT with gentle shaking. Add 50 µL of Stop Solution to each well and read at 450 nm immediately. Calculate the mean absorbance for each set of duplicate standards and samples, and subtract the average zero standard optical density. Plot the standard curve using Sigma plot software, with standard concentration on the x-axis and absorbance on the y-axis. Finally, the best-fit straight line was drawn through the standard points. The concentrations of all serum proteins detected were determined according to the standard curve.
2.3 Immunohistochemistry, IHC
Hematoxylin and eosin-stained sections of all formalin-fixed paraffin-embedded (FFPE) resected CRC tumor tissues were reviewed by two pathologists to confirm the presence of adequate lesional tissue for tissue microarray (TMA) construction and to evaluate pathological features including tumor histology, grade, abundance of tumor-infiltrating lymphocytes, vascular, and pattern of advancing border. Tissue microarrays were constructed using three 2-mm cores of representative tumor tissue from each case, along with multiple cores of non-neoplastic colonic tissue as controls. Immunohistochemistry (IHC) was performed on 6-µm section cut from TMA block using an automated staining platform (Bond Rx, Leica Microsystems, Bannockburn, IL, USA) using anti-PD-L1 antibody. The paraffin sections were deparaffinized with xylene and rehydrated through a graded alcohol series. Antigen retrieval was accomplished by boiling citrate buffer and endogenous peroxidase activity was blocked with 3% H2O2 followed by staining with anti-PD-L1 antibody (clone:E1L3N #13684, Cell Signaling Technology, Danvers, MA, USA) at 37°C for 90min at a dilution of 1:150. Bound antibody was then visualized using the EnVision KitTM (#K5007, Agilent, Santa Clara, CA, USA) according to the manufacturer’s directions. PD-L1 expression on tumor cells was evaluated using a three-tiered grading system: 0 =≤5% of the tumor cells; 1=549% of tumor cells; and 2=≥50% tumor cells with membranous staining of any intensity. Cytoplasmic staining was not considered in this study. Scores of 1 and 2 were considered to be positive for PD-L1 expression.
2.4 Follow-up
All CRC patients underwent regular follow-ups at our hospital every 3 months for the first 2 years, every 6 months in years 35, and annually after that. The primary endpoints were overall survival (OS) and disease-free survival (DFS). OS was calculated from the date of surgery to either the date of death or the last follow-up. DFS was defined as the time from surgery to the time of recurrence (local or distant) or the date of the last follow-up.
2.5 Statistical analysis
The 95% confidence interval (CI) of all results was calculated. Continuous variables were presented on Mean and Standard Deviation (SD) while categorical variables as Number (n) and Percentage (%). Survival analysis was conducted using the KaplanMeier method and Cox proportional hazard regression. The multivariate Cox model included all variables with P < 0.10 in the univariate model. Fisher’s exact test was used to assess the association of PD-L1 expression with clinicopathological features. MannWhitney U test was performed by SPSS software (version 25.0, IBM, Armonk, NY, USA). Statistical significance was determined when as P < 0.05.
3. Results
3.1 Patient characteristics
A total of 386 patients were included in the study. By the end of the follow-up period, 257 (66.58%) patients had relapsed, and 178 (46.1%) patients had died of cancer-related causes. The median DFS and OS times for the whole population were 44.8 months (95% CI 22.3-53.7 months) and 53.2 months (95% CI 38.6-58.1 months), respectively. A summary of patients characteristics is presented in Table 1.
|
Control Group (n=400) |
CRC without lymph node metastasis (n=194) |
CRC with lymph node metastasis (n=192) |
p-value |
|
|
Age, years |
||||
|
18~65 |
166 (41.50) |
81 (41.75) |
78 (40.63) |
0.958 |
|
>65 |
234 (58.50) |
113 (58.25) |
114 (59.37) |
|
|
Gender |
||||
|
male |
231 (57.75) |
112 (57.73) |
109 (56.77) |
0.589 |
|
female |
169 (42.25) |
82 (42.27) |
83 (43.23) |
|
|
Tumor size |
||||
|
<5.0cm |
- |
123 (63.40) |
119 (61.98) |
0.585 |
|
≥5.0cm |
- |
71 (36.60) |
73 (38.02) |
|
|
Tumor differentiation |
||||
|
Well |
- |
58 (29.90) |
57 (29.69) |
0.784 |
|
Moderate |
- |
108 (55.67) |
106 (55.21) |
|
|
Poor |
- |
28 (14.43) |
29 (15.10) |
|
|
T status |
||||
|
T1 |
- |
20 (10.31) |
19 (9.89) |
0.835 |
|
T2 |
- |
22 (11.34) |
23 (11.98) |
|
|
T3 |
- |
93 (47.94) |
92 (47.92) |
|
|
T4 |
- |
59 (30.41) |
58 (30.21) |
|
|
Stage |
||||
|
I |
- |
42 (21.65) |
41 (21.35) |
0.547 |
|
II |
- |
63 (32.47) |
64 (33.33) |
|
|
III |
- |
89 (45.88) |
87 (45.31) |
Table 1: Patient characteristics.
3.2 Serum PD-L1 levels in CRC patients
The serum level of PD-L1 was detectable in all patients and control group. The median values in control group, CRC without lymph node metastasis and CRC with lymph node metastasis were 229.22 (range 122.99390.6 pg/mL), 400.77 (range 300.89671.63 pg/mL) and 414.29 pg/mL (range 342.46594.63 pg/mL), respectively. We observed that the level of serum PD-L1 was significantly elevated in CRC patient compared with the control group, and serum PD-L1 level in CRC group with lymph node metastasis is higher than the CRC group without lymph node metastasis (all p<0.001, Figure 1).
3.3 Correlation between the serum PD-L1 levels and clinicopathological features
There is no correlation between the serum level of PD-L1 from patient with CRC and the size of tumor and degree of tumor differentiation (all p>0.05, Table 2). Serum PD-L1 level was significantly increased with the clinicopathologic staging (all p<0.001, Figure 2).
|
n |
PD-L1 [ ], pg/mL |
p-value |
|
|
Tumor size |
|||
|
<5.0cm |
242 |
367.63±53.2 |
0.088 |
|
≥5.0cm |
144 |
391.51±55.5 |
|
|
Tumor differentiation |
|||
|
Well |
115 |
370.36±58.7 |
0.341 |
|
Moderate |
214 |
381.12±61.5 |
|
|
Poor |
57 |
397.07±64.1 |
|
|
T status |
|||
|
T1 |
39 |
319.21±59.0 |
0.001 |
|
T2 |
45 |
340.56±53.1 |
|
|
T3 |
185 |
373.48±56.0 |
|
|
T4 |
117 |
427.13±57.9 |
|
|
Stage |
|||
|
I |
83 |
335.25±54.5 |
0.001 |
|
II |
127 |
371.92±58.7 |
|
|
III |
176 |
415.86±56.6 |
Table 2: Serum PD-L1 levels and clinicopathological features.
3.4 PD-L1 expression in primary tumor and metastatic lymph nodes
The positive rate of PD-L1 within the metastatic lymph nodes of CRC is significantly higher compared with the primary lesions, and the CRC without lymph node metastasis. PD-L1 positive rates from CRC without lymph node metastasis, primary lesions of CRC with lymph node metastasis, and metastatic lymph nodes of CRC were 57.73%, 70.83% and 88.02%, respectively (Table 3).
|
Location |
PD-L1 positive rate |
p-value |
|
|
CRC with lymph node metastasis (n=192) |
Primary lesions |
136 (70.83%) |
0.0028 |
|
CRC without lymph node metastasis (n=194) |
Tumor tissues |
112 (57.73%) |
|
|
CRC with lymph node metastasis (n=192) |
Metastatic lymph nodes |
169 (88.02%) |
0.001 |
|
CRC with lymph node metastasis (n=192) |
Primary lesions |
136 (70.83%) |
Table 3: Comparison of PD-L1 positive rate between CRC without lymph node metastasis and CRC with lymph node metastasis.
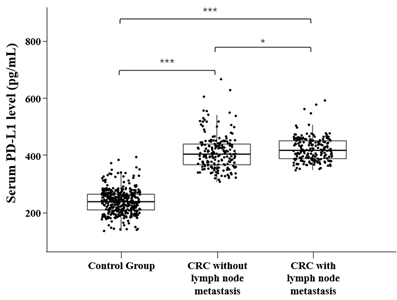
Figure 1: Serum PD-L1 levels in Control Group and CRC patients.
*p < 0.05, ***p < 0.001, Mann–Whitney U test
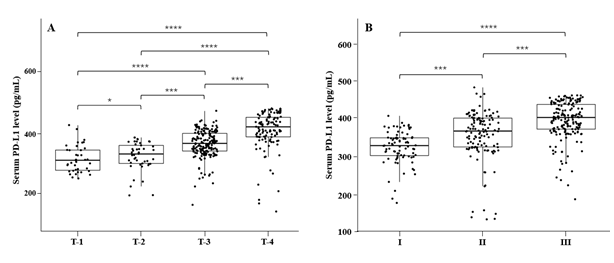
Figure 2: Correlation beteen serum PD-L1 levels and clinicopathological features
*p < 0.05, ***p < 0.001, ****p < 0.0001, Mann–Whitney U test
3.5 Association of PD-L1 expression and prognosis of CRC patients
The five-year survival rate of CRC patients enrolled in this study (n=386) was 53.89% (208/386), and the median survival time was 56.8 months. CRC patients with positive PD-L1 had less median survival time (44.8 months) and lower five-year survival rate (46.98%) compared with the PD-L1 negative patients that had 53.2 months of median survival time and 68.57% of five-year survival rate. The Cox proportional hazards regression analysis showed that the tumor sizes, serum PD-L1 levels and clinicopathologic stages were not correlated with the CRC prognosis (all p>0.45, Table 4). However, the univariate analysis indicated that the PD-L1 expression on tumor cells, the degree of tumor differentiation, and lymph node metastasis were statistically associated with the prognosis of CRC patient (all p<0.02, Table 4). The multivariate analysis further demonstrated that the PD-L1 expression on tumor cells and lymph node metastasis were independent prognostic factors of CRC (all p<0.005, Table 4).
|
HR (95% CI) |
p-value |
|
|
Univariate analysis |
||
|
Tumor size |
0.92 (0.474~1.234) |
0.465 |
|
Tumor differentiation |
1.68 (1.264~2.456) |
0.017 |
|
T-status |
1.10 (0.842~1.876) |
0.512 |
|
P-stage |
1.37 (0.453~2.764) |
0.654 |
|
Lymph node metastasis |
3.08 (2.358~4.852) |
0.005 |
|
PD-L1 expression on tumor cells |
1.83 (1.118~3.468) |
0.006 |
|
Serum PD-L1 levels |
1.12 (0.718~2.071) |
0.508 |
|
Multivariate analysis |
||
|
Lymph node metastasis |
1.79 (1.124~2.276) |
0.006 |
|
PD-L1expressiom on tumor cells |
1.77 (1.149~2.877) |
0.007 |
Table 4: Prognosis analysis (Cox proportional hazards model).
4. Discussion
In present study, we performed a comprehensive analysis of PD-L1 in a cohort of CRC including the patients with lymph node metastasis (n=192) and the patients without lymph node metastasis (n=194), together with a healthy control (n=400). Notably, the examination of PD-L1 levels in serum revealed that serum PD-L1 was significantly elevated in CRC patient compared with the healthy control. Thus, our observations have the implication that serum PD-L1 may be served as a potential biomarker for the diagnosis of CRC, together with other markers analyzed in currently clinical practices including CEA, CA19-9 and CA24-2 [28]. We didn’t identify the prognosis value of serum PD-L1 in present study although a few reports had addressed the association of serum PD-L1 with the prognosis of patients, including the study with contrasting result in lung cancer [25-26] and no correlation in gastric cancer and nasopharyngeal carcinoma [29]. Serum PD-L1 may act as a paracrine negative immune regulator within the tumor [30], and derive from protumor inflammatory responses, antitumor immune-responses and intrinsic splicing activities in tumor cells.
Our results also demonstrated that the level of serum PD-L1 is higher in CRC patients with lymph node metastasis compared to CRC patients without lymph node metastasis. We found that serum PD-L1 isn’t associated with clinical characteristics such as age, gender, tumor size and tumor differentiation, and prognosis. Serum PD-L1 is more a general marker of an inflammatory status than just a marker of active, immuno-suppressive cancer cells. It reflects the function of PD-L1 expressed in inflamed tissues. However, serum PD-L1 contributes to immune regulation together with PD-L1 on tumor cells. Thus, additional studies on the role of serum PD-L1 are needed to prob the diagnosis potential for early detection, predictive value for treatment response and prognostic function in CRC.
PD-L1 expression on tumor cells has been recognized as a key factor of immune evasion through down-regulation of the active T-cell-mediated immune response [4-5]. Our study showed that PD-L1 expression on tumor cells and lymph node metastasis, as the two independent prognostic factors of CRC, were significantly associated with a poor prognosis. Higher risk of immune evasion is attribute to the PD-L1 expression and lymphocyte attack. Early study suggests that PD-L1 expression is the consequence of higher oncogenic signaling in some CRC. This is in line with a previous study reporting up-regulation of PD-L1 expression by EGFR activation and BRAFV600E [31-32]. We also identified that patients with negative PD-L1 had better five-year survival rate and median survival time in comparison with the PD-L1 positive patients regardless the tumor size, clinicopathologic stage and serum PD-L1 level. To our best knowledge, most of the published works primarily addressed the prognostic relevance of PD-L1, whereas little is known about their predictive value as well as their relationship with molecular genetic alterations in CRC. The identification of patients that are likely to receive a benefit from PD-1/PD-L1 inhibition therapy remains a challenge. The lack of detection of common CRC related genes including EGFR, KRAS, BRAF and p53 in tumor together with CEA, CA19-9 and CA24-2 in serum is the limitation of this study.
Our future work will focus on the integration of PD-L1 level in serum and PD-L1 expression on tumor cells in a separate cohort to investigate the role of serum PD-L1 on diagnosis for early detection of CRC, prediction to the therapy, risk evaluation of lymph node metastasis, and prognosis of patients. The examination of CRC related genes and common serum biomarkers mentioned above will also be taken to support new finding and elucidate the molecular mechanism of therapeutics.
Acknowledgments
The authors are grateful to Linzhen Song for excellent assistance in data analysis.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethical Approval and Ethical Standards
This study was approved by ChangXing People Hospitals’s Ethics Committee (no. 2019ZLK-072 dated July 30, 2016) and conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Informed Consent
All patients gave written informed consent for the collection of tissue and blood for research.
Author Contributions
WS, YX, SL, HW: protocol/project development.
JG, YX: data collection and management.
HW, WS: data analysis.
HW: manuscript writing/editing.
All authors contributed to the article and approved the submitted version.
References
- Rebecca LS, Kimberly DM, Hannah EF, et al. Cancer statistics, 2022. CA Cancer J Clin 72 (2022): 7-33.
- Franke AJ, Skelton WP, Starr JS, et al. Immunotherapy for Colorectal Cancer: A Review of Current and Novel Therapeutic Approaches. J Natl Cancer Inst 111 (2019): 1131-1141.
- Vatandoust S, Price TJ, Karapetis CS. Colorectal cancer: Metastases to a single organ. World J. Gastroenterol 21 (2015): 11767-11776.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12 (2012): 252264.
- Dermani FK, Samadi P, Rahmani G, et al. PD-1/PD-L1 immune checkpoint: Potential target for cancer therapy. J Cell Physiol 234 (2019): 1313-1325.
- Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity 48 (2018): 434-452.
- Gandini S, Massi D and Mandalà M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Crit Rev Oncol Hematol 100 (2016): 88-98.
- Sánchez-Magraner L, Miles J, Baker CL, et al. High PD-1/PD-L1 Checkpoint Interaction Infers Tumor Selection and Therapeutic Sensitivity to Anti-PD-1/PD-L1 Treatment. Cancer Res 80 (2020): 4244-4257.
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 378 (2018): 1976-1986.
- Chalabi M, Fanchi LF, Dijkstra KK, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med 26 (2020):566-576.
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 382 (2020):1894-1905.
- Myint ZW, Goel G. Role of modern immunotherapy in gastrointestinal malignancies: A review of current clinical progress. J Hematol Oncol 10 (2017): 1-12.
- Taube JM, Young GD, McMiller TL, et al. Differential Expression of Immune-Regulatory Genes Associated with PD-L1 Display in Melanoma: Implications for PD-1 Pathway Blockade. Clin Cancer Res 21 (2015): 3969-3976.
- Hussain SA, Birtle A, Crabb S, et al. From Clinical Trials to Real-life Clinical Practice: The Role of Immunotherapy with PD-1/PD-L1 Inhibitors in Advanced Urothelial Carcinoma. Eur Urol Oncol 1 (2018):486-500.
- Toh JWT, de Souza P, Lim SH, et al. The Potential Value of Immunotherapy in Colorectal Cancers: Review of the Evidence for Programmed Death-1 Inhibitor Therapy. Clin Colorectal Cancer 15 (2016): 285-291.
- Ganesh K, Stadler ZK, Cercek A, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol 16 (2019): 361-375.
- Rosenbaum MW, Bledsoe JR, Morales-Oyarvide V, et al. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod Pathol 29 (2016):1104-1112.
- Shiraliyeva N, Friedrichs J, Buettner R, et al. PD-L1 expression in HNPCC-associated colorectal cancer. Pathol Res Pract 213 (2017): 1552-1555.
- Bae SU, Jeong WK, Baek SK, et al. Prognostic impact of programmed cell death ligand 1 expression on long-term oncologic outcomes in colorectal cancer. Oncol Lett 16 (2018): 5214-5222.
- Gatalica Z, Snyder C, Maney T, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev 23 (2014): 29652970.
- Finkelmeier F, Canli Ö, Tal A, et al. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer 59 (2016): 152-159.
- Asanuma K, Nakamura T, Hayashi A, et al. Soluble programmed death-ligand 1 rather than PD-L1 on tumor cells effectively predicts metastasis and prognosis in soft tissue sarcomas. Sci Rep 10 (2020):9077.
- Ito M, Yajima S, Suzuki T, et al. High serum PD-L1 level is a poor prognostic biomarker in surgically treated esophageal cancer. Cancer Med 9 (2020):1321-1327.
- Liao G, Zhao Z, Qian Y, et al. Prognostic Role of Soluble Programmed Death Ligand 1 in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Front Oncol 11 (2021): 774131.
- He J, Pan Y, Guo Y, et al. Study on the Expression Levels and Clinical Significance of PD-1 and PD-L1 in Plasma of NSCLC Patients. J Immunother 43 (2020): 156164.
- Okuma Y, Hosomi Y, Nakahara Y, et al. High plasma levels of soluble programmed cell death ligand 1 are prognostic for reduced survival in advanced lung cancer. Lung Cancer 104 (2017): 16.
- Takahashi N, Iwasa S, Sasaki Y, et al. Serum levels of soluble programmed cell death ligand 1 as a prognostic factor on the first-line treatment of metastatic or recurrent gastric cancer. J Cancer Res Clin Oncol 142 (2016): 17271738.
- Carpelan-Holmstrom M, Louhimoet J, Stenman UH, et al. CEA, CA 242, CA 19-9, CA 72-4 and hCGbeta in the diagnosis of recurrent colorectal cancer. Tumour Biol 25 (2004): 228-234.
- Takahashi N, Iwasa S, Sasaki Y, et al. Serum levels of soluble programmed cell death ligand 1 as a prognostic factor on the first-line treatment of metastatic or recurrent gastric cancer. J Cancer Res Clin Oncol 142 (2016): 1727-1738.
- Mahoney KM, Shukla SA, Patsoukis N, et al. A secreted PD-L1 splice variant that covalently dimerizes and mediates immunosuppression. Cancer Immunol. Immunother 68 (2019): 421432.
- Chen N, Fang W, Zhan J, et al. Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients with EGFR Mutation. J Thorac Oncol 10 (2015): 910923.
- Feng D, Qin B, Pal K, et al. BRAFV600E-induced, tumor intrinsic PD-L1 can regulate chemotherapy-induced apoptosis in human colon cancer cells and in tumor xenografts. Oncogene. 38 (2019): 67526766.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 74.74%
Acceptance Rate: 74.74%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 