Vitamin D status in immigrants in Norway
Muhammad M Ahmed1, Haakon E Meyer1,2 Mette Brekke3 and Ahmed A Madar1*
1Department of Community Medicine and Global Health, Institute of Health and Society, University of Oslo, 0318 Oslo, Norway
2Division of Mental and Physical Health, Norwegian Institute of Public Health, 0213 Oslo, Norway
3General Practice Research Unit, Institute of Health and Society, University of Oslo, 0318, Oslo, Norway
*Corresponding Author: Ahmed A Madar, Department of Community Medicine and Global Health, Institute of Health and Society, University of Oslo, 0318 Oslo, Norway
Received: 26 February 2021; Accepted: 06 March 2021; Published: 25 March 2021
Article Information
Citation: Muhammad M Ahmed, Haakon E Meyer, Mette Brekke and Ahmed A Madar. Vitamin D status in immigrants in Norway. Journal of Food Science and Nutrition Research 4 (2021): 023-036.
DOI: 10.26502/jfsnr.2642-11000059
View / Download Pdf Share at FacebookAbstract
Background: High prevalence of poor vitamin D status has been reported among immigrants in Norway, but there is limited information on vitamin D status among immigrants from the Middle East and sub-Saharan Africa.
Aim: The aim of this study was to describe vitamin D status and potentially associated factors among adults with different regional backgrounds.
Methods: Its cross-sectional study conducted at different immigrant centers in Oslo and adjacent municipalities. Participants: 251 adults (18-50 years) with Middle Eastern, sub-Saharan African and South Asian backgrounds were recruited from January-March 2011. Venous blood samples were collected and serum 25-hydroxyvitamin D (s-25(OH)D) and parathyroid hormone (p-PTH) were analyzed in one batch.
Results: The overall mean s-25(OH)D was 28.9 nmol/l (range 5 to 130 nmol/l). Over 90% had a serum concentration below 50 nmol/L, while around 9% had below 12.5 nmol/l (females 7% and males 2%). Median p-PTH level was within reference values, however, 31% were above the reference value. The mean s-25(OH)D was higher among those with sub-Saharan African backgrounds compared to the other two geographical groups. Sociodemographic factors such as gender and education did not influence s-25(OH)D levels. There was no association between s-25(OH)D levels and diet, sun exposure or clothing habits in males or females.
Conclusion: This study revealed that immigrants with South Asian, Middle Eastern and Sub-Saharan African backgrounds in Oslo have low s-25(OH)D levels. Measures should be taken to ensure adequate vitamin D intake among immigrant groups throughout the year.
Keywords
<p>25-Hydroxyvitamin D, S-25(OH)D levels, Immigrants, Norway</p>
Article Details
1. Introduction
Vitamin D is a fat-soluble steroidal prohormone which is involved in calcium homeostasis and is required for normal bone formation and mineralization. The main sources of vitamin D come from selected food commodities, such as fatty fish and fortified food, from supplements and from cutaneous synthesis. For cutaneous synthesis of vitamin D, ultraviolet B exposure is required [1].Vitamin D deficiency is a worldwide problem and is related to numerous hazardous health risks [2-5]. Recently, its potential role in the prevention and treatment of infections has also been highlighted. In a meta-analysis of 25 randomised controlled trials, it was reported that the risk of acute respiratory tract infections was reduced in those receiving vitamin D supplements, and s-25(OH)D deficient individuals supplemented with s-25(OH)D supplements showed strongest effect of such supplements in such risk reduction [6].
In the Nordic countries, immigrants with South Asian, Middle Eastern and Sub-Saharan African backgrounds have poor s-25(OH)D levels and have much lower s-25(OH)D levels compared to their Nordic peers [7-11]. In Norway, high prevalence of vitamin D deficiency (s-25(OH)D) below 25 nmol/L) has been reported among immigrants [12-16]. The main causes for their poor s-25(OH)D levels are attributed to low consumption of vitamin D–rich foods, low intake of vitamin D supplements and low ultraviolet B exposure [13-17]. Serum concentration of 25-hydroxyvitamin D (s-25(OH)D) is the standard assessment of vitamin D status in the body. However, there is no universal standard for the normal cut-off points for s-25(OH)D. To facilitate comparison, we chose to use a s-25(OH)D concentration <12.5 nmol/L as severely deficient nmol/L, a concentration <25 nmol/L as moderate deficiency, and a concentration ≥50 nmol/L as sufficient [18].
Existing information on s-25(OH)D levels in subgroups of the immigrant population in Norway is limited, particularly in those from the Middle East and Sub-Saharan Africa [19]. According to SSB Norway statistics 2020, 18.2 % of the Norwegian population comprises of immigrants and Norwegians born to immigrant parents, a substantial portion of which have the same geographical background as the present study population. The aim of this study is to assess the s-25(OH)D levels and associated factors among immigrants with South Asian, Middle Eastern and Sub-Saharan African backgrounds currently living in Norway.
2. Materials and Methods
2.1 Study design and participants
We present here an extended analysis of baseline data of a randomized double-blinded placebo-controlled study among immigrants with South Asian, Middle Eastern and Sub-Saharan African backgrounds presently living in Norway [14]. As reported previously [14], adults with geographical origins from the Middle East, Sub-Saharan Africa and South Asia were recruited to participate in the study in Oslo and adjacent municipalities (at a latitude of 60°N) between January and March 2011. The study was conducted in 11 different local immigrants’ activity centers. Information meetings were organized with leaders in different immigrant organizations. Detailed information about the study was advertised at various arenas including mosques, immigrant organizations, Tamil resources and immigrant’s local activity centers. Additional recruitment of participants was done at Health Centers with high a proportion of immigrant visitors. Those who agreed to participate and fulfilled the eligibility criteria provided written informed consent.
2.2 Inclusion and exclusion criteria
Healthy individuals aged between 18 and 50 years and who were either born in or had parents born in South Asia, Sub-Saharan Africa, or the Middle East were eligible to participate in the study. Pregnant women and patients with malabsorption, kidney problems, cancer, tuberculosis, sarcoidosis, osteoporosis or recent fracture were excluded. Persons treated for medical conditions were excluded from the study, as were individuals receiving hormone replacement therapy. Other exclusion criteria were use of vitamin D supplements more than four times per week and use of tanning beds in the three months prior to study; however, no participants were excluded for these reasons.
2.3 Data collection
The collection of background information and anthropometric measurements of the study group has been described in detail previously [14]. In brief, background information on age, gender, country background, and how long they have lived in Norway were collected. Weight and height were measured by trained personnel, with participants wearing light clothing for accurate estimation of body weight. An interviewer-administered questionnaire was used to collect the data regarding background information, dietary habits and sun exposure patterns. The same team collected all the data in the different centers. Communication with participants was in Norwegian language, with interpreters provided when needed.
2.4 Diet
Information about dietary intake of vitamin D and supplements was obtained through a simple interviewer-administered food frequency questionnaire (FFQ) which was pilot tested among the target population. The FFQ included questions about consumption of vitamin D-containing foods (fish, eggs, butter, margarine, and fortified food) and frequency of consumption. In Norway, all butter and margarine are fortified with vitamin D (0,4 µg D-vitamin per 100 gram). Only one type of milk (extra skimmed milk) is fortified with vitamin D (1 µg D-vitamin per 100 gram), and frequency of its consumption and number of glasses consumed was noted. The use of supplements such as cod liver oil, fish capsules and vitamin D containing tablets, including the frequency and number of tablets or spoons taken each time, was noted.
2.5 Sun exposure
Information about dressing habits, such as use of a burka, hijab, niqab and long-sleeved clothes, and sun bathing during summer months was included in the questionnaire. For sun bathing duration (less than 10 minutes and more than 10 minutes per day) was also noted.
2.6 Blood sampling and analysis
Blood samples were collected from the study participants after inclusion. All non-fasting venous blood samples were taken by the same biochemist. Serum and plasma were separated and frozen in several aliquots at -20°C at the same day and within 1-2 weeks all samples were stored at -80°C until they were analyzed in one batch at Fürst Medical Laboratory (www.furst.no). The Fürst Medical Laboratory is accredited by the International Organization for Standardization and is part of the vitamin D quality assessment scheme (DEQAS).
Levels of s-25(OH)D were analyzed with high-pressure liquid chromatography tandem mass spectrometry (HPLC-MS-MS) with the Waters Acquity UPLC and Waters triple quadrupole MS instruments. In-house standards at four levels ranging from 25–200 nmol/L were calibrated against external MS-standards from Recipe (Germany), product no. MS7013, traceable to National Institute of Standards and Technology (NIST). A deuterised internal standard with C26,27 hexadeuterium-labelled 25(OH) D3, which was purchased from Synthetica (Norway) was used to calculate both 25(OH) D2 and 25(OH) D3. The CV (reproducibility within the laboratory, 4 instruments) for serum 25(OH) D3 was 8% at a concentration of 55.2 nmol/L and 6% at a concentration of 195.1 nmol/L. Both s-25(OH)D2 and -D3 were measured. However, s-25(OH)D2 levels were generally negligible, and we combined measures for s-25(OH)D2 and -D3 and presented as s-25(OH)D overall in the analysis. Plasma parathyroid hormone (PTH) was analyzed with chemiluminescence (Advia Centaur XP; Siemens HMSD, USA) with an analytical coefficient of variation of 8.2% and reference range of 1.2–8.4 pmol/L.
2.7 Ethics
The study was approved by the Regional Committee for Medical and Health Research Ethics, including an approval to establish a biobank. (study code: 2010/1982). All participants provided written informed consent.
2.8 Statistical analysis
Sample size was based on prior research investigating the effect of vitamin D supplementation on muscle strength and power (12). Analysis of the data was performed using IBM SPSS statistical software (V.22 SPSS Inc., Chicago, IL, USA). Descriptive statistics are presented as means and standard deviations. A linear regression model was used to access association between potential predictors and s-25(OH)D. Results were considered statistically significant at a p-value below 0.05. As the assumptions for the independent samples t-test were not met, non-parametric alternatives including the Mann-Whitney U test and Kruskal-Wallis test were used. Statistical analysis was done using Spearman’s bivariate correlation, ANOVA and linear regression models.
3. Results
301 healthy individuals aged 18-50 years showed interest of participation and were screened for eligibility of the study, and 50 persons did not meet the inclusion criteria. Ultimately, 251 persons were included in the study.
The study participants (n=251) had a mean age of 37 years (SD 8). Most study participants were female (n=182, 72.5%), and those with Sub-Saharan African backgrounds dominated (n=120, 47.8%) among the study participants. Only six persons (all female) were born in Norway, and the mean age among this group was 25 years. Basic characteristics of the study participants are given in table 1.
|
South Asia N=95 |
Middle East N=36 |
Sub-Sahara Africa N=120 |
All N=251 |
|
|
Age, years (mean, SD) |
40(5) |
37(8) |
35(9) |
37 (8) |
|
Gender, female (n, %) |
55 (58) |
34 (94.5) |
93 (77.5) |
182(72.5) |
|
Women |
||||
|
Education, years (n, %) |
||||
|
Up to 10 years |
8 (14.5) |
20 (58.8) |
57 (61.2) |
85 (46.7) |
|
Time lived in Norway, years (mean, SD) |
13.7 (5.9) |
14.7 (8.4) |
10.9 (4.6) |
12.5 (6.1) |
|
Vitamin D supplements (n, %)† |
||||
|
Yes |
17 (30.9) |
8 (23.5) |
21 (22.5) |
46 (25.3) |
|
Fatty fish intake ≥twice a week (n, %) |
29 (52.4) |
10 (29.4) |
26 (27.9) |
65(35.7) |
|
Using butter or margarine daily (n, %) |
42 (76.3) |
25 (73.5) |
72 (77.4) |
139(76.3) |
|
Using fortified milk daily (n, %) |
3 (5.45) |
0 |
5 (4.1) |
8 (3.2) |
|
>10 min exposing sun on face/arms (n, %) |
32 (58) |
21 (62) |
43 (46) |
96 (53) |
|
Body mass index (kg/m2) |
26.6 (3.7) |
27.8 (5.8) |
29.3 (5.8) |
28.2 (5.4) |
|
Mostly covered (n, %)≠ |
9 (16.3) |
19 (55.8) |
68 (73.11) |
96 (52.7) |
|
Gender, male (n, %) |
40 (42) |
2 (5.5) |
27 (22.5) |
69 (27.5) |
|
Men |
||||
|
Education, years (n, %) |
||||
|
Up to 10 years |
14 (35) |
2 (100) |
2 (7.4) |
18 (26.1) |
|
Time lived in Norway, years (mean, SD) |
19.7 (4.9) |
19 (5.6) |
9.4 (6.5) |
15.6 (7.5) |
|
Vitamin D supplements (n, %)† |
||||
|
Yes |
13 (32.5) |
1 (50) |
8 (29.5) |
22 (31.9) |
|
Fatty fish intake ≥twice a week (n, %) |
19 (47.5) |
0 |
4 (14.8) |
23 (33.3) |
|
Using butter or margarine daily (n, %) |
29 (72.5) |
1 (50) |
21 (77.7) |
51 (73.9) |
|
Using fortified milk daily (n, %) |
1(2.5) |
0 |
2 (7.4) |
3 (4.3) |
|
>10 min exposing sun on face/arms (n, %) |
22 (55) |
2 (100) |
11 (41) |
35 (51) |
|
Body mass index (kg/m2) |
25.9 (2.7) |
31.9 (0.9) |
23.9 (4.5) |
25.3 (3.7) |
* Data are mean (SD) unless specified otherwise
†Amount of vitamin D from supplements consumed by the participants over 7 days was not calculated # Cover, head, arms, and legs with clothes
Table 1: Baseline characteristics of men and women aged 18-50 years (N=251) with South Asian, Middle Eastern and Sub-Saharan African backgrounds living in Norway (January-March 2011)*.
The participants had been living in Norway for a mean duration of 13.3 years, and approximately 41% have up to 10 years while 21% had higher education (university level). Nearly half of participants reported consumption of fatty fish more than twice per week. Only 11 (4%) of the study participants reported use of vitamin D fortified milk.
More than 52% of the participants reported that they spent more than 10 minutes/day outdoors while exposing sun directly on their face and/or arms during the summertime. Around 50% of 182 female participants (30.2% South Asians, 7.2% Middle Eastern and 12% Sub-Saharan African) reported covering their whole body, including legs, hands, and face, while outside their houses. Two thirds of participants reported use of fortified butter and margarine daily.
3.1 S-25(OH)D levels
S-25(OH)D concentration ranged from 5 to 130 nmol/L, with a mean of 28.9 nmol/l (SD 18.7). Overall, 90% of the study participants had a s-25(OH)D level below 50 nmol/l, and 54 and 55% of females and males, respectively, had a s-25(OH)D level below 25 nmol/l. Table 2 describes S-25(OH)D levels based on s-25(OH)D levels and other biochemical results among study participants. There was no significant difference of mean s-25(OH)D levels between male and females. However, the lowest s-25(OH)D was seen among women with Middle Eastern backgrounds. A statistically significant difference in s-25(OH)D was found between groups, with those from Sub-Saharan Africa having the highest mean s-25(OH)D levels compared to the other two geographical groups (P=0.002). The mean concentration of p-PTH was 7.8 (SD 3.8) pmol/l and 7.1 (SD 3.6) pmol/l for females and males, respectively. This was within reference values (1.2–8.4 pmol/L), but 31% (32 and 27 % of females and males) were above the upper reference value. p-PTH levels were comparable across the three geographical groups, and there was no significant difference in mean and median values of p-PTH across the three geographical groups.
|
South Asian N= 95 |
Middle Eastern N= 36 |
Sub-Saharan Africa N=120 |
All N= 251 |
|
|
Women |
N =55 |
N=34 |
N=93 |
N = 182 |
|
S-25(OH)D (nmol /L), mean (SD) |
25.4 (14.6) |
21.3 (11.8) |
34.8 (23.3) |
29.4 (19.9) |
|
S-25(OH)D (nmol /L) n (%) |
||||
|
≥ 50 |
3 (5.5) |
2 (5.9) |
16 (17.2) |
21 (11.5) |
|
<50 |
17 (30.9) |
7 (20.6) |
40 (43) |
64 (35.2) |
|
<25 |
31 (56.4) |
20 (58.8) |
28 (30.1) |
79 (43.4) |
|
<12.5 |
4 (7.3) |
5 (14.7) |
9 (9.7) |
18 (9.9) |
|
Plasma PTH (pmol/l), mean (SD)* |
6.3 (2.5) |
7.5 (2.9) |
8.9 (4.4) |
7.8 (3.8) |
|
S-Calcium, (mmol/l) mean (SD)# |
2.3 (0.08) |
2.3 (0.09) |
2.3 (0.09) |
2.3 (0.09) |
|
Men |
N=40 |
N=2 |
N=27 |
N=69 |
|
S-25(OH)D (nmol /L), mean (SD) |
26.4 (12.1) |
35 (17) |
28.8(18.9) |
27.6 (15.1) |
|
S-25(OH)D (nmol /L) n (%) |
||||
|
≥ 50 |
2(5) |
- |
3 (11.1) |
5 (7.2) |
|
<50 |
16(40) |
1(50) |
10 (37) |
27 (39.1) |
|
<25 |
21(52.5) |
1(50) |
10 (37) |
32 (46.4) |
|
<12.5 |
1(2.5) |
- |
4 (14.8) |
5 (7.2) |
|
Plasma PTH (pmol/l), mean (SD)* |
6.9 (3.1) |
5 (0.9) |
7.7 (4.3) |
7.1 (3.6) |
|
S-Calcium, (mmol/l) mean (SD)# |
2.3 (0.1) |
2.3 (0.02) |
2.4 (0.09) |
2.3 (0.09) |
*Reference range: plasma PTH: 1.2–8.4 pmol/L,
#Reference range: serum calcium 2.15–2.51 mmol/L
Table 2: S-25(OH)D levels based on s-25(OH)D concentration among men and women aged 18-50 years (N=251) with South Asian, Middle Eastern and Sub-Saharan African backgrounds living in Norway (January-March 2011).
3.3 Factors associated with s-25(OH)D
3.3.1 Diet We did not find any significant association between dietary factors such as fatty fish, fortified milk, butter/ margarine, and s-25(OH)D levels (table 3a and 3b). Further, despite of the fact that 75.7% of the study participants reported use of vitamin D fortified butter and margarine daily, we did not find any association between its use and s-25(OH)D levels.
|
Explanatory variables |
Difference in |
95% CI |
P-values |
Difference in s-25(OH)D (nmol/l)†† |
95% CI |
P-values |
|
s-25(OH)D (nmol/l)† |
||||||
|
Fish intake* |
-1.3 |
(-7.2, 4.5) |
0.66 |
-1.7 |
(-7.7, 4.4) |
0.58 |
|
< 2 times per week to > 2 times per week |
||||||
|
Fortified milk# |
7.5 |
(-6.7, 21.8) |
0.3 |
8.5 |
(-5.9, 23) |
0.25 |
|
No/Yes |
||||||
|
Butter/margarine‡ |
2.9 |
(-4, 9.8) |
0.41 |
3.5 |
(-3.5, 10.5) |
0.32 |
|
No/Yes |
||||||
|
>10 min exposing sun on face/ arms|| |
4 |
(-2.5, 10.6) |
0.23 |
2.7 |
(-2.9, 8.4) |
0.35 |
|
Veiled¶ |
5.8 |
(0.0, 11.6) |
0.04 |
0.3 |
(-6.8, 7.3) |
0.94 |
|
No/Yes |
||||||
|
BMI |
0.4 |
(-0.2, 0.9) |
0.2 |
-0.01 |
(-0.63, 0.63) |
0.9 |
|
Education‡‡ |
1.3 |
(-6.0, 8.7) |
0.72 |
1 |
(-6.6, 8.6) |
0.79 |
|
≤ 10 years compared to > 10 years |
||||||
|
South Asian |
-9.4 |
(-17.8 , -3.1) |
0.004 |
-10 |
(-16.3, -3.8) |
0.002 |
|
compared to Sub- Saharan Africa |
||||||
|
Middle Eastern compared to Sub-Saharna Africa |
-13.5 |
(-20.9, -6.0) |
<0.001 |
-13.7 |
(-20.9, -6.4) |
<0.001 |
*Fish Intake coded as 0=Less than 2 times per week,1= More than 2 times per week
#Fortified milk coded as 0=No, 1=Yes‡ Butter/margarin 0=No, 1=Yes
||10 min exposing sun on face/arms coded as 0= No, 1=Yes.
¶Veiled coded as 0=No, 1=Yes
‡‡Education coded as 1≤ 10 years, 2> 10 years
†Unadjusted regression coefficient by Linear Regression
††Adjusted regression coefficient for age by Linear Regression
Table 3a: Association between s-25(OH)D (nmol/l) and potentially associated factors, women (n=182) (B estimates and 95% con?dence intervals).
|
Explanatory variables |
Difference in s-25(OH)D (nmol/l)† |
95% CI |
P-values |
Difference in s-25(OH)D (nmol/l)†† |
E95% CI |
P-values |
|
Fish intake* |
-1.8 |
(-9.1, 5.5) |
0.62 |
-2.6 |
(-10.1, 5.0) |
0.5 |
|
< 2 times per week to > 2 times per week |
||||||
|
Fortified milk# |
6 |
(-11.9, 23.9) |
0.5 |
5.7 |
(-12.9, 24.2) |
0.54 |
|
No/Yes |
||||||
|
Butter/margarine‡ |
2 |
(-6.3, 10.3) |
0.62 |
2.5 |
(-6.1, 11) |
0.57 |
|
No/Yes |
||||||
|
>10 min exposing sun on face/ arms|| |
-0,7 |
(-0.06, -0.43) |
0.67 |
-0.13 |
(-7.1, 6.8) |
0.97 |
|
Veiled¶ |
2.5 |
(-15.4 , 20.4) |
0.78 |
1.5 |
(-14.8 , 17.3) |
0.88 |
|
No/Yes |
||||||
|
BMI |
0.22 |
(-0.75, 1.2) |
0.65 |
0.06 |
(-0.99, 1.1) |
0.9 |
|
Education‡‡ |
-6.7 |
(-13.4, 1.0) |
0.09 |
-6.4 |
(-13.9, 1.2) |
0.1 |
|
≤ 10 years compared to > 10 years |
||||||
|
South Asia |
-2.5 |
(-9., 4.8) |
0.44 |
-7.2 |
(-16.2, 1.8) |
0.12 |
|
compared to Sub- Sahara Africa |
||||||
|
Middle East compared to Sub-Sahara Africa |
6.1 |
(-15.2, 27.5) |
0.32 |
0.09 |
(-22.0, 22.1) |
0.99 |
*Fish Intake coded as 0=Less than 2 times per week,1= More than 2 times per week
#Fortified milk coded as 0=No, 1=Yes
‡Butter/margarine 0=No, 1=Yes
||10 min exposing sun on face/arms coded as 0= No, 1=Yes.
¶Veiled coded as 0=No, 1=Yes
‡‡Education coded as 1= up to 10 years, 2= Above 10 years
†Unadjusted regression coefficient by Linear Regression
††Adjusted regression coefficient for age by Linear Regression
Table 3b: Association between s-25(OH)D (nmol/l) and potentially associated factors, men (n=69) (B estimates and 95% con?dence intervals).
3.3.2 Sun exposure We did not find any significant association between daily sun exposure with s-25(OH)D for female and male study participants, including when adjusted for age (Table 3). Non-veiled women had slightly lower s-25(OH)D levels compared to those who reported being veiled (27.4, and 32.1 nmol/l), but this was not significant (p= 0.15). There was a significant association between geographical background and s-25(OH)D levels which persisted after adjustment for age among females. There was no significant association among the male participants of the study. Higher education (university level) was not associated with with s-25(OH)D among study participants (p=0.24). There was no signi?cant association between s-25(OH)D levels, length of stay in Norway or BMI in both females and males in the study participants. The lack of association persisted across the different geographical groups.
3.3.3 S-25(OH)D and p-PTH There was a signi?cant negative correlation between s-25(OH)D and p-PTH for males (Spearman correlation, r -0.3, P=0.01) but it did not reach significance in females (Spearman correlation, r -0.12, P=0.1). The same trend was seen among females and males in each of the three different geographical areas. Furthermore, p-PTH concentration began to rise between 20-25nmol/L s-25(OH)D levels (Figure 1).
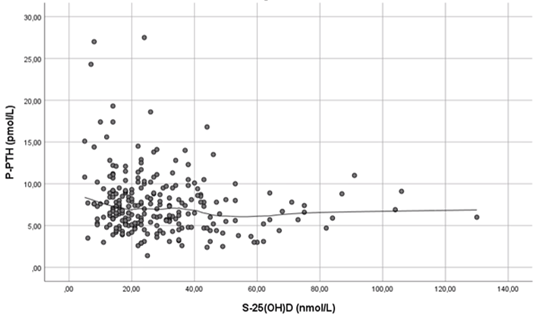
Figure 1: Association (Spearman correlation; r -0.17, P=0·009) between s-25(OH)D and p-PTH Line was fitted using losess in SPSS version 25(SPSS Inc., Chicago IL, USA).
4. Discussion
In this study, we found that both male and female immigrants with South Asian, Middle Eastern and Sub-Saharan African backgrounds in Norway had poor s-25(OH)D levels. The mean s-25(OH)D concentration was 28.6 nmol/l for the whole group, in line with findings from previous similar studies among immigrants in Norway [12, 13, 15, 20, 21]. Elevated p-PTH was also common among study participants, and there was an inverse association between s-25(OH)D and p-PTH in both women and men. This is also in line with similar studies among non-Western immigrants in Scandinavian counties [22-24] and in elderly populations in China [25] and Lebanon [26]. The study population is relatively young, with an average study population age of only 37 years. We did not find an association between age and s-25(OH) D across the geographical groups, nor for length of stay in Norway. This is line in with what was previously found among immigrants in Norway [14]. Also in line with previous studies, we did not find an association between s-25(OH)D and consumption of fatty fish [13, 22, 27], fortified extra low fat milk [28, 29], butter and margarine [29], education or BMI [30]. We did not find associations between sun exposure and s-25(OH)D status in immigrants in Norway. This is in line with previously reported findings among immigrant women in Norway [22]. This might be attributed to the fact that daily sun exposure was assessed at the end of winter, when it can be presumed that few persons spend extended time outdoors in a watery sun with low intensity of solar UV radiation in Norway and the high melanin in the study participants as previously found [31].
Generally, women who wear veils have a higher risk of vitamin D deficiency than women who do not wear any veil [32]. Middle Eastern women with western clothing styles have been reported to have higher s-25(OH)D levels compared to veiled women [33], while a former Norwegian study found no association between clothing habits and s-25(OH)D status among non-Western immigrants[22]. Wearing long sleeved clothes is attributed to poor s-25(OH)D status among immigrant in Sweden [10] due to reduced sun exposure[34]. In our study, we did not find an association between using a veil and s-25(OH)D levels.
Men were more educated as compared to women in our study, and there was no association between education status and s-25(OH)D levels among female or male participants in the study, in contrast to prior findings of a positive association with s-25(OH)D status in women in various immigrant research studies in Norway [13, 22] and Italy [35].
This study overall found a lack of associations between factors such as consumption of fortified food, fatty fish or sun exposure with s-25(OH)D levels. This demonstrates that even when carefully selected and relevant questions are asked about factors previously reported to be associated with vitamin D concentrations, these questions may not provide reliable information on vitamin D sources among immigrant populations.
In this study, women with Middle Eastern and men with South Asian backgrounds had the lowest mean s-25(OH)D levels. In contrast, immigrants from Sub-Saharan Africa residing in Sweden and Australia had the lowest s-25(OH)D status compared to other immigrant groups [10, 36], explained by the fact that darker skin requires higher doses of UV radiation for cutaneous production of s-25(OH)D as compared to lighter skin types [37].
4.1 Strengths and weaknesses of the study
The major strength of the present study is that we have recruited study participants from a range of backgrounds, and the same team collected all the data in the different centers. All blood samples were analyzed in one batch at the same laboratory, to avoid analytical variability.
The study also has some limitations. The study participants were recruited to participate in an intervention study, which may have attracted participants who were motivated to maintain or improve their health. Another limitation is that the food frequency questionnaire (FFQ) largely depends on the participant’s memory and perceptions and may be affected by recall bias, although it has been used in similar previous studies where it was validated. Furthermore, the assessment was performed at the end of winter when it would be presumed that few persons spent time in a watery sun with a low zenith angle, Therefore, all groups may have had low UV exposure, and comparisons between veiled and non-veiled groups may not have detected true differences in the effect of veiled clothing on vitamin D status. The data may also lack power for testing gender-specific differences, particularly among males, because of the higher participation rate among females.
In addition to bone health, currently, there is a growing body of circumstantial evidence that links vitamin D to reduce risk and severity of COVID-19 and other acute respiratory infections [38, 39]. Therefore, a package of public health measures addressing all aspects of vitamin D deficiency in immigrant populations in the Western countries are needed.
5. Conclusion
Both female and male immigrants in Norway with South Asian, Middle Eastern and Sub-Saharan African backgrounds have poor vitamin D status. The prevalence of poor vitamin D status is higher among those with Middle Eastern and South Asian backgrounds. Health authorities in Norway should implement measures to ensure adequate vitamin D intake among groups at risk of vitamin D deficiency, and measures should be taken to ensure adequate vitamin D intake throughout the year.
Acknowledgement
This study was designed with the participation of various health personnel, which facilitated the successful completion of this study. We would like to thank Kirsten Valebjørg Knutsen who contributed to the planning and data collection of this study. We are also grateful for the contribution of the research group, including Eva Kristensen in data collection. The authors also thank Niki Marjerrison for language editing and proofreading of this article. Finally, thank you to the study participants who agreed to take part in this study, for their time and contributions.
Author Contributions
A.A.M. M.B and H.E.M. designed the study. A.A.M. carried out the data collection, M.M.A performed data analysis and prepared the manuscript. H.E.M, M.B and A.A.M critically reviewed the draft and contributed to the interpretation of the findings, and all authors approved the final version of the manuscript.
Conflicts of Interest: The authors declare no conflict of interest.
Funding
Non-financial support for all authors
Ethical Standards Disclosure
"This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the Regional Committee for Medical and Health Research Ethics, Norway. Written informed consent was obtained from all participants.
References
- Lamberg-Allardt C, Brustad M, Meyer HE. Vitamin D- A systematic literature review for the 5th edition of the Nordic Nutrition Recommendations. Food & nutrition research 57 (2013): 226-271.
- Fiscaletti M, Stewart P, Munns C. The importance of vitamin D in maternal and child health: a global perspective. Public health reviews 38 (2017): 1-17.
- Hayes A and Cashman KD. Food-based solutions for vitamin D deficiency: putting policy into practice and the key role for research. Proceedings of the Nutrition Society. 76 (2017): 54-63.
- Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Reviews in Endocrine and Metabolic Disorders 18 (2017): 153-165.
- Pilz S, Gaksch M, Hartaigh BO. Vitamin D in preventive medicine. Anticancer research 35 (2015): 1161-1170.
- Martineau AR, Jolliffe DA, Hooper RL. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. (2017): 35-40.
- Björk A, Andersson Å, Johansson G. Evaluation of sun holiday, diet habits, origin and other factors as determinants of vitamin D status in Swedish primary health care patients: a cross-sectional study with regression analysis of ethnic Swedish and immigrant women. BMC family practice 14 (2013): 129.
- Adebayo FA, Itkonen ST, Lilja E. Prevalence and determinants of vitamin D deficiency and insufficiency among three immigrant groups in Finland: Evidence from a population-based study using standardised 25-hydroxyvitamin D data. Public Health Nutrition. 23 (2020): 1254-1265.
- Bärebring L, Schoenmakers I, Glantz A. Vitamin D status during pregnancy in a multi-ethnic population-representative Swedish cohort. Nutrients. 8 (2016): 655.
- Granlund L, Ramnemark A, Andersson C. Prevalence of vitamin D deficiency and its association with nutrition, travelling and clothing habits in an immigrant population in Northern Sweden. European journal of clinical nutrition 70 (2016): 373-379.
- Itkonen ST, Andersen R, Björk AK. Vitamin D status and current policies to achieve adequate vitamin D intake in the Nordic countries. Scandinavian Journal of Public Health (2020).
- Eggemoen ÅR, Falk RS, Knutsen KV. Vitamin D deficiency and supplementation in pregnancy in a multiethnic population-based cohort. BMC pregnancy and childbirth. 16 (2016): 7-10.
- Holvik K, Meyer H, Haug E. Prevalence and predictors of vitamin D deficiency in five immigrant groups living in Oslo, Norway: the Oslo Immigrant Health Study. European journal of clinical nutrition. 59 (2005): 57-63.
- Knutsen KV, Madar AA, Lagerløv P. Does vitamin D improve muscle strength in adults? A randomized, double-blind, placebo-controlled trial among ethnic minorities in Norway. The Journal of Clinical Endocrinology & Metabolism. 99 (2014): 194-202.
- Madar A , Klepp K, Meyer H. Effect of free vitamin D 2 drops on serum 25-hydroxyvitamin D in infants with immigrant origin: a cluster randomized controlled trial. European journal of clinical nutrition 63 (2009): 478- 484.
- Knutsen KV, Brekke M, Gjelstad S. Vitamin D status in patients with musculoskeletal pain, fatigue and headache: a cross-sectional descriptive study in a multi-ethnic general practice in Norway. Scandinavian journal of primary health care 28 (2010): 166-171.
- Grønborg IM, Tetens I, Christensen T. Vitamin D-fortified foods improve wintertime vitamin D status in women of Danish and Pakistani origin living in Denmark: a randomized controlled trial. European journal of nutrition. 59 (2020): 741-753.
- Lips P. Which circulating level of 25-hydroxyvitamin D is appropriate? The Journal of steroid biochemistry and molecular biology. 89 (2004): 611-614.
- Ross AC. The 2011 report on dietary reference intakes for calcium and vitamin D. Public health nutrition. 14 (2011): 938-939.
- Brunvand L & Haug E. Vitamin D deficiency amongst Pakistani women in Oslo. Acta obstetricia et gynecologica Scandinavica. 72 (1993): 264-268.
- Henriksen C, Brunvand L, Stoltenberg C. Diet and vitamin D status among pregnant Pakistani women in Oslo. European journal of clinical nutrition. 49 (1995): 211-218.
- Madar AA, Stene LC, Meyer HE. Vitamin D status among immigrant mothers from Pakistan, Turkey and Somalia and their infants attending child health clinics in Norway. British journal of nutrition. 101 (2008): 1052-1058.
- Andersen R, Mølgaard C, Skovgaard LT. Pakistani immigrant children and adults in Denmark have severely low vitamin D status. European Journal of Clinical Nutrition. 62 (2008): 625-634.
- Wicherts IS, Boeke AJP, Vander Meer IM. Sunlight exposure or vitamin D supplementation for vitamin D-deficient non-western immigrants: a randomized clinical trial. Osteoporosis International. 22 (2011): 873-882.
- Aleteng Q, Zhao L, Lin H. Optimal Vitamin D status in a middle-aged and elderly population residing in Shanghai, China. Med Sci Monit. 23 (2017): 6001-6011.
- Gannagé?Yared MH, Chemali R, Yaacoub N. Hypovitaminosis D in a sunny country: relation to lifestyle and bone markers. Journal of bone and mineral research. 15 (2000): 1856- 1862.
- Bratlie M, Hagen IV, Helland A. Five salmon dinners per week were not sufficient to prevent the reduction in serum vitamin D in autumn at 60° north latitude: a randomised trial. British Journal of Nutrition. 123 (2020): 419-427.
- Nasjonalt råd for ernæring: Meyer H, Brunvand L, Brustad M, et al. Tiltak for å sikre en god vitamin D-status i befolkningen (Proposals to secure a good vitamin D-status in the population), Rapport IS-1408. Norwegian Nutrition Council Oslo Norway: Norwegian Nutrition Council (2006).
- Madar A , Stene L, Meyer H. Vitamin D status among immigrant mothers from Pakistan, Turkey and Somalia and their infants attending child health clinics in Norway. The British journal of nutrition. 101(2008): 1052-1058.
- Midtbø LK, Nygaard LB, Markhus MW. Vitamin D status in preschool children and its relations to vitamin D sources and body mass index-Fish Intervention Studies-KIDS (FINS-KIDS). Nutrition. 70 (2020): 110-595.
- Cardoso S, Santos A, Guerra RS, et al. Association between serum 25-hidroxyvitamin D concentrations and ultraviolet index in Portuguese older adults: a cross-sectional study. BMC Geriatrics. 17 (2017): 256.
- Yosephin B, Riyadi H, Anwar F, et al. Is vitamin D deficiency associated with using veil in female garment workers? Asian Pacific Journal of Tropical Disease. 6 (2016): 481-485.
- Van Schoor N, Lips P. Worldwide vitamin D status. Vitamin D: Elsevier (2018): 15-40.
- Pearce SH, Cheetham TD. Diagnosis and management of vitamin D deficiency. BMJ. 340 (2010): 56-64.
- Isaia G, Giorgino R, Rini G. Prevalence of hypovitaminosis D in elderly women in Italy: clinical consequences and risk factors. Osteoporosis International. 14 (2003): 577-582.
- Horton-French K, Dunlop E, Lucas RM. Prevalence and Predictors of Vitamin D Deficiency among African Immigrants Living in Australia. International journal of environmental research and public health 16 (2019): 28-55.
- Clemens T, Henderson S, Adams J. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. The Lancet. 319 (1982): 74-76.
- Mitchell F. Vitamin-D and COVID-19: do deficient risk a poorer outcome?. The Lancet Diabetes & Endocrinology. 8 (2020): 10-16.
- Martineau AR, David A, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data, BMJ 35 (2017).


 Impact Factor: * 3.8
Impact Factor: * 3.8 Acceptance Rate: 77.96%
Acceptance Rate: 77.96%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 