Clinical Experience using Osimertinib in Patients with Recurrent Malignant Gliomas Containing EGFR Alterations
Marin Abousaud1, Naqeeb M Faroqui2, Glenn Lesser3, Roy E Strowd3, Shakti H Ramkissoon4,5, Madan Kwatra6, Kristin S Houston3, Fang-Chi Hsu7, Annette Carter3, Robin Petro3, Alisha T DeTroye3 *
1Department of Pharmacy, Emory Healthcare, Atlanta, GA, USA
2Department of General Surgery, Wellstar Atlanta Medical Center, Atlanta, GA, USA
3Wake Forest Baptist Health Comprehensive Cancer Center, Department of Internal Medicine, Section on Hematology and Oncology, Winston-Salem, NC, USA
4Wake Forest Baptist Health Comprehensive Cancer Center, Department of Pathology, Winston-Salem, NC, USA
5Foundation Medicine, Morrisville, NC, USA
6Department of Anesthesiology, Duke University Medical Center, Durham, NC, USA
7Department of Biostatistics and Data Science, Division of Public Health Sciences, Wake Forest School of Medicine, Winston-Salem, NC, USA
*Corresponding Author: Alisha T DeTroye, MMS, PA-C, DFAAPA, Department of Internal Medicine-Section on Hematology and Oncology, Wake Forest Baptist Health, Winston-Salem, NC 27157, USA
Received: 10 March 2021; Accepted: 02 April 2021; Published: 29 April 2021
Article Information
Citation: Marin Abousaud, Naqeeb M Faroqui, Glenn Lesser, Roy E Strowd, Shakti H Ramkissoon, Madan Kwatra, Kristin S Houston, Fang-Chi Hsu, Annette Carter, Robin Petro, Alisha T DeTroye. Clinical Experience using Osimertinib in Patients with Recurrent Malignant Gliomas Containing EGFR Alterations. Journal of Cancer Science and Clinical Therapeutics 5 (2021): 210-220.
View / Download Pdf Share at FacebookAbstract
Background: EGFR alterations are commonly observed in malignant gliomas (MG). Osimertinib, an irreversible EGFR-tyrosine kinase inhibitor, effectively penetrates the blood brain barrier and achieves therapeutic concentrations in brain tissue.
Materials and Methods: This retrospective chart review identified six patients with recurrent MG and EGFR alterations who received osimertinib.
Results: Four patients were assessed for response. One patient had a partial response, two patients achieved stable disease and one was refractory. One patient with an EGFR vIII rearrangement remained on treatment for 236 days and a second patient with an EGFR vIII mutation remained on treatment for 294 days and continued on treatment at the time of analysis. Thrombocytopenia occurred in two patients, one patient developed grade 1 diarrhea and pneumonia, and another patient developed grade 1 mucositis.
Conclusion: Osimertinib had a tolerable safety profile in this heavily pretreated brain tumor population. Osimertinib may benefit select patients with recurrent MG containing EGFR alterations.
Keywords
<p>Osimertinib; EGFR; <em>EGFR vII</em>I; Tyrosine kinase inhibitor; Glioma; Glioblastoma; GBM; Astrocytoma; Targeted therapy; Precision oncology</p>
Article Details
1. Introduction
Gliomas are the most prevalent malignant primary brain tumor in adults, accounting for approximately 30% of all central nervous system tumors and 80% of all malignant brain tumors [1]. Maximal safe resection followed by radiation therapy with concurrent and/or adjuvant chemotherapy is considered the standard of care for malignant gliomas (MGs) [2]. As with other cancers, there has been a need for the development of effective targeted agents for gliomas, since most patients will progress and require further treatment. Finding effective treatments for these primary malignant brain tumors has been challenging due to their chemo- and radio-resistant properties, paucity of targetable genomic aberrations, and the presence of the blood brain barrier (BBB) which potentially limits the entry of systemically administered therapeutics.
In recent decades, the epidermal growth factor receptor (EGFR) signaling pathway has garnered a great deal of attention due to its role in cancer pathogenesis and the availability of novel therapies that specifically and effectively target this pathway [3, 4]. Several studies have suggested a correlation between EGFR alterations and glioma tumor growth, survival, invasion, and angiogenesis [5, 6]. EGFR alterations are more commonly observed in patients with glioblastomas (GBM) in comparison to low-grade gliomas [4]. Many EGFR variants have been identified in gliomas, including amplification, overexpression, insertion-deletion (indel), point mutations, rearrangements, and other aberrations [7]. Based on The Cancer Genome Atlas (TCGA) program, the four major EGFR alterations identified in GBM are: 1. EGFR with a large deletion in the extracellular domain (EGFR vIII); 2. EGFR with kinase domain duplication (EGFR-KDD); 3. wildtype (wt) EGFR amplification; and 4. EGFR fused with SEPT-14 (EGFR-SEPT14) [8, 9].
In patients who overexpress EGFR, 50-60% of them can also have the EGFR vIII mutation. EGFR vIII is characterized by a gene rearrangement that deletes exons 2-7 which results in ligand-independent (constitutive) phosphorylation and activation of the EGFR receptor and signaling pathway [4, 5].
EGFR-tyrosine kinase inhibitors (EGFR-TKIs), such as gefitinib, erlotinib, afatinib, and dacomitinib, have been studied in EGFR-altered gliomas but have yielded minimal to no clinical benefit and short durations of response [10-14]. Unlike the other EGFR-TKIs, osimertinib (AZD9291), a third-generation, irreversible EGFR-TKI commonly used to treat EGFR-mutant lung cancer, is able to effectively penetrate the BBB and achieve therapeutic concentrations in brain tissue, making it an attractive option for gliomas with EGFR alterations [15, 16]. Additionally, osimertinib has been shown to be a more tolerable agent in comparison to the other first- and second-generation EGFR-TKIs [16]. There have been in vitro and in vivo studies supporting the preclinical activity of osimertinib in EGFR expressing GBM, particularly with GBM that express EGFR vIII [9, 17]. Due to the scarcity of studies describing the impact of osimertinib on gliomas in humans, we report our clinical experience of using osimertinib in patients with gliomas containing EGFR alterations.
2. Materials and Methods
This was an observational, single-center, retrospective chart review that was approved by the Wake Forest University Health Sciences Institutional Review Board (Approval Number: IRB00065716). A report was generated from the EPIC electronic medical record for patients who received osimertinib for gliomas with EGFR alterations within the Wake Forest Baptist Comprehensive Cancer Center (WFBCCC) system. Data was extracted from medical records and stored in REDCap (Research Electronic Data Capture), which is HIPAA compliant and includes audit trails to ensure patient confidentiality.
To be eligible for the study, patients were required to have histologically confirmed glioma with known EGFR alterations identified by next generation sequencing (NGS) (Foundation Medicine, Inc., Cambridge, MA, USA) and have received at least one dose of osimertinib. As previously described, DNA extracted from formalin-fixed, paraffin-embedded tumor tissues were assayed by adaptor ligation hybrid capture-based NGS [18, 19]. Sequencing data was analyzed for genomic alterations, including short variant alterations (base substitutions, insertions, and deletions), copy number alterations (focal amplifications and homozygous deletions), and select gene fusions or rearrangements [18, 19].
Patients received osimertinib 80 mg by mouth once daily and continued treatment until disease progression, the development of unacceptable side effects, medical complications, or death. For the majority of patients, osimertinib was not covered by insurance due to its off-label use in MGs. Through the Precision Oncology program at WFBCCC, team members were able to facilitate the process of obtaining osimertinib for off-label indication for these patients.
2.1 Endpoints
Study aims were to describe our experience using osimertinib in patients with gliomas with EGFR alterations and to assess the safety and tolerability of osimertinib in this patient population. Other outcome measures included best response, time to disease progression, and toxicity while on osimertinib.
2.2 Definitions
Best response was assessed on brain MRIs using the Response Assessment in Neuro-Oncology (RANO) criteria (complete response [CR], partial response [PR], stable disease [SD], progression of disease [PD]/refractory) [20]. Time to progression was defined as time from date of treatment initiation to date of disease progression or death from any cause. Best response and disease progression were determined by the treating physician’s and radiologist’s interpretation of the imaging and clinical course documented in the patient’s medical record. Toxicity was evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0 [21].
2.3 Statistical analysis
The sample size was small. Descriptive statistics were utilized for demographic data and all outcome measures. Response rates were summarized by count and frequency.
3. Results
From January 1, 2018 to February 4, 2021, six patients were identified for inclusion in the study. The median age at diagnosis was 56.5 years (range: 46-74) and they were primarily males (83.3%). Four patients had a pathologic diagnosis of GBM while two patients were diagnosed with anaplastic astrocytomas. Three patients had a gross total resection during their first surgery, two had stereotactic biopsies, and one had a subtotal resection. The median number of prior surgeries, recurrences, and prior regimens before osimertinib treatment was 2 (range: 2-3), 2 (range: 1-2), and 2.5 (range: 2-3), respectively. Three of the six patients were MGMT unmethylated, two patients were methylated, and one patient’s MGMT status was unknown. The median Karnofsky performance status (KPS) score at the start of osimertinib therapy was 70.
All patients had previously received radiation plus temozolomide prior to osimertinib. The majority of patients began osimertinib as a third line of treatment and three of the patients were taking it concurrently with another agent: bevacizumab (two patients) or temozolomide (one patient). The median length of time from patient consent to initiation of osimertinib was 40 days. All of the patients received glucocorticoids concurrently with osimertinib. Two patients had a potential drug-drug interaction with osimertinib; however, this did not lead to any osimertinib dose adjustments or toxicities. Both patients were on carbamazepine; one patient had only received one dose of osimertinib while on carbamazepine and the other patient took carbamazepine approximately two times per week as needed for trigeminal neuralgia. Based on the discretion of the treating provider, a dose adjustment for osimertinib was not warranted since carbamazepine was taken sparingly.
Genomic profiling revealed that all patients were IDH1/2 wild type. EGFR amplification was detected in five patients, four of which showed co-occurring EGFR vIII rearrangements characterized by intragenic deletion of exons 2-7. Other structural rearrangements identified included one patient with EGFR vII (deletion of exons 14-15) and one with EGFR vIVa (deletion of exons 25-27) (Figure 1 and Table 1).
Consistent with their classification as MGs, all tumors showed homozygous deletion of CDKN2A/B and TERT promoter mutations. A subset of tumors also harbored EGFR single nucleotide variations (SNV) or point mutations such as G598V, R108K, T263P and A289V, as well as co-occurring mutations (Figure 1 and Table 1).
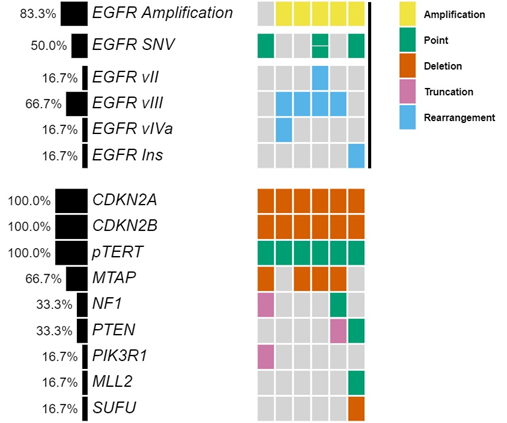
Figure 1: Co-mutation plot of six patients treated with osimertinib highlights heterogeneity of EGFR alterations and genomic profiles.
|
Pt |
IDH1/2 Status |
EGFR Alterations |
Co-occurring Alterations |
TMB (mut/Mb) |
MSI Status |
|
1 |
Wild type |
(G598V) |
CDKN2A/B homozygous del, NF1 (P228fs*53), PIK3R1 (L449fs*3), MTAP homozygous del, pTERT (-146C>T) |
1 |
Stable |
|
2 |
Wild type |
Amplification, vIII, vIVa |
CDKN2A/B homozygous del, pTERT (-124C>T) |
5 |
Stable |
|
3 |
Wild type |
Amplification, vIII |
CDKN2A/B homozygous del, MTAP homozygous del (exons 2-8), pTERT (-124C>T) |
3 |
Stable |
|
4 |
Wild type |
Amplification, (R108K), (T263P), vII, vIII |
CDKN2A/B homozygous del, MTAP homozygous del, pTERT (-124C>T) |
3 |
Stable |
|
5 |
Wild type |
Amplification, vIII |
CDKN2A/B homozygous del, NF1 (K1661fs*36), PTEN truncation intron 7, MTAP homozygous del, pTERT (-124C>T) |
3 |
Stable |
|
6 |
Wild type |
Amplification, (A289V), exon 20 insertion (D770_N771insSVD) |
CDKN2A/B homozygous del, PTEN splice site (1027-2A>G), SUFU homozygous del (exons 11-12), MLL2 (R2635Q), pTERT (-124C>T) |
2 |
Stable |
|
Abbreviations: Pt – patient, TMB – tumor mutational burden, MSI – microsatellite instability |
|||||
Table 1: Genomic landscape of malignant gliomas treated with osimertinib.
Of the six patients, only four could be assessed for response. Out of the four patients, one patient achieved partial response, two patients achieved a best response of stable disease while on osimertinib and one patient was refractory to treatment. The patient with PR has an EGFR vIII mutation and continues on osimertinib after 294 days in conjunction with temozolomide and Optune. One patient with an EGFR vIII mutation and SD on osimertinib remained on treatment for 236 days prior to progression. The other patient progressed after 77 days of treatment. The patient who was on osimertinib for 77 days had a transient improvement on imaging, which may also have reflected an increase in dexamethasone dosing. Neuroimaging findings for the patient with PR and longest SD on osimertinib are shown in Figures 2 and 3.
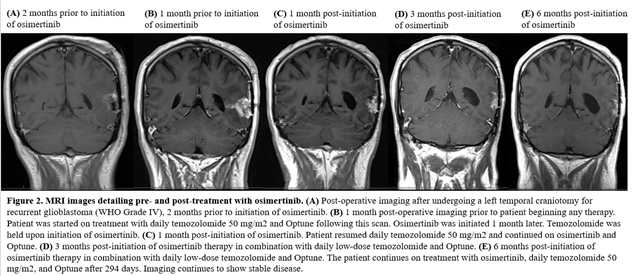
Figure 2:
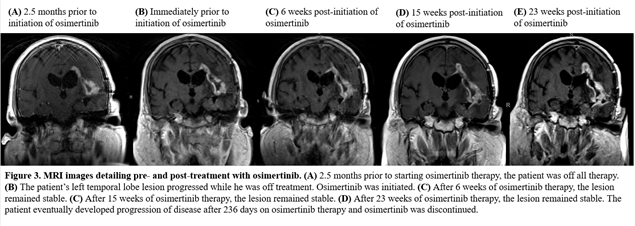
Figure 3:
In terms of the safety profile of osimertinib, four of six patients experienced an adverse event. One patient had grade 2 thrombocytopenia, one patient had grade 3 thrombocytopenia (was on concurrent bevacizumab therapy), one patient had grade 1 mucositis and one patient had grade 1 diarrhea and pneumonia. Three patients experienced mild myelosuppression that was felt to be unrelated to osimertinib. No rash was noted for any of the patients. Most patients did not have baseline and follow up electrocardiogram (EKGs) or echocardiograms (ECHOs) performed to monitor for QTc prolongation and cardiomyopathy/decreased left ventricular ejection fraction, respectively. For the one patient whom had a baseline and follow up EKG obtained, no QTc prolongation was observed while on osimertinib. A summary of patient demographics with their corresponding responses and tolerability can be found in Table 2.
Table 2: Baseline demographics and corresponding response and tolerability.
4. Discussion
Treatment options are limited for MG patients who recur after standard chemoradiation. As oncology shifts towards precision medicine and personalized therapy, EGFR is an appealing therapeutic target in various cancer types due to its role in tumor growth and survival [3-6]. After the FLAURA trial reported practice changing outcomes with osimertinib in EGFR-mutant lung cancer, including patients with CNS involvement, osimertinib became an even more attractive agent to consider in patients with MGs with EGFR alterations [16].
Unlike lung cancer, gliomas contain a wide variety of EGFR alterations, which make it difficult to target. This study highlights the molecular heterogeneity of EGFR alterations in MG patients; the two most common EGFR alterations identified in this study were EGFR amplification and EGFR vIII mutation. EGFR vIII rearrangements are the most common EGFR rearrangement and are reported in 24-57% of GBM [22, 23].
Two patients in our study with the EGFR vIII mutation remained on osimertinib for extended duration (236 and 294 days). Based on in vitro and in vivo studies, osimertinib has demonstrated preclinical activity in GBM with EGFR vIII rearrangements [9, 17, 24]. The extended period of treatment and disease stability of our EGFR vIII patients demonstrates the potential activity of osimertinib in select patients with EGFR vIII altered gliomas. Our study highlights the importance of identifying and characterizing EGFR alterations in MGs to determine in which patients treatment with osimertinib be considered.
Obtaining osimertinib for these patients is currently a challenge, as most insurance companies will not cover its costs due to its off-label use in MGs. Even under the best circumstances, obtaining an off-label drug may be quite time consuming. At WFBCCC, a unique precision medicine program is offered to match tumor genetics with potential novel treatments. In general, these results are used for later lines of therapy when standard therapy has been exhausted.
Tumor genetic information is obtained, ideally, from a new tissue sample after all prior lines of therapy, to better define the individual’s tumor and explore treatment options when necessary. This dedicated multi-disciplinary team expedites consent, outcome of benefit determination, and options for drug assistance and coverage that resulted in a median of 40 days from the time osimertinib was prescribed to receipt of the medication.
Although this study is limited in sample size, this heavily pretreated brain tumor population tolerated osimertinib therapy and half of the patients were able to achieve either PR or SD ranging from 77 to 294 days. To our knowledge, this is the largest study reporting on the clinical activity and tolerability of osimertinib in humans with MGs. Makhlin et al recently reported promising clinical activity with osimertinib in one patient with EGFR-mutant GBM [25]. Formal clinical trials are needed to evaluate osimertinib’s safety and efficacy in MGs as well as whether there is a benefit in combination with other therapeutic agents in the recurrent setting.
A future additional clinical trial consideration is whether osimertinib should be combined with temozolomide as a frontline, newly diagnosed treatment option in selected patients.
5. Conclusion
Overall, osimertinib may benefit select patients with recurrent MGs harboring EGFR alterations while having a tolerable safety profile.
Further clinical investigations are needed to assess the efficacy and safety of osimertinib in this brain tumor population, particularly in patients whose tumors express EGFR vIII rearrangements, and to identify which EGFR alterations may sensitize tumors to this BBB penetrant EGFR-TKI.
Disclosures
- SH Ramkissoon is an employee of Foundation Medicine.
- RE Strowd has nothing to disclose related to this manuscript. Dr. Strowd serves a consultant for Monteris Medical Inc, Novocure, and Nanobiotix; he receives an editorial stipend as Section Editor of the Resident and Fellow Section of Neurology® and has received research/grant support from the National Institutes of Health (R21-CA229027), Department of Defense (BC181274), American Society for Clinical Oncology (ASCO CDA-2018), Southeastern Brain Tumor Foundation (SBTF-2018), and Jazz Pharmaceuticals (JAZZ-VT10820).
- All other authors have no financial disclosures.
References
- Goodenberger ML, Jenkins RB. Genetics of adult glioma. Cancer Genetics 205 (2012): 613-621.
- Nabors LB, Portnow J, Ahluwalia M, et al. National Comprehensive Cancer Network (NCCN) Guidelines: Central Nervous System Cancers, Version 2.2020. J Natl Compr Canc Netw (2020).
- Gullick WJ. Prevalence of aberrant expression of the epidermal growth factor receptor in human cancers. Br Med Bull 47 (1991): 87-98.
- Hatanpaa KJ, Burma S, Zhao D, et al. Epidermal Growth Factor Receptor in Glioma: Signal Transduction, Neuropathology, Imaging, and Radioresistance. Neoplasia 12 (2010): 675-684.
- Huang PH, Xu AM, White FM. Oncogenic EGFR signaling networks in glioma. Sci Signal 2 (2009).
- Vivanco I, Robins HI, Rohle D, et al. Differential sensitivity of glioma- versus lung cancer-specific EGFR mutations to EGFR kinase inhibitors. Cancer Discov 2 (2012): 458-471.
- Saadeh FS, Mahfouz R, Assi HI. EGFR as a clinical marker in glioblastomas and other gliomas. The International Journal of Biological Markers 33 (2018): 22-32.
- Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell 155 (2013): 462-477.
- Chagoya G, Kwatra SG, Nanni CW, et al. Efficacy of osimertinib against EGFRvIII+ glioblastoma. Oncotarget 11 (2020): 2074-2082.
- Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med 353 (2005): 2012-2024.
- Chakravarti A, Wang M, Robins HI, et al. RTOG 0211: a phase 1/2 study of radiation therapy with concurrent gefitinib for newly diagnosed glioblastoma patients. Int J Radiat Oncol Biol Phys 85 (2013): 1206-1211.
- Prados MD, Chang SM, Butowski N, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol 27 (2009): 579-584.
- Reardon DA, Nabors LB, Mason WP, et al. Phase I/randomized phase II study of afatinib, an irreversible ErbB family blocker, with or without protracted temozolomide in adults with recurrent glioblastoma. Neuro-Oncology 17 (2015): 430-439.
- Sepulveda-Sanchez JM, Vaz MA, Balana , et al. Phase II trial of dacomitinib, a pan–human EGFR tyrosine kinase inhibitor, in recurrent glioblastoma patients with EGFR amplification. Neuro Oncol 19 (2017): 1522-1531.
- Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 4 (2014): 1046-1061.
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 378 (2018): 113-125.
- Liu X, Chen X, Shi L, et al. The third-generation EGFR inhibitor AZD9291 overcomes primary resistance by continuously blocking ERK signaling in glioblastoma. Journal of Experimental and Clinical Cancer Research 38 (2019): 219.
- Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol 31 (2013): 1023-1031.
- Williams EA, Montesion M, Sharaf R, et al. CYLD-mutant cylindroma-like basaloid carcinoma of the anus: a genetically and morphologically distinct class of HPV-related anal carcinoma. Mod Pathol (2020).
- Wen PY, Macdonald DR, Reardon DA, et al. Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Working Group. J Clin Oncol 28 (2010): 1963-1972.
- Common Terminology Criteria for Adverse Events v.5.0 (NCI-CTCAE) (2020).
- Ekstrand AJ, James CD, Cavenee WK, et al. Genes for epidermal growth factor receptor, transforming growth factor α, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res 51 (1991): 2164-2172.
- Wong AJ, Ruppert JM, Bigner SH, et al. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci USA 89 (1992): 2965-2969.
- Chen C, Cheng CD, Wu H, et al. Osimertinib successfully combats EGFR-negative glioblastoma cells by inhibiting the MAPK pathway. Acta Pharmacol Sin 42 (2021): 108-114.
- Makhlin I, Salinas RD, Zhang D, et al. Clinical activity of theEGFRtyrosine kinase inhibitor osimertinib inEGFR-mutant glioblastoma.CNS Oncol 8 (2019): 43-48.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 74.74%
Acceptance Rate: 74.74%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 