DICKKOPF-1 (DKK-1) Gene Associations in Human Cancers by Vitamin D and Sulforaphane
Sharmin Hossain1*, Zhenhua Liu2, Richard J. Wood2
1National Institute on Aging (NIA/NIH), Baltimore, Maryland, USA
2Department of Nutrition, University of Massachusetts, Amherst, Massachusetts, USA
*Corresponding Author: Dr. Sharmin Hossain, National Institute on Aging (NIA/NIH), Baltimore, Maryland, USA
Received: 06 July 2020; Accepted: 28 July 2020; Published: 03 August 2020
Article Information
Citation: Sharmin Hossain, Zhenhua Liu, Richard J. DICKKOPF-1 (DKK-1) Gene Associations in Human Cancers by Vitamin D and Sulforaphane. Journal of Cancer Science and Clinical Therapeutics 4 (2020): 237-244.
View / Download Pdf Share at FacebookAbstract
Dickkopf Wnt Signaling Pathway Inhibitor-1 gene (DKK-1) encodes a member of the Dickkopf family of proteins and is involved primarily in the embryonic development and bone formation in adults. Based on its known relationship to Wnt signaling pathway, we set out to determine how this gene is affected by dietary agents- vitamin D and sulforaphane (SFN). Cells were exposed for 24 hours to vitamin D3 [1,25(OH)2D3] (100nM) either alone or in combination with L-sulforaphane and TSA (20μM and 1μM respectively) at 70% confluency. We hypothesized that DKK-1 expression will be increased in both cancer cells using our select treatments. In proliferating Caco-2 cells, treatment with 1,25(OH)2D3 had nearly a 3-fold increase in DKK-1 (p<0.05) expression while in MCF-7 cells saw no effect of the treatments. Co-treating the cells with both compounds seemed to attenuate the D effect, implying competing needs along the signaling pathway and require further investigation.
Keywords
<p>Sulforaphane; Trichostatin A; Vitamin-D receptor; DICKKOPF-1 (DKK-1) gene; Colon cancer; Breast cancer</p>
Article Details
Abbreviations:
1,25(OH)2D3- 1,25 di-hydroxyvitamin D; BC- Breast Cancer; CRC- Colorectal Cancer; SEM- Standard Error of Means; SFN- Sulforaphane; TSA- Trichostatin A; VDR- Vitamin-D receptor
1. Introduction
Dickkopf Wnt Signaling Pathway Inhibitor-1 gene (DKK-1) encodes a member of the Dickkopf family of proteins[1] and has been reported to be involved primarily in the embryonic development and bone formation in adults. Much of the DKK-1 literature, therefore, reflect research on primary [2] and secondary hyperparathyroidism [3], rheumatoid and psoriatic arthritis [4-7], non-alcoholic fatty liver disease [8], osteoporosis [9], ankylosing spondylitis [10], systemic sclerosis[11] and alopecia [12]. The role of DKK-1 in cancer revolves primarily around components of Wnt-signaling pathway. DKK-1 binds to the low-density lipoprotein-6 or LRP6 co-receptor and inhibits β-catenin-dependent (canonical) Wnt signaling, serving as a potent Wnt antagonist. Although linked quite frequently to Wnt-signaling in cancer pathology, very little is known about DKK-1 as a gene and its possible linkages to other systems functions in humans. Aberrant activation of Wnt-β catenin pathway in most human cancers has been well-documented assuming its role in cell proliferation and invasion [13]. Overexpression of DKK-1 has been associated with uncontrolled Wnt signaling in hepatoblastomas [14] and human endothelial colony-forming cells [15] demonstrating its role in tumoral angiogenesis.
The family of Wnt glycoproteins regulates development and homeostasis under normal conditions. However, the signaling pathways are severely disrupted in cancer. The evidence pool on DKK-1 in breast cancer (BC) is rather small, and non-existent with vitamin D as a treatment. In an animal study of metastatic BC to the bones, the researchers found DKK-1 to be expressed 6 times more in cells with osteolytic lesions in animals[16]. Studies on human subjects with and without BC and bone metastasis reported significantly high levels of DKK-1 in the serum samples of patients with breast cancer (P=0.016) compared to controls [16, 17] and poor BC prognosis [18]. Uncontrolled DKK-1 expression and/or downregulation have been reported in different BC cell lines[19, 20] suggesting potential targets in reversing the effect in BC [21, 22]. One study investigating the inhibitory effect of human mesenchymal stem cells (Z3 cells) on the growth of human MCF-7 breast cancer cells demonstrated that β-catenin was down-regulated followed by the over expression of DKK-1 in Z3 cells compared to MCF-7. The inhibitory effect of Z3 cells on MCF-7 cells were attenuated after neutralizing DKK-1. Therefore, DKK-1 secreted by Z3 cells involves the inhibition via the Wnt pathway. [23] In MCF-7 cells, the downregulation of DKK-1 has been linked with higher cell proliferation by losing control of Wnt/β-catenin signaling pathway, giving a new mechanistic insight.[20].
Traditionally the main recognized function of vitamin D has been calcium and phosphate homeostasis. Nevertheless, the importance of vitamin D3 as a protective agent against various cancers is quite significant. The active form of vitamin D, (1,25-dihydroxyvitamin D3) or vitamin D3 and the nuclear vitamin D receptor (VDR) are responsible for regulating several genes involved in cell differentiation and cell proliferation [24]. However, there is only 10 studies investigating DKK-1 with vitamin D and only three of them focused on colon cancer. Our current study adds to existing but rather limited research base of DKK-1 in relation to vitamin D response in two different human cancer cell lines.
DKK-1 is expressed at high level in colon cancer cell lines such as Caco-2 or HT-29. The association between CRC and vitamin D3 was first suggested in ecologic studies, but further was confirmed by observational studies in humans and experimental studies in both animal and cell culture studies [25]. In human SW480-ADH colon cancer cells, vitamin D3 increased DKK-1 mRNA and protein expressions in a dose dependent manner and induced certain histone modification patterns, further influencing the expressions [26]. Studies in humans, on the other hand, have looked at DNA methylation and risk of colorectal cancer, to elucidate any associated epigenetic mechanisms. In a Canadian cohort of colorectal cancer patients, dietary vitamin D intake was strongly negatively associated with DKK-1 methylation [27]. Results from laboratory and human study findings contradict each other, which further emphasizes the need to explore these associations.
In addition to mainstream dietary macro and micronutrients, recent research has been focused on investigating the role of phytochemicals in cancer prevention. Phytochemicals are naturally present compounds that have demonstrated anti-inflammatory, anti-hypertensive, antioxidant as well as anti-carcinogenic properties, and some example phytochemical groups are lycopene, genistein, sulforaphane, (-)-epigallocatechin-3-gallate (EGCG), curcumin, etc. Such compounds have been shown to modify self-renewal properties of cancer stem cells, but no studies to date have investigated specific genes in relation to individual phytochemicals by their specific beneficial properties [28]. Sulforaphane (SFN) is classified as a histone deacetylase (HDAC) inhibitor[29-31] present abundantly in food sources like broccoli, Brussel sprouts, cabbages, cauliflowers etc. HDACs are a class of enzyme responsible for regulating gene transcription[32]. HDAC inhibitors such as SFN increase transcription of key genes and play roles in regulatory pathways that ultimately discourage oncogenesis from taking place[29-31]. No studies to date have looked at the effect of vitamin D and SFN on DKK-1 in human breast or colon cancer cells.
This study is the first to investigate the importance of DKK-1 regulation by vitamin D and SFN, separately and in combination in breast and colon cancer cells in human. Based on our previous findings, we anticipate that treating cancer cells with vitamin D will increase DKK-1 expression and further increase in the presence of SFN. Comparing two invasive cancers side-by-side would also provide valuable understanding of any regulatory differences by cell types and possibly lead to the development therapies targeting DKK-1 pathways, which could improve the prognosis of innumerable cancer sufferers.
2. Materials and Methods
2.1 Cell culture
Both cells lines- breast cancer (MCF-7) and colorectal cancer (Caco-2) were obtained from American Type Culture Collection (ATCC). Cells were treated on day 7 from the date of seeding into 6-well plates. The experiment included six different treatment groups namely- DMSO control (0.1%), 1,25 (OH)2D3 (100nM), SFN (20 µM), TSA (1µM), 1,25 (OH)2D3 + SFN and 1,25 (OH)2D3 + TSA. After treatment, the cells were incubated for 24 hours before harvesting. MCF-7 cells were obtained from Arcaro lab. 1, 25 (OH)2D3 was purchased from Enzo Life Sciences, Plymouth Meeting, PA. Trichostatin A and SFN were purchased from Sigma-Aldrich, St. Louis, MO. Real-time PCR materials, including all primers and master mix were purchased from Applied Biosystems, Foster City, CA. Cell culture dishes were bought from Costar, Cambridge, MA. All other cell culture materials and materials for RNA isolation and cDNA synthesis were purchased from Life Technologies, Grand Island, NY.
2.2 RNA isolation and reverse transcription
At the completion of the treatment period, cells were washed with saline, then treated with 1 mL Trizol per well of a 6-well plate, and cells were scraped to be harvested for subsequent RNA extraction. Cells were frozen at -80°C until RNA was ready to be isolated in collection tubes. To isolate the RNA, 200 µL chloroform was added to each homogenate for phase separation. Isopropanol (500 µL) was then used to precipitate the RNA. Washing three times with 1 mL of 75% ethanol was followed by the addition of 40 µL of Diethyl pyrocarbonate (DEPC) water. The samples were then incubated for 10 minutes at 56°C to ensure maximum RNA solubility. RNA samples were then quantified using a Nanodrop machine (λ260/280). cDNA was prepared using the SuperScript III First Strand Synthesis System for RT-PCR (Life Technologies, Grand Island, NY).
For all experiments, mRNA expressions of GAPDH, DKK-1were measured by ViiA™ 7 Real-Time PCR System (Applied Biosystems, Foster City, CA). Reactions consisted of 10 µL of SYBR GREEN Gene Expression Master Mix (Applied Biosystems, Foster City, CA) with forward and reverse primers (10nM each) and 10 µL of DNAse/RNAse Free Water with 100 ng of cDNA. Duplicates were set up for each sample. The levels of DKK-1were measured by the comparative ΔΔCT method (ThermoFisher Scientific, 2016). For each sample, the relative abundance of GAPDH and the target genes were obtained. The difference between those two abundances (target gene- GAPDH) is the ΔCT for that sample which were the values used for statistical analysis. The mean ΔCT values were then normalized against the mean ΔCT values of the control sample, Dimethyl Sulfoxide (DMSO), resulting in a ΔΔCT value. 2 was raised to the – ΔΔCT to produce a relative quotient (RQ) for each sample, with the RQ of the control being one. RQ values were used to create the mRNA expression curves for each gene. We used the same sequences as reported by Zhou et.al, for DKK-1 [20]. GAPDH primer sequences are as below:
GAPDH-F CATGGGTGTGAACCATGAGA
GAPDH-R GGGTGCTAAGCAGTTGGT
2.4 Statistical analyses
Results from real-time PCR were analyzed using one-way ANOVA with a p-value of <0.05 used as the cut-off for statistical significance. The analysis was setup as a randomized block design with each experiment as a block. Brown-Forsythe Tests was also used to assess significant differences among the test standard deviations from the control (DMSO), also using a P-value of <0.05 as a cut-off. All statistical analyses and figures were generated using GraphPad Prism (v 8.4.0) and results are presented as fold-increase with corresponding p-values.
3. Results
We hypothesized that the expression of DKK-1 will be increased by 1,25(OH)2D3 alone, and further enhanced by the addition of SFN because of its known HDAC inhibitory function, in both cell lines. In proliferating Caco-2 cells, ANOVA (p<0.0001) and Brown-Forsythe tests (p=0.03) reveal significant differences across the treatment groups compared to control. DKK-1 expression (N=6) was increased by 1,25(OH)2D3 as hypothesized (3.30- fold, p<0.05). However, the addition of SFN did not make a significant change in DKK-1 expression (2.875- fold, p=0.0012) presumably because SFN alone had no effect on DKK-1 (0.91- fold, p=0.56). The expression followed similar patterns as 1,25(OH)2D3 in D+TSA group (2.885- fold, p=0.0012) and TSA alone (5.80-fold, p<0.0001) with nearly twice as much the expression in DKK-1, compared to 1,25(OH)2D3 alone. The results were quite different in MCF-7 cells as seen from the overall results of the ANOVA (p=0.73) and Brown-Forsythe (p=0.65) tests. Not only were there no effect of any of the treatment groups, but the addition of SFN to 1,25(OH)2D3 seemed to inhibit DKK-1 expression. In MCF-7 cells, the results were- 1,25(OH)2D3 alone (0.40 fold, p= 1.00), SFN alone ( 1.64 fold, p=0.95), D+SFN (-0.66 fold, p=1.00), D+TSA (2.5- fold, p=0.81) and TSA alone (2.69-fold, p=0.76) group without statistical significance. The results for RT-PCR experiments are presented in Figure 1.
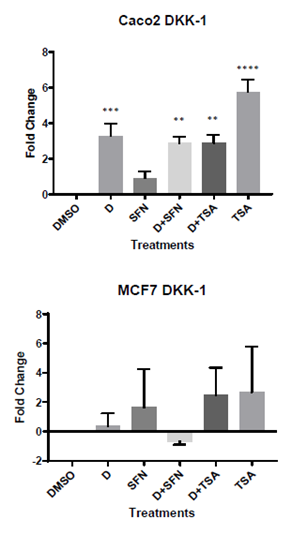
Figure 1: mRNA expressions of DKK-1 in proliferating Caco-2 and MCF-7 cells. A) DKK-1 expression was significantly increased by D alone in proliferating Caco-2 cells (>3-fold increase) and the effect remained similar in D+SFN and D+TSA groups. While TSA alone showed the maximum increase in DKK-1 expression, our target HDAC inhibitor SFN had no effect; B) DKK-1 expressions were not affected by any of the treatment groups in MCF-7 cells. Results are from N>3 experiments for both genes and *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 represent significance.
4. Discussion
The study findings suggest that vitamin D might be attenuating effects of SFN in DKK-1 expression in two different human cancer cell lines. This is the first study to use proliferating colorectal cancer cells, Caco-2, as a model- which is not usual for its research application. In a separate study, we have shown that Caco-2 cells behave differently under different conditions- proliferating vs. differentiated (Revise and resubmit: Journal of Food Science and Agriculture JSFA-19-3273). We presumed Caco-2 proliferating cells retain properties of any invasive, metastatic cancers of other human organs, and hence used as a comparison cell line to MCF-7. Our HDAC inhibitor findings are similar to what we have demonstrated before, gene expressions related to HDAC inhibition could be cell-specific[33] and requires further investigation.
The vitamin D receptor (VDR) antagonizes β-catenin signaling. In vitro, VDR activation in breast cancer cells reduced β-catenin activation and transcriptional activity leading to elevated expression of the extracellular Wnt inhibitor DKK-1 protein, and a reduction in the interaction of β-catenin with the cyclin D1 promoter. Expression of a stabilized form or β-catenin ablated the protective effects of VDR activation. Collectively, these findings delineate a protective role for VDR signaling through disruption of β-catenin activation[34].
The ability of various bioactive food components to influence the balance between proliferative and quiescent cells by regulating DKK-1 may account for their biological response [28]. There is no data on the effect of SFN alone on DKK-1 to elucidate some of the underlying mechanisms. Undoubtedly, additional studies are needed to clarify the physiological role of such bioactive components in cancer management.
In the study of colonic SHZ-88 cells, the growth, migration, and invasion were significantly inhibited with upregulation of DKK-1 and down regulation of Wnt/ β-catenin target genes. Mesenchymal stem cells derived from the perichondrium inhibit the growth of breast cancer cells through the Wnt/β-catenin signaling pathway in vitro and in vivo via regulation of DKK-1 [35]. Studies on colorectal cancer cells suggest that vitamin D3 activates DKK-1 transcription in an indirect manner, suggesting a novel mechanism of Wnt inhibition [13]. In an immunodeficient mouse study, exogenous expression of E-cadherin resulted in a 17-fold increase in DKK-1 mRNA expression. Vitamin D3 is also known to inhibit β-catenin transcriptional activity by promoting vitamin D receptor (VDR) binding to β-catenin and the induction of E-cadherin expression. The increase in the expression of DKK-1 mRNA and protein, however, acts as a tumor suppressor in human colon cancer cells. In contrast, vitamin D3 represses DKK-4 transcription by inducing direct VDR binding to its promoter. DKK-4 as a novel target of the Wnt/β-catenin pathway has been shown to be up-regulated in colorectal tumors and increase cell migration and invasion. Together, these results show that vitamin D3 exerts a complex set of regulatory actions leading to the inhibition of the Wnt/β-catenin pathway in colon cancer cells [26]. This report demonstrates that DKK-1 expression varies by cancer cell types with same nutrient interventions- highlighting a more heterogeneous nature of DKK-1 responses. This also points to the need for comparative studies that simultaneously measure the potential pro- and anti-carcinogenic effects of DKK-1 activation.
Future studies might investigate whether vitamin D and other bioactive compounds influence DKK-1 stimulation. Such research would further elucidate the relationship between DKK-1 and natural bioactive compounds in cancer prognosis.
5. Conclusion
Insights into the biology of breast and colon cancers have been gained from the investigation of genes commonly overexpressed in these cells in cancer and have led to paradigms that have informed the study of epigenetic alterations. These insights are important in developing new diagnostic and prognostic assays and potential therapies for cancer.
Acknowledgements
We would like to thank Dr. Liu (Department of Nutrition) and Dr. Kathleen Arcaro (Department of Veterinary and Animal Sciences) for their generous support in this research.
Source of Funding
This research was funded by the United States Department of Agriculture (USDA); Hatch Grant (No. MAS 00992).
Conflict of Interest
None.
References
- GeneCards DKK1 Gene (Protein Coding) Dickkopf WNT Signaling Pathway Inhibitor 1. Weizmann Institute of Science.
- Viapiana O, et al. Sclerostin and DKK1 in primary hyperparathyroidism. Calcif Tissue Int 92 (2013): 324-329.
- Ho TY, et al. Evaluation of the association of Wnt signaling with coronary artery calcification in patients on dialysis with severe secondary hyperparathyroidism. BMC Nephrol 20 (2019): 345.
- Rossini M, et al. In patients with rheumatoid arthritis Dickkopf-1 serum levels are correlated with parathyroid hormone bone erosions and bone mineral density. Clin Exp Rheumatol 33 (2015): 77-83.
- Adami G, et al. Effects of TNF Inhibitors on Parathyroid Hormone and Wnt Signaling Antagonists in Rheumatoid Arthritis. Calcif Tissue Int 99 (2016): 360-364.
- Fassio A, et al. In psoriatic arthritis Dkk-1 and PTH are lower than in rheumatoid arthritis and healthy controls. Clin Rheumatol 36 (2017): 2377-2381.
- Yolbas S, et al. Paricalcitol inhibits the Wnt/beta-catenin signaling pathway and ameliorates experimentally induced arthritis. Turk J Med Sci 48 (2018): 1080-1086.
- Polyzos SA, et al. Circulating sclerostin and Dickkopf-1 levels in patients with nonalcoholic fatty liver disease. J Bone Miner Metab 34 (2016): 447-456.
- Wanby P, et al. Serum levels of the bone turnover markers dickkopf-1 sclerostin osteoprotegerin osteopontin osteocalcin and 25-hydroxyvitamin D in Swedish geriatric patients aged 75 years or older with a fresh hip fracture and in healthy controls. J Endocrinol Invest 39 (2016): 855-863.
- Orsolini G, et al. Parathyroid hormone is a determinant of serum Dickkopf-1 levels in ankylosing spondylitis. Clin Rheumatol 37 (2018): 3093-3098.
- Taylan A, et al. Osteoprotegrin interacts with biomarkers and cytokines that have roles in osteoporosis skin fibrosis and vasculopathy in systemic sclerosis: A potential multifaceted relationship between OPG/RANKL/TRAIL and Wnt inhibitors. Mod Rheumatol 29 (2019): 619-624.
- Lim YY, et al. Potential relationship between the canonical Wnt signalling pathway and expression of the vitamin D receptor in alopecia. Clin Exp Dermatol 39 (2014): 368-375.
- Aguilera O, et al. The Wnt antagonist DICKKOPF-1 gene is induced by 1alpha 25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis 2007. 28(9): 1877-84.
- Wirths O, et al. Overexpression of human Dickkopf-1 an antagonist of wingless/WNT signaling in human hepatoblastomas and Wilms' tumors. Lab Invest 83 (2003): 429-434.
- Smadja DM, et al. The Wnt antagonist Dickkopf-1 increases endothelial progenitor cell angiogenic potential. Arterioscler Thromb Vasc Biol 30 (2010): 2544-2552.
- Voorzanger-Rousselot N, et al. Increased Dickkopf-1 expression in breast cancer bone metastases. Br J Cancer 97 (2007): 964-970.
- Voorzanger-Rousselot N, et al. Assessment of circulating Dickkopf-1 with a new two-site immunoassay in healthy subjects and women with breast cancer and bone metastases. Calcif Tissue Int 84 (2009): 348-354.
- Zhou SJ, et al. Serum Dickkopf-1 expression level positively correlates with a poor prognosis in breast cancer. Diagn Pathol 9 (2014): 161.
- Mikheev AM, et al. Dickkopf-1 mediated tumor suppression in human breast carcinoma cells Breast Cancer Res Treat 112 (2008): 263-273.
- Zhou XL, et al. Downregulation of Dickkopf-1 is responsible for high proliferation of breast cancer cells via losing control of Wnt/beta-catenin signaling Acta Pharmacol Sin 31 (2010): 202-210.
- Rachner TD, et al. Dickkopf-1 is regulated by the mevalonate pathway in breast cancer Breast Cancer Res 16 (2014): R20.
- Kim HY, et al. CBX7 inhibits breast tumorigenicity through DKK-1-mediated suppression of the Wnt/beta-catenin pathway Faseb j 29 (2015): 300-313.
- Qiao L, et al. Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling Cancer Lett 269 (2008): 67-77.
- Di Rosa M, et al. Vitamin D3 insufficiency and colorectal cancer Crit Rev Oncol Hematol 88 (2013): 594-612.
- Jacot W, et al. Increased prevalence of vitamin D insufficiency in patients with breast cancer after neoadjuvant chemotherapy Breast Cancer Res Treat 134 (2012): 709-717.
- Pendas-Franco N, et al. Vitamin D and Wnt/beta-catenin pathway in colon cancer: role and regulation of DICKKOPF genes Anticancer Res 28 (2008): 2613-2623.
- Rawson JB, et al. Vitamin D intake is negatively associated with promoter methylation of the Wnt antagonist gene DKK1 in a large group of colorectal cancer patients Nutr Cancer 64 (2012): 919-928.
- Kim YS, et al. Cancer stem cells: potential target for bioactive food components J Nutr Biochem 23 (2012): 691-698.
- Rajendran P, et al. HDAC turnover CtIP acetylation and dysregulated DNA damage signaling in colon cancer cells treated with sulforaphane and related dietary isothiocyanates Epigenetics 8 (2013): 612-623.
- Myzak MC, et al. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc-minus mice Faseb j 20 (2006): 506-508.
- Myzak MC, et al. Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects Exp Biol Med (Maywood) 232 (2007): 227-234.
- Kouzarides T, Acetylation: a regulatory modification to rival phosphorylation? Embo j 19 (2000): 1176-1179.
- Hossain S, Liu Z, Wood RJ. Histone deacetylase activity and vitamin D-dependent gene expressions in relation to sulforaphane in human breast cancer cells J Food Biochem 44 (2020): e13114.
- Johnson AL, Zinser GM, Waltz SE. Vitamin D3-dependent VDR signaling delays ron-mediated breast tumorigenesis through suppression of beta-catenin activity Oncotarget 6 (2015): 16304-16320.
- Li M, et al. Perichondrium mesenchymal stem cells inhibit the growth of breast cancer cells via the DKK-1/Wnt/beta-catenin signaling pathway Oncol Rep 36 (2016): 936-944.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 74.74%
Acceptance Rate: 74.74%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 