Efficacy of Anti-Estrogen Therapy in Estrogen Receptor Positive High-Grade Serous Ovarian Carcinoma: A Systematic Review
Phyllis van der Ploeg1,2*, Meggy P.M. Ottenheijm1, Laura A.M. van Lieshout1,3, Anja van de Stolpe4, Steven L. Bosch5, Anna M.J. Thijs6, Ruud L.M. Bekkers1,2, Jurgen M.J. Piek1
1Department of Obstetrics and Gynecology, Catharina Hospital, Eindhoven, The Netherlands
2GROW School for Oncology and Developmental Biology, Maastricht University, Maastricht, The Netherlands
3Radboud Institute for Health Sciences, Department of Obstetrics and Gynecology, Radboud University Medical Center, Nijmegen, The Netherlands
4Molecular Diagnostics, Philips Research, Eindhoven, The Netherlands
5Laboratory for Pathology and Medical Microbiology (Stichting PAMM), Eindhoven, The Netherlands
6Department of Oncology, Catharina Hospital, Eindhoven, The Netherlands
*Corresponding Author: Dr. Phyllis van der Ploeg, Department of Obstetrics and Gynecology, Catharina Hospital, Eindhoven, The Netherlands
Received: 07 July 2020; Accepted: 03 August 2020; Published: 20 August 2020
Article Information
Citation: Phyllis van der Ploeg, Meggy P.M. Ottenheijm, Laura A.M. van Lieshout, Anja van de Stolpe, Steven L. Bosch, Anna M.J. Thijs, Ruud L.M. Bekkers, Jurgen M.J. Piek. Efficacy of Anti-Estrogen Therapy in Estrogen Receptor Positive High-Grade Serous Ovarian Carcinoma: A Systematic Review. Journal of Cancer Science and Clinical Therapeutics 4 (2020): 283-303.
View / Download Pdf Share at FacebookAbstract
Therapy targeting the estrogen receptor (ER) pathway is being explored as a treatment option in ovarian carcinoma. However, studies on the efficacy of anti-estrogen therapy include a broad range of histological subtypes and/or do not select patients based on ER status. This systematic review provides an analysis of literature on the clinical benefit rate (CBR) of anti-estrogen therapy in ER positive high-grade serous carcinomas (HGSC) and on the correlation between ER expression by immunohistochemistry and clinical response. We did not find studies with populations consisting solely of ER positive HGSC. However, we included six studies reporting on 407 evaluable patients of whom 376 were HGSC (92%) and 302 were confirmed ER positive (80%). Anti-estrogen therapy resulted in a CBR of 27-65% and an overall response rate of 0-16%. No correlation was found between ER expression and clinical response. Therefore, ER protein expression alone is not a specific predictor of response. This may result from the incorrect assumption that ER expression equals ER pathway activity, since in the absence of ER activating mutations, ER pathway activity depends on availability of the estradiol ligand. In order to apply effective ER targeted therapy, it is important to develop better predictors to identify (non)-responders.
Keywords
<p>High-grade serous ovarian carcinoma; Anti-estrogen targeted therapy; Clinical benefit rate</p> <gdiv></gdiv>
Article Details
1. Introduction
Ovarian carcinoma reflects a heterogenous disease compromised of five histological subtypes, namely high-grade serous, low-grade serous, endometrioid, clear cell and mucinous, that all differ in their cell-of-origin, pathogenesis and prognosis [1]. High-grade serous carcinoma (HGSC) is the most common subtype (70%) of ovarian carcinoma, of which 80% of the patients is diagnosed with advanced-stage disease due to asymptomatic and rapid tumor progression [2, 3]. Current treatment consisting of debulking surgery and (neo)adjuvant chemotherapy with carboplatin and paclitaxel frequently results in complete remission [4]. However, most patients will experience relapse of disease, which is often complicated by resistance to platinum containing chemotherapy. As only few options then remain, research focusses on the use of alternative therapies, such as targeting the estrogen receptor (ER) signaling pathway [5]. The ER signaling pathway can be initiated through direct or indirect estradiol signaling [6]. As a steroid hormone, estradiol can directly enter the cytoplasm where it can bind nuclear ER monomers (ER and ER ) and induces receptor dimerization. The transcription factor complex translocates to the nucleus where it binds to ER response elements in gene promoter regions and activates transcription of ER target genes. Alternatively, estradiol can bind a G-protein coupled receptor on the plasma membrane and hereby activate intracellular second messengers. In this manner estradiol can indirectly influence activation of other signaling pathways, such as the phosphoinositide 3-kinase (PI3K) signaling pathway [6]. ER signaling pathway activity can be inhibited by selective estrogen receptor modulators (SERMs; e.g. tamoxifen), selective estrogen receptor downregulators (SERDs; e.g. fulvestrant) or aromatase inhibitors (e.g. anastrozole, letrozole or exemestane) [7]. Tamoxifen is able to act both as a partial agonist and antagonist [8]. Like estradiol, it can bind to ER but the induced transcriptional activation of ER is lower. Tamoxifen competes with estradiol for ER binding and antagonizes the effect of estradiol, but in the absence of estradiol it will act as a partial agonist [8-10]. Fulvestrant has a pure antagonistic effect as it binds reversibly to ER monomers, which prevents receptor dimerization and thereby stimulates degradation of ER [11]. Aromatase is an essential molecule in the formation of estradiol and inhibition by anastrozole or letrozole leads to blockage of the final step in the steroid biosynthetic pathway to generate estradiol [12]. As a result, aromatase inhibitors are able to almost completely block estradiol production by aromatase-expressing cells.
Ovarian carcinoma is considered a hormone-dependent disease as estrogens caused proliferation of ovarian cancer cells in vivo and in vitro [13, 14]. However, the exact mechanism of action of estrogens in ovarian carcinoma is not fully understood. The use of anti-estrogen therapy is a well-established treatment for hormone-dependent breast cancer [15]. Oral administration and low toxicity makes anti-estrogens an attractive therapy option, which has also been studied in ovarian carcinoma during the past decades [16, 17]. A meta-analysis concluded that anti-estrogen therapy in ovarian cancer was associated with a modest clinical benefit rate (CBR) of 41% (95% confidence interval (CI): 34-48) [17]. CBR was defined as the total proportion of patients who had achieved complete response (CR), partial response (PR) and stable disease (SD). The selected trials in this meta-analysis included women with a broad range of ovarian cancer histological subtypes and multiple studies did not select patients based on ER status, resulting in a heterogenic population.
Immunohistochemical (IHC) ER protein staining of formalin fixed paraffin embedded sections is widely used to identify ER protein expression. A study from the Ovarian Tissue Analysis Consortium investigated ER positivity based on ³50% stained tumor cell nuclei in 2,933 ovarian carcinomas [18]. They found strong ER expression in 60% of the HGSC. However, in contrast to breast cancer, the predictive value of ER status on anti-estrogen response has not been well-established for HGSC. Additional ER scoring methods such as the histoscore method, which takes intensity and percentage of stained tumor cells into account, have been developed [19]. The first phase II trial with ovarian cancer patients treated with letrozole found a significant correlation between response and increasing ER histoscores (p<0.001) [20]. Another phase II trial with letrozole also reported a significant correlation (p=0.028), suggesting that patients with high ER histoscores respond better to anti-estrogen therapy [21]. However, this correlation has not been found in other studies [22, 23]. The conflicting findings may result from the inclusion of ER negative patients in these studies, as these patients may have obscured the relation between ER expression and response to anti-estrogen therapy. In this systematic review, we aim to analyze the literature on the CBR of anti-estrogen therapy in a homogenic population of ER positive metastatic or recurrent HGSC. Additionally, we aim to correlate ER expression based histoscores to clinical response.
2. Methods
2.1. Search strategy
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [24]. Studies were identified by performing a literature search in the electronic databases PubMed, the Cochrane Library and ClinicalTrails.gov. The search query combined synonyms and Mesh terms for ‘ovarian carcinoma’ and ‘anti-estrogen therapy’. The full search is shown as supplementary material (Table S1: Search strategy). The last search was conducted on December 13th, 2019.
2.2. Inclusion criteria
Studies were eligible for inclusion when reporting clinical response rates of anti-estrogen therapy in ER positive metastatic or recurrent HGSC patients. Anti-estrogen therapy included the following drugs: tamoxifen, anastrozole, letrozole, exemestane and fulvestrant. In order to review the latest clinical results, only studies published during the last 10 years were included. Language was restricted to English and full study results had to be available. All clinical studies were included with the exception of case reports. Reviews and meta-analysis were not eligible for inclusion, but reference lists were carefully screened for any additional inclusions.
2.3. Data extraction and quality assessment
Eligibility assessment was performed independently by two reviewers (P.v.d.P. and M.P.M.O.) and any disagreements were resolved by discussion with a third reviewer (J.M.J.P.). Data was extracted using pre-designed standardized data collection forms which included publication details, study design, sample size, study population, histologic subtype, type of treatment, method of response measurement, clinical outcomes, type of ER measurement, ER status and measured correlation between ER status and therapy response.
Two reviewers (P.v.d.P. and M.P.M.O.) independently assessed risk of bias using the ROBINS-I tool for non-randomized studies [25]. Risk of bias was scored as high, low or unclear risk and assessed for the following domains: confounding, selection of participants, co-interventions, missing data, measurement of outcome, selective reporting and other sources of bias. To use the ROBINS-I tool, a hypothetical ‘target’ trial is necessary to assess bias. We defined this trial as a phase II or III clinical trial of anti-estrogen therapy in ER positive metastatic or recurrent HGSC. Criteria that this trial should meet are as follows: 1) Baseline information should include tumor histology, number of prior lines of (chemo)therapy and ER status. 2) Detailed information on the intervention is required and no co-interventions would be allowed during the treatment period. 3) CBR should be measured after a minimum of three months by GCIG and/or RECIST criteria.
2.4. Statistical analysis
The primary outcome measure was CBR, defined as the percentages of patients that achieved CR, PR and SD. Secondary outcome measures were overall response rate (ORR), defined as the percentages of patients with CR and PR, median progression-free survival (PFS) and median duration of response. For CBR and ORR 95% CI were calculated using the modified Wald method [26].
3. Results
3.1. Search results
We identified a total of 461 references from PubMed, Cochrane Library and clinicaltrials.gov, of which 434 remained after removal of duplicates. After title and abstract screening 386 articles were excluded. Full-text screening of the remaining 48 articles resulted in the exclusion of an additional 41 articles as they did not meet the inclusion criteria, mostly because they lacked data on ER status. There were no clinical studies available with a population consisting entirely of ER positive HGSC. However, we identified seven articles describing six individual clinical studies reporting on anti-estrogen therapy in ovarian cancer populations partially consisting of ER positive HGSC. The screening and selection process and reasons for exclusion are illustrated in a PRISMA flowchart (Figure 1).
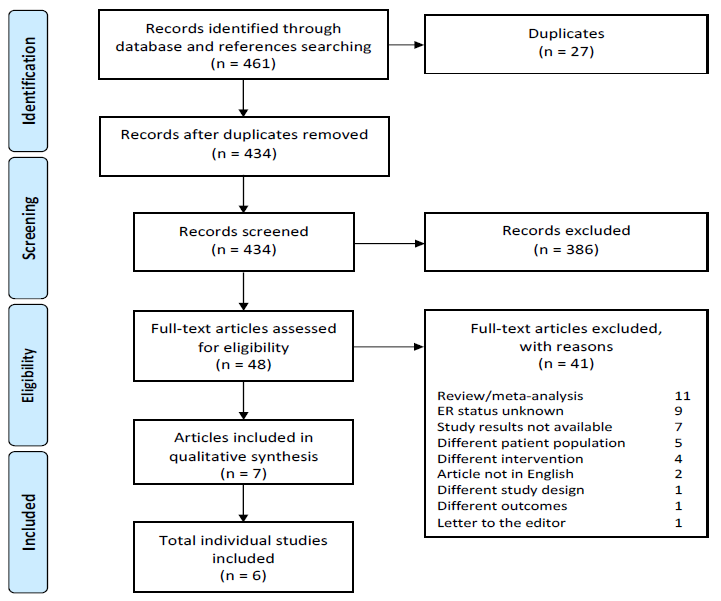
Figure 1: PRISMA flowchart of systematic literature search and selection of studies. ER: estrogen receptor.
3.2. Description of included studies
The sample size of included studies ranged from 19 to 164 evaluable patients, resulting in a total of 407 included ovarian cancer patients, 376 of whom were diagnosed with HGSC (92%). ER positivity was confirmed in 302 out of 376 HGSC (80%). Study characteristics are summarized in Table 1 and a detailed description of the included studies is given below.
Argenta et al. [27, 28] reported two studies on one phase II clinical trial of fulvestrant treatment in 31 (26 evaluable) ER positive recurrent ovarian carcinoma patients, 16 (62%) of which were HGSC. Treatment regimen consisted of 500 mg intramuscular (IM) on day 1, followed by 250 mg IM on day 15, day 29 and every 28 days thereafter. Primary endpoint was CBR at 90 days based on modified Rustin and RECIST criteria. ER positivity was based on >10% stained tumor cell nuclei in primary tumor tissue and ER histoscores were obtained in 24 patients (92%) using the archived paraffin-embedded blocks.
Bonaventura et al. [29] performed a phase II clinical trial of 53 (49 evaluable) platinum-resistant recurrent ovarian carcinoma treated with anastrozole (1.0 mg daily). Most patients were HGSC, but the exact percentage was not specified. CBR was determined by GCIG or RECIST 1.1 criteria every three months of treatment. All patients were ER positive based on >10% stained tumor cell nuclei and tumor tissue blocks for ER histoscore assessment were available in 34 patients (69%) with clinical response data.
Colon-Otero et al. [30] presented a phase II clinical trial enrolling 20 patients with platinum-resistant or -sensitive relapsed ovarian cancers treated with a combination of letrozole (2.5 mg per oral (PO) daily) and the mammalian target of rapamycin (mTOR) inhibitor everolimus (10 mg PO daily). Nineteen patients were evaluable for response evaluation, 17 of which were HGSC (89%). Primary endpoint was PFS at 12 weeks based on CA125 tumor marker concentrations or radiological assessments by RECIST 1.1. All patients were defined ER positive, however no information was given about the threshold for ER positivity or whether this was based on primary or recurrent tumor tissue.
Kok et al. [31] reported a phase II clinical trial of anastrozole (1.0 mg PO daily) in asymptomatic ER positive recurrent ovarian cancer patients based on CA125 progression. These patients normally await (chemo)therapy until symptoms occur. The study population consisted of 52 evaluable patients of whom 40 were HGSC (77%). Primary endpoint was CBR at three months based on GCIG or RECIST 1.1 criteria. ER positivity was based on >10% stained tumor cell nuclei and histoscores were calculated retrospectively in 28 patients (54%) on available archival tissue blocks.
The study of George et al. [32] was a retrospective cohort study in 97 platinum-resistant or -sensitive relapsed ovarian carcinoma patients, 90 of which were HGSC (93%). Patients were treated with tamoxifen 20 or 40 mg PO daily (44%) or letrozole 2.5 mg PO daily (56%). CBR was measured after three months based on GCIG or RECIST 1.1 criteria. Fifty-two percent of the population was classified ER positive, although a threshold of percentage positive ER stained tumor cell nuclei was not mentioned. In 47% ER status was unknown and 1% was ER negative. The authors did not specify if IHC staining was conducted on primary or recurrent material and ER histoscores were not calculated.
Table 1: Patient and treatment characteristics of studies including high-grade serous carcinoma treated with anti-estrogen therapy.
Stanley et al. [33] conducted a retrospective cohort study in 267 (164 evaluable for CA125 response) platinum-resistant or – sensitive relapsed HGSC patients treated with letrozole (78%) or tamoxifen (22%). Patients received at least four weeks of treatment. Response after 12 weeks was based on modified GCIG criteria due to variable frequency of CA125 measurements. ER histoscores were calculated in 225 patients (148 evaluable for CA125 response) of which the majority (85%) was based on primary tumor tissue prior to chemotherapy.
3.3. Risk of bias in included studies
Studies were subjected to a comprehensive quality assessment for the risk of bias on seven predefined domains and reviewers’ judgements of each domain were summarized in Table 2.
3.3.1. Bias due to confounding: We considered four studies to be at low risk of bias due to confounding related to the detailed description of the patient characteristics [27-31]. Populations consisted of recurrent or metastatic ER positive disease with at least one prior line of chemotherapy. In two studies a proportion of the population had unknown ER status and therefore these studies were considered to be at high risk of bias [32, 33].
3.3.2. Bias in selection of participants: Five studies we rated at low risk of bias due to selection of participants as the inclusion- and exclusion criteria and selection process were descripted in detail and reasons for exclusion were mentioned [27-31, 33]. One study did not sufficiently describe the exclusion criteria and selection process and thus was rated as at unclear risk of bias [32].
3.3.3. Bias in classification of intervention: We rated three studies as at low risk of bias due to classification of intervention as the intervention was described in detail and no co-interventions were allowed [27, 28, 30, 32]. Three studies did not sufficiently describe the intervention and/or did not describe if the use of co-interventions during the study period was excluded. These studies were considered to be at unclear risk of bias [29, 31, 33].
3.3.4. Bias due to deviations from intended intervention: We judged two studies to be at low risk of bias due to deviations from intended intervention as treatment delay did not occur and reasons for dose modifications were specified [27, 28, 30]. Three studies did not describe if deviations from intended intervention, dose modifications or treatment delay occurred and were rated as at unclear risk of bias [29, 31, 33]. One study was considered to be at high risk of bias as duration of anti-estrogen therapy use was not defined and a substantial proportion (18%) of the population received both tamoxifen and letrozole as a single agent during the study period [32].
3.3.5. Bias due to missing or incomplete data: Four studies sufficiently described number of included and evaluable patients and were judged as low risk of bias due to missing or incomplete data [27-31]. One study was considered as unclear risk of bias as they did not specify the number of exclusions based on missing response data [32]. One study was considered to be at high risk of bias as only 64% of the patients were evaluable for CA125 response [33].
3.3.6. Bias in measurement of outcome: We judged four studies as at low risk of bias due to measurement of outcome related to a detailed description of criteria for response evaluation [27-31]. Two retrospective studies were rated as at high risk of bias as timepoints of response measurements were not standardized [32, 33].
3.3.7. Bias in selection of reported results: One study reported the outcomes according to the published protocol and thus we considered it at low risk of bias in selection of reported results [27, 28]. Four studies were rated at unclear risk of reporting bias: two studies referred to the same protocol [29, 31] and for the other studies we were not able to find a published protocol [32, 33]. One study was rated to be at high risk of reporting bias as the primary objective in the study protocol stated to compare the PFS of combination therapy with letrozole and everolimus with results from a previously reported phase II study with letrozole monotherapy [30]. However, results were not compared as the article does not refer to a previously conducted phase II trial.
3.3.8. Other sources of bias: We identified no other sources of bias.
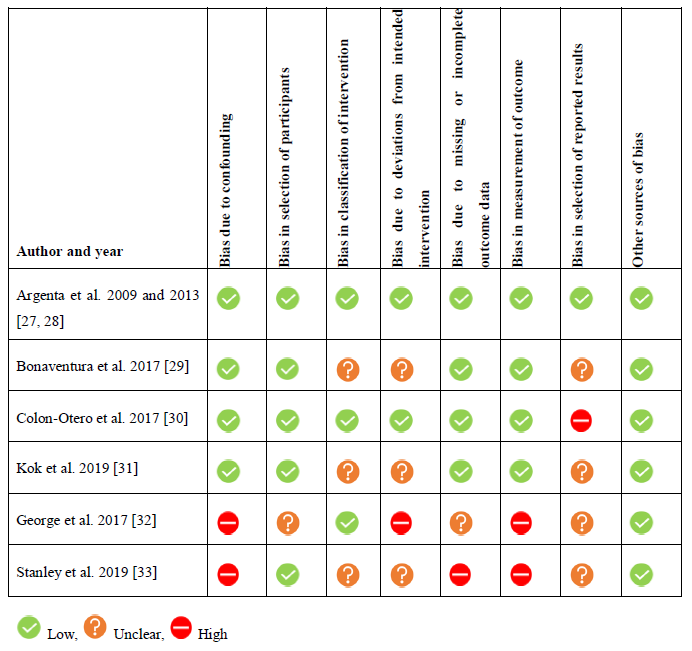
Figure 1: PRISMA flowchart of systematic literature search and selection of studies. ER: estrogen receptor.
Table 2: Risk of bias of included studies by reviewers’ judgement.
3.4. Clinical outcome of anti-estrogen therapy
The included studies in this systematic review reported a CBR ranging between 27-65% and an ORR ranging between 0-16% after approximately three months of anti-estrogen therapy (Table 3, Figure 2). The median PFS ranged between 2.0-3.9 months and the mean duration of response ranged between 2.8-6.5 months. Argenta et al. investigated the effect of fulvestrant injections in patients with multiple recurrent disease and observed a CBR of 50% (95% CI: 32-68) based on computer tomography evaluation [27, 28]. Both Bonaventura et al. and Kok et al. reported on anastrozole therapy and observed a CBR of 27% (95% CI: 16-40) and 35% (95% CI: 23-48), respectively [29, 31]. The study population of Bonaventura et al. included platinum-resistant recurrent patients compared to asymptomatic recurrent patients in the study of Kok et al. [29, 31]. The combination of the aromatase inhibitor letrozole with the mTOR inhibitor everolimus resulted in a CBR of 53% (95% CI: 32-73) in platinum-resistant or -sensitive relapsed patients in the study of Colon-Otero et al. [30]. Two of the included trials reported results from both tamoxifen and letrozole therapy in platinum-resistant or -sensitive relapsed patients. George et al. reported an overall CBR of 60% (95% CI: 50-69). They found a CBR of 65% (95% CI: 50-78) with tamoxifen therapy, which was not statistically higher than the 56% CBR (95% CI: 42-68) of letrozole therapy (p=0.140) [32]. Though, the median duration of response in the group with partial responders was longer with letrozole therapy compared to tamoxifen therapy (26.0 versus 11.5 months, respectively, p=0.030). Stanley et al. reported an overall CBR of 40% (95% CI: 32-47) but did not find a significant difference between tamoxifen (33%, 95% CI: 20-50) or letrozole (41%, 95% CI: 33-50) CBR (p=0.495) [33]. They noticed a longer median duration of letrozole therapy compared to tamoxifen (126 versus 98 days, respectively, p=0.006 in univariable analysis, p=0.255 in multivariable analysis).
3.5. Correlation between ER histoscores and therapy response
Four of the included studies obtained ER histoscores and correlated this to anti-estrogen therapy response (Table 3) [28, 29, 31, 33]. In total, ER histoscores of 234 ovarian cancer patients were reported, which in most cases was assessed on archived primary tumor tissue instead of a tissue sample of recurrent disease. Argenta et al. demonstrated significantly higher ER histoscores in subjects responding to fulvestrant therapy compared to non-responders (p=0.020) [28]. Bonaventura et al. clustered histoscores into groups of ER histoscores < 100 (n=13), 100 to 200 (n=11) and > 200 (n=10) and correlated this to a CBR of 31%, 18% and 50%, respectively [29]. The highest response rates were seen in patients with the highest histoscores although a statistically significant difference could not be objectified. A paradoxical difference was found in the lowest histoscore group were the CBR was superior to the middle-range histoscore group, which might indicate that an increasing ER histoscore is not directly proportional to therapy response. Kok et al. were also unable to find a significant correlation, as they found a CBR of 25% in patients with ER histoscores of 0-100 (n=8), 50% in the group with ER histoscores of 101-200 (n=12) and 25% in the group with ER histoscores of 201-300 (n=8) [31]. In the retrospective cohort of Stanley et al. a positive trend in response was seen with increasing ER histoscores. They reported a CBR of 34% in patients with ER histoscores of 0-150 (n=12), 40% in the group with ER histoscores of 151-200 (n=14), 48% in the group with ER histoscores of 201-250 (n=21) and 47% in the group with ER histoscores of 251-300 (n=16) (p=0.404) [33]. The authors included an additional analysis in which they also included patients with a delayed stable disease (patients whose CA125 tumor maker rose and afterwards stabilized) to the responding group. Then, the group with the highest ER histoscores (251-300, n=22) had a significant higher CBR of 65% compared to 37% in the lowest ER histoscores group (0-150, n=13) (p=0.040).
Table 3: Estrogen receptor (ER) targeted therapy response and ER status in high-grade serous carcinoma (HGSC). CR: complete remission, PR: partial response, SD: stable disease, ORR: overall response rate, CBR: clinical benefit rate, PFS: progression free survival, IHC: immunohistochemistry.
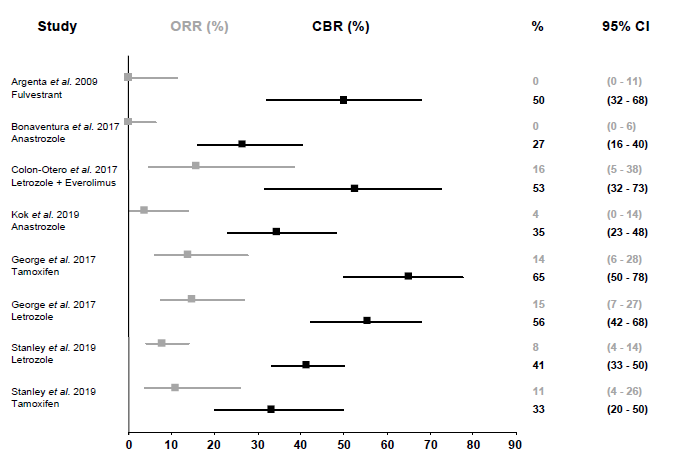
Figure 2: Overall response rate (ORR) and clinical benefit rate (CBR) of anti-estrogen therapy. ORR and CBR are shown with 95% confidence intervals (CI). ORR is defined as the proportion (%) of patients with complete or partial response. CBR is defined as the proportion (%) of patients with complete response, partial response or stable disease.
4. Discussion
4.1. Clinical benefit of anti-estrogen therapy in HGSC
This systematic review aims to analyze studies reporting on the clinical benefit of anti-estrogen therapy in ER positive metastatic or recurrent HGSC. Although there were no studies including solely ER positive HGSC patients, we were able to identify six individual clinical studies reporting on anti-estrogen therapy in ovarian cancer populations partially consisting of ER positive metastatic or recurrent HGSC. The included studies reported a CBR ranging between 27-65% and an ORR ranging between 0-16% in a population consisting of 92% recurrent or metastatic HGSC, 80% of whom were confirmed ER positive.
4.2. Predicting anti-estrogen therapy response by ER expression by immunohistochemistry
We analyzed the literature to find a correlation between ER expression and clinical response in order to identify the specificity of this marker as a predictor of anti-estrogen therapy response. Of the four studies reporting ER histoscores, two studies were not able to find a significant correlation [29, 31]. The other two studies did report significant differences between groups but were unable to provide strong evidence for a correlation between increasing ER histoscores and improved clinical response [27, 28, 33]. Argenta et al. found a significant difference in mean ER histoscore between the responding and non-responding group [27, 28]. Furthermore, Stanley et al. reported a statistical significant difference in CBR between two ER histoscore groups, but they did not report a significant correlation between increasing histoscores and better clinical response [33]. Additionally, this difference was only significant when patients with a delayed stable disease were included to the response data. These results do suggest that clinical response is more likely in HGSC with the highest levels of ER histoscores (>250), but lower histoscores seem to be inconclusive in predicting therapy response. To conclude, we had insufficient data to identify ER expression by IHC as a specific predictor for anti-estrogen therapy response, which could be caused by the relatively small number of patients with available ER histoscores.
Another possible explanation for the lack of support for a correlation between ER expression and therapy response might be the use of primary tumor samples for ER expression assessment, while anti-estrogen therapy was administered to metastatic or recurrent disease after (multiple lines of) chemotherapy. Both the metastatic process and cytotoxic regimes may result in a change in tumor driving signaling pathways in recurrent disease compared to the primary tumor. ER expression discordance between untreated primary tumor and tumor tissue taken after chemotherapy treatment has been demonstrated in HGSC by Van Kruchten et al. [34]. They found differing ER expression in tumor tissue taken at diagnosis compared to tumor obtained at debulking surgery after neo-adjuvant chemotherapy. In two patients IHC ER staining appeared to be negative at diagnosis, while the subsequent tumor sample taken after chemotherapy showed positive ER expression. Furthermore, ER expression instability has been reported in 35% of paired primary and recurrent HGSC patients, of which 14% showed loss and 21% gain of ER expression [35]. In addition, discrepancies in ER expression between primary tumor and corresponding distant metastases have been found. Several studies investigated primary breast cancers and corresponding distant metastases and reported changes in ER status in 15-40% of the patients [36]. Visualization of ER expression by a fluorescent estradiol tracer on positron emission tomography (PET) imaging in metastatic breast cancer patients revealed heterogenous ER expression between primary tumor and metastases in up to 45% of the patients [37]. ER expression discordance may have been caused by tumor evolution, although heterogeneity in ER expression within a tumor as an alternative cause cannot be excluded. Despite this uncertainty as to the cause, results support the importance of ER status re-assessment in the actual tumor to be treated, preferably by taking multiple histological samples, to improve identification of patients sensitive to anti-estrogen therapy.
Moreover, it could be questioned if receptor staining is the most adequate predictor of signaling pathway activity. In absence of ER the direct ER signaling pathway is inactive. However, positive nuclear ER staining does not automatically imply that the ER signaling pathway is transcriptionally active, since pathway activity depends on availability of the estradiol ligand [38, 39], or alternatively (but rare) an activating ER mutation [40]. The potential clinical relevance of the discrepancy between positive ER staining and actual ER pathway activity has recently been investigated using a mRNA based ER pathway activity test which provides an ER pathway activity score based on computational interpretation of the expression values of the ER target genes [41-44]. In three ER positive breast cancer cohorts, low ER pathway activity scores were found in breast cancer patients who failed to respond to aromatase inhibitors and showed progressive disease [42]. The ER pathway activity test was also used to investigate the correlation between ER IHC staining and actual ER signaling pathway activity in a metastatic breast cancer cohort [43]. While low ER expression was always associated with very low ER pathway activity scores, indicating an inactive ER pathway, cases with high ER expression appeared to have a wide variation in ER pathway scores. These results were confirmed in another retrospective breast cancer cohort in which metastatic disease was treated with tamoxifen [44]. Although all primary tumor samples were ER positive, 41% of the patients had high ER pathway activity scores which was associated with longer time to progression of metastases (p=0.005). No correlation was observed between ER expression and ER pathway activity (p=0.400). These results provide evidence that positive ER expression not necessarily means an activated ER signaling pathway and that ER expression alone is not a sufficiently specific predictor of anti-estrogen therapy response.
4.3. Mechanism of action and clinical response of anti-estrogen agents
Our results do not demonstrate distinct superiority of one type of anti-estrogen therapy over another. The CBR of tamoxifen and letrozole treatment were comparable [32, 33]. However, letrozole in general was associated with longer median duration of response in two studies investigating both agents [32, 33]. This might be explained by the partial agonistic effect of tamoxifen, which activates the ER pathway to some extent and carries a risk for tumor progression [9, 10].
Within the group of aromatase inhibitors, it is suggested that letrozole is more effective than anastrozole [45, 46]. A pharmacodynamic study in breast cancer patients reported letrozole to be more effective in inhibiting aromatase activity and circulating estradiol levels compared to anastrozole [47]. Whether this also translated in improved survival rates was recently studied in a phase II randomized trial designed to compare the efficacy of letrozole and anastrozole in breast cancer patients [48]. No statistically significant difference in disease-free and overall survival between letrozole and anastrozole therapy was observed. In our review, comparison of letrozole and anastrozole therapy in HGSC shows a CBR of 41-56% compared to 27-35%, respectively [29, 31-33]. However, these results are based on indirect comparison of studies with heterogeneity in study design (retrospective cohorts versus phase II clinical trials) and patient population (platinum-resistant or -sensitive versus asymptomatic recurrent disease). Therefore, no conclusion can be drawn about the superiority of one aromatase inhibitor over another in HGSC.
One of the included studies investigated the use of fulvestrant; an ER downregulator which stimulates degradation of ER [27, 28]. Fulvestrant resulted in a CBR of 50% according to RECIST criteria [27, 28]. Fulvestrant can be effective in case of activating mutations in the ESR1 gene encoding for ER [49, 50]. Mutations in ESR1 confer ligand-independent transcriptional activity of the ER transcription factor. ESR1 mutations can arise de novo prior to anti-estrogen therapy [40]. However, acquired ESR1 mutations frequently occur as a resistance mechanism to aromatase inhibitor therapy, as seen in metastatic breast cancer patients, were mutations were found in approximately 20% of the patients [51-53]. In contrast to breast cancer, little is known about ESR1 mutations in ovarian cancer. A recent study identified ESR1 alterations in 2.1% of the studied ovarian cancers (n=5,594) [40]. As the majority of the investigated samples might be from primary disease, further research is needed to explore the prevalence of ESR1 mutations in metastatic ovarian cancers.
Taken together, the different mechanisms of actions of anti-estrogen agents emphasize the importance of knowledge on functionality of the ER signaling pathway in order to select the appropriate anti-estrogen agents. In case of positive ER expression in the absence of estradiol, tamoxifen could act as a partial agonist and might stimulate tumor progression. In case of ESR1 mutations, treatment with aromatase inhibitors probably results in non-responding patients as ER activation is independent from the estradiol ligand.
4.4. Anti-estrogen therapy in combination with a mTOR inhibitor
In the study of Colon-Otero et al., letrozole was combined with the mTOR inhibitor everolimus [30]. mTOR is a member of the PI3K/AKT/mTOR signaling pathway which plays a critical role in cell growth and proliferation and is frequently activated in ovarian cancer [54-56]. The rationale for combined therapy is that PI3K/AKT/mTOR pathway activation could diminish the effect of anti-estrogen therapy, as the PI3K/AKT/mTOR pathway may activate estradiol-independent transcription of ER [57]. This second tumor-driving pathway may cause primary resistance to anti-estrogens. A phase III clinical trial in advanced breast cancer patients treated with exemestane in combination with everolimus after progression on aromatase inhibitors resulted in a significant prolonged PFS by more than twofold compared to exemestane alone [58]. The combination of letrozole and everolimus in the study of Colon-Otero et al. resulted in a relative promising CBR of 53%, but it must be noted that this study had a smaller sample size compared to the other included studies in this review [30]. A lower CBR has been reported in a phase I clinical trial with 50 ER positive advanced gynecologic and breast malignancies treated with anastrozole and everolimus [59]. Two of the six serous ovarian carcinoma patients (33%) had SD for ³6 months. However, this study was not included in this review as it could not be confirmed that the serous carcinoma were high-grade. The molecular alterations and crosstalk between the PI3K/AKT/mTOR and ER signaling pathways suggest a promising role for combinations of these inhibitors, which should be further explored in clinical trials focusing on selecting optimal patient populations.
4.5. Relation between ER expression and tumor differentiation
Although the majority of the patient populations of the included studies in this review represent HGSC, small numbers of endometrioid, low grade serous (LGSC), clear cell, transitional cell carcinoma, carcinosarcoma and granulosa cell tumors were included. The Ovarian Tissue Analysis Consortium reported significant differences in ER expression between histologic subtypes [18]. Strong ER expression was found in 71% of LGSC and in 60% of HGSC and endometrioid carcinoma in contrast to a mere 14% in clear cell carcinoma. Apart from ER expression, these subtypes differ clearly in cell type of origin and clinical behavior [60, 61]. Noticeably, relatively high response rates were observed in better-differentiated carcinomas. A phase II study treating patients with ER positive recurrent or metastatic LGSC (89%) and serous borderline tumors (11%) with anastrozole found a CBR of 64% for ³6 months [62]. In addition, a retrospective cohort study with a homogenous population of 64 patients diagnosed with recurrent LGSC treated with anti-estrogen therapy reported a CBR of 71% [63]. These improved response rates may be related to the inverse relationship between ER expression and tumor grade, in which low-grade tumors have high ER expression, which may reflect higher ER pathway activity. In line with this, studies in breast cancer patients reported loss of ER expression by increasing histologic grade [64-67]. The inverse association between ER expression and tumor grade was also found in two independent endometrial carcinoma cohorts, in which ER pathway activity was also measured, using the aforementioned ER pathway activity test [68]. In this study, higher ER expression as measured with ER IHC staining was associated with higher ER pathway activity scores. These results suggest that indeed the inverse correlation between ER staining and grade extends to an inverse relation between actual ER pathway activity and tumor grade. This provides a logical explanation for the better response to anti-estrogen therapy in lower grade ovarian cancer types with higher ER expression: the ER pathway is probably more active providing an effective therapy target.
4.6. Efficacy of anti-estrogen therapy in ER negative patients
Although we focused on studies with ER positive populations in this review, interesting results have been reported in studies that included ER negative patients as well. Del Carmen et al. compared time to disease progression after anastrozole therapy between patients with ER positive (n=32) and negative (n=13) asymptomatic recurrent or persistent ovarian carcinoma [69]. They found a median time to progression of 72 days in the ER positive group compared to 125 days in the ER negative group. Although these results suggest a superior response in ER negative patients, survival analysis showed no differences in time to progression between the ER positive and negative group. In line with this, a retrospective cohort of Stasenko et al. also was not able to find a significant improved PFS in ER positive patients compared to ER negative patients. They reported a median time to progression of 4.0 months in the ER positive group (n=44) compared to 2.0 months in the negative group (n=19) (p=0.360) [23]. In addition, a meta-analysis including a heterogenic ovarian cancer population with ER positive and negative patients reported subgroup analysis for hormone receptor status [17]. They found a CBR of 46% in ER positive and/or progesterone receptor positive patients, 44% in exclusively ER positive patients and 37% in patients with unknown receptor status, which was not statistically significant (p=0.540) [17]. The fact that these studies did not report a significant superior therapy response in ER positive patients compared to ER negative patients, cautions against deciding on anti-estrogen therapy based on ER expression assessment on a single tissue sample from the primary tumor. It is important to acknowledge that tumor heterogeneity could lead to sampling errors and that tumor tissue from primary diagnosis is not representative for the recurrent tumor.
4.7 Recommendations
Further research on the identification of responding patients for anti-estrogen therapy should focus on the use of mRNA levels of target genes of the ER transcription factor. We mentioned an ER pathway activity test based on computational interpretation of the expression levels of several ER target genes [41]. In our opinion, measuring a panel of ER specific target genes would be a more appropriate approach to predict ER signaling pathway activity.
Furthermore, we would like to address the lack of large prospective trials comparing anti-estrogen therapy with standard next-line treatment. There has been only one phase III randomized controlled trial comparing tamoxifen to chemotherapy treatment in platinum-resistance ovarian carcinoma patients [70]. Unfortunately, determination of ER status was not incorporated in the study design, resulting in exclusion of this study from our analysis. Although, the authors report a better PFS after chemotherapy treatment compared to tamoxifen treatment, there was no difference in overall survival between the treatment groups [70]. In addition, tamoxifen treatment was associated with less toxicity and better quality of life. These results suggest a role for anti-estrogens in ovarian carcinoma treatment but emphasize the need for further prospective trials comparing standard treatment to anti-estrogen therapy in selected ovarian carcinoma patients with a functionally active ER pathway.
5. Conclusion
In our systematic review we included six clinical studies and found a clinical benefit to anti-estrogen therapy in 27-65% of the population consisting for 92% of recurrent or metastatic HGSC, of which 80% was confirmed ER positive. Complete and partial response rates are low, as we found an ORR of 0-16%. No correlation was found between ER expression and clinical response. Therefore, ER protein expression alone is not a specific predictor of response. Treatment with anti-estrogen therapy is probably only effective when the ER pathway is functionally active, which depends on availability of the estradiol ligand or alternatively (but rare) on an activating ER mutation. The currently used IHC ER staining is insufficiently specific for identification of transcriptionally active ER in HGSC, probably resulting in treatment of a population of non-responder patients. Even worse, in the absence of estradiol, tamoxifen may exert its partial agonistic action and may potentially stimulate tumor progression. As ER expression remains an unreliable response predictor, it is of great importance to measure actual ER pathway activity in order to improve therapy response.
Supplementary Material
Table S1: Search strategy.
Author Contributions
Conceptualization, J.M.J.P., P.v.d.P. and M.P.M.O.; Methodology, P.v.d.P. and M.P.M.O.; Formal Analysis, P.v.d.P. and M.P.M.O.; Data interpretation, P.v.d.P., M.P.M.O., L.A.M.v.L., A.v.d.S., S.L.B., A.M.J.T., R.L.M.B. and J.M.J.P.; Writing – Original Draft Preparation, P.v.d.P.; Writing – Review & Editing, P.v.d.P, M.P.M.O., L.A.M.v.L., A.v.d.S., S.L.B., A.M.J.T., R.L.M.B. and J.M.J.P. All authors have approved the content of the article.
Funding
This systematic review did not receive direct funding.
Conflict of Interest
P.v.d.P. is employee of Catharina hospital, but her research work is funded by the Catharina Research fund and Molecular Diagnostics, Philips Research. A.v.d.S. is employee of Molecular Diagnostics, Philips Research. The other authors declare no conflict of interest.
References
- Karnezis AN, Cho KR, Gilks CB, et al. The disparate origins of ovarian cancers: pathogenesis and prevention strategies. Nat Rev Cancer. 17 (2017): 65-74.
- Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 68 (2018): 284-296.
- Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 14 (2017): 9-32.
- Lisio MA, Fu L, Goyeneche A, et al. High-Grade Serous Ovarian Cancer: Basic Sciences, Clinical and Therapeutic Standpoints. Int J Mol Sci. 20 (2019).
- Voutsadakis IA. Hormone Receptors in Serous Ovarian Carcinoma: Prognosis, Pathogenesis, and Treatment Considerations. Clin Med Insights Oncol. 10 (2016): 17-25.
- Fuentes N, Silveyra P. Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol. 2019 116: 135-170.
- Sommeijer DW, Sjoquist KM, Friedlander M. Hormonal treatment in recurrent and metastatic gynaecological cancers: a review of the current literature. Curr Oncol Rep. 15 (2013): 541-548.
- Frasor J, Stossi F, Danes JM, et al. Selective estrogen receptor modulators: discrimination of agonistic versus antagonistic activities by gene expression profiling in breast cancer cells. Cancer Res. 64 (2004): 1522-1533.
- Berry M, Metzger D, Chambon P. Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J. 9 (1990): 2811-2818.
- McInerney EM, Katzenellenbogen BS. Different regions in activation function-1 of the human estrogen receptor required for antiestrogen- and estradiol-dependent transcription activation. J Biol Chem. 271 (1996): 24172-24178.
- Carlson RW. The history and mechanism of action of fulvestrant. Clin Breast Cancer. 2005 6 Suppl 1: S5-8.
- Miller WR. Aromatase inhibitors: mechanism of action and role in the treatment of breast cancer. Semin Oncol. 30 (2003): 3-11.
- Armaiz-Pena GN, Mangala LS, Spannuth WA, et al. Estrous cycle modulates ovarian carcinoma growth. Clin Cancer Res. 15 (2009): 2971-2978.
- Chan KK, Leung TH, Chan DW, et al. Targeting estrogen receptor subtypes (ERalpha and ERbeta) with selective ER modulators in ovarian cancer. J Endocrinol. 221 (2014): 325-336.
- Manna PR, Molehin D, Ahmed AU. Dysregulation of Aromatase in Breast, Endometrial, and Ovarian Cancers: An Overview of Therapeutic Strategies. Prog Mol Biol Transl Sci. 144 (2016): 487-537.
- Williams C, Simera I, Bryant A. Tamoxifen for relapse of ovarian cancer. Cochrane Database Syst Rev. (2010): CD001034.
- Paleari L, Gandini S, Provinciali N, et al. Clinical benefit and risk of death with endocrine therapy in ovarian cancer: A comprehensive review and meta-analysis. Gynecol Oncol. 146 (2017): 504-513.
- Sieh W, Kobel M, Longacre TA, et al. Hormone-receptor expression and ovarian cancer survival: an Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 14 (2013): 853-8562.
- Kirkegaard T, Edwards J, Tovey S, et al. Observer variation in immunohistochemical analysis of protein expression, time for a change? Histopathology. 48 (2006): 787-794.
- Bowman A, Gabra H, Langdon SP, et al. CA125 response is associated with estrogen receptor expression in a phase II trial of letrozole in ovarian cancer: identification of an endocrine-sensitive subgroup. Clin Cancer Res. 8 (2002): 2233-2239.
- Smyth JF, Gourley C, Walker G, et al. Antiestrogen therapy is active in selected ovarian cancer cases: the use of letrozole in estrogen receptor-positive patients. Clin Cancer Res. 13 (2007): 3617-3622.
- Papadimitriou CA, Markaki S, Siapkaras J, et al. Hormonal therapy with letrozole for relapsed epithelial ovarian cancer. Long-term results of a phase II study. Oncology. 66 (2004): 112-117.
- Stasenko M, Plegue M, Sciallis AP, et al. Clinical response to antiestrogen therapy in platinum-resistant ovarian cancer patients and the role of tumor estrogen receptor expression status. Int J Gynecol Cancer. 25 (2015): 222-228.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 339 (2009): b2700.
- Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 355 (2016): i4919.
- Agresti AC, B.A. Approximate is better than ‘exact’ for interval estimation of binomial proportions. Am Stat. 52 (1998): 119-126.
- Argenta PA, Thomas SG, Judson PL, et al. A phase II study of fulvestrant in the treatment of multiply-recurrent epithelial ovarian cancer. Gynecol Oncol. 113 (2009): 205-209.
- Argenta PA, Um I, Kay C, et al. Predicting response to the anti-estrogen fulvestrant in recurrent ovarian cancer. Gynecol Oncol. 131 (2013): 368-373.
- Bonaventura A, O'Connell RL, Mapagu C, et al. Paragon (ANZGOG-0903): Phase 2 Study of Anastrozole in Women With Estrogen or Progesterone Receptor-Positive Platinum-Resistant or -Refractory Recurrent Ovarian Cancer. Int J Gynecol Cancer. 27 (2017): 900-906.
- Colon-Otero G, Weroha SJ, Foster NR, et al. Phase 2 trial of everolimus and letrozole in relapsed estrogen receptor-positive high-grade ovarian cancers. Gynecol Oncol. 146 (2017): 64-68.
- Kok PS, Beale P, O'Connell RL, et al. PARAGON (ANZGOG-0903): a phase 2 study of anastrozole in asymptomatic patients with estrogen and progesterone receptor-positive recurrent ovarian cancer and CA125 progression. J Gynecol Oncol. 30 (2019): e86.
- George A, McLachlan J, Tunariu N, et al. The role of hormonal therapy in patients with relapsed high-grade ovarian carcinoma: a retrospective series of tamoxifen and letrozole. BMC Cancer. 17 (2017): 456.
- Stanley B, Hollis RL, Nunes H, et al. Endocrine treatment of high grade serous ovarian carcinoma quantification of efficacy and identification of response predictors. Gynecol Oncol. 152 (2019): 278-285.
- van Kruchten M, de Vries EF, Arts HJ, et al. Assessment of estrogen receptor expression in epithelial ovarian cancer patients using 16alpha-18F-fluoro-17beta-estradiol PET/CT. J Nucl Med. 56 (2015): 50-55.
- Feng Z, Wen H, Ju X, et al. Hormone receptor expression profiles differ between primary and recurrent high-grade serous ovarian cancers. Oncotarget. 8 (2017): 32848-3255.
- Foukakis T, Astrom G, Lindstrom L, et al. When to order a biopsy to characterise a metastatic relapse in breast cancer. Ann Oncol 23 (2012): x349-x353.
- van Kruchten M, de Vries EGE, Brown M, et al. PET imaging of oestrogen receptors in patients with breast cancer. Lancet Oncol. 14 (2013): e465-e475.
- Katzenellenbogen BS, Bhardwaj B, Fang H, et al. Hormone binding and transcription activation by estrogen receptors: analyses using mammalian and yeast systems. J Steroid Biochem Mol Biol. 47 (1993): 39-48.
- van Hemert F, Dam-de Veen C, Konings S, et al. A novel dual antibody staining assay to measure estrogen receptor transcriptional activity. bioRxiv. (2020): 2020.04.14.021782.
- Gaillard SL, Andreano KJ, Gay LM, et al. Constitutively active ESR1 mutations in gynecologic malignancies and clinical response to estrogen-receptor directed therapies. Gynecol Oncol. 154 (2019): 199-206.
- Verhaegh W, van Ooijen H, Inda MA, et al. Selection of personalized patient therapy through the use of knowledge-based computational models that identify tumor-driving signal transduction pathways. Cancer Res. 74 (2014): 2936-2945.
- Inda MA, Blok EJ, Kuppen PJK, et al. Estrogen Receptor Pathway Activity Score to Predict Clinical Response or Resistance to Neoadjuvant Endocrine Therapy in Primary Breast Cancer. Mol Cancer Ther. 19 (2020): 680-689.
- Yang SR, van de Stolpe A, van Brussel A, et al. Does hormone expression by IHC predict ER pathway activity? An analysis in a metastatic breast cancer patient cohort [abstract]. Proceedings of the 2018 San Antonio Breast Cancer Symposium, AACR, San Antonio, Texas, United States of America, 2018 Dec 4-8 Publisher: Cancer Res 79 (2019): Abstract nr P5-11-06.
- Sieuwerts AM, Inda MA, Smid M, et al. ER and PI3K Pathway Activity in Primary ER Positive Breast Cancer Is Associated with Progression-Free Survival of Metastatic Patients under First-Line Tamoxifen. Cancers (Basel). 12 (2020).
- Riemsma R, Forbes CA, Kessels A, et al. Systematic review of aromatase inhibitors in the first-line treatment for hormone sensitive advanced or metastatic breast cancer. Breast Cancer Res Treat. 123 (2010): 9-24.
- Geisler J. Differences between the non-steroidal aromatase inhibitors anastrozole and letrozole--of clinical importance? Br J Cancer. 104 (2011): 1059-1066.
- Geisler J, Haynes B, Anker G, et al. Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol. 20 (2002): 751-757.
- Smith I, Yardley D, Burris H, et al. Comparative Efficacy and Safety of Adjuvant Letrozole Versus Anastrozole in Postmenopausal Patients With Hormone Receptor-Positive, Node-Positive Early Breast Cancer: Final Results of the Randomized Phase III Femara Versus Anastrozole Clinical Evaluation (FACE) Trial. J Clin Oncol. 35 (2017): 1041-1048.
- Spoerke JM, Gendreau S, Walter K, et al. Heterogeneity and clinical significance of ESR1 mutations in ER-positive metastatic breast cancer patients receiving fulvestrant. Nat Commun. 7 (2016): 11579.
- Gosden JR, Middleton PG, Rout D. Localization of the human oestrogen receptor gene to chromosome 6q24-q27 by in situ hybridization. Cytogenet Cell Genet. 43 (1986): 218-220.
- Jeselsohn R, Yelensky R, Buchwalter G, et al. Emergence of constitutively active estrogen receptor-alpha mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res. 20 (2014): 1757-1767.
- Toy W, Shen Y, Won H, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 45 (2013): 1439-1445.
- Robinson DR, Wu YM, Vats P, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet. 45 (2013): 1446-1451.
- Mabuchi S, Kuroda H, Takahashi R, et al. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol Oncol. 137 (2015): 173-179.
- Li H, Zeng J, Shen K. PI3K/AKT/mTOR signaling pathway as a therapeutic target for ovarian cancer. Arch Gynecol Obstet. 290 (2014): 1067-1078.
- Gasparri ML, Bardhi E, Ruscito I, et al. PI3K/AKT/mTOR Pathway in Ovarian Cancer Treatment: Are We on the Right Track? Geburtshilfe Frauenheilkd. 77 (2017): 1095-1103.
- Ciruelos Gil EM. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat Rev. 40 (2014): 862-871.
- Piccart M, Hortobagyi GN, Campone M, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2dagger. Ann Oncol. 25 (2014): 2357-2362.
- Wheler JJ, Moulder SL, Naing A, et al. Anastrozole and everolimus in advanced gynecologic and breast malignancies: activity and molecular alterations in the PI3K/AKT/mTOR pathway. Oncotarget. 2014 5 (10): 3029-3038.
- Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 34 (2010): 433-443.
- Gockley A, Melamed A, Bregar AJ, et al. Outcomes of Women With High-Grade and Low-Grade Advanced-Stage Serous Epithelial Ovarian Cancer. Obstet Gynecol. 129 (2017): 439-447.
- Tang M, O'Connell RL, Amant F, et al. PARAGON: A Phase II study of anastrozole in patients with estrogen receptor-positive recurrent/metastatic low-grade ovarian cancers and serous borderline ovarian tumors. Gynecol Oncol. 154 (2019): 531-538.
- Gershenson DM, Sun CC, Iyer RB, et al. Hormonal therapy for recurrent low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol. 125 (2012): 661-666.
- Maynard PV, Davies CJ, Blamey RW, et al. Relationship between oestrogen-receptor content and histological grade in human primary breast tumours. Br J Cancer. 38 (1978): 745-748.
- Pichon MF, Broet P, Magdelenat H, et al. Prognostic value of steroid receptors after long-term follow-up of 2257 operable breast cancers. Br J Cancer. 73 (1996): 1545-1551.
- Thike AA, Chng MJ, Fook-Chong S, et al. Immunohistochemical expression of hormone receptors in invasive breast carcinoma: correlation of results of H-score with pathological parameters. Pathology. 33 (2001): 21-25.
- Gupta D, Gupta V, Marwah N, et al. Correlation of Hormone Receptor Expression with Histologic Parameters in Benign and Malignant Breast Tumors. Iran J Pathol. 10 (2015): 23-34.
- Van der Putten LJ, van Brussel A, van Weelden WJ, et al. Estrogen receptor pathway activity in endometrial carcinomas and its relation to tumor grade and recurrence [abstract]. Proceedings of the American Association for Cancer Research Annual Meeting 2018, AACR, Chicago, Illinois, United States of America 14-18 Publisher: Cancer Res 78 (2018): Abstract nr 2656.
- del Carmen MG, Fuller AF, Matulonis U, et al. Phase II trial of anastrozole in women with asymptomatic mullerian cancer. Gynecol Oncol. 91 (2003): 596-602.
- Lindemann K, Gibbs E, Avall-Lundqvist E, et al. Chemotherapy vs tamoxifen in platinum-resistant ovarian cancer: a phase III, randomised, multicentre trial (Ovaresist). Br J Cancer. 116 (2017): 455-463.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 74.74%
Acceptance Rate: 74.74%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 