“Double Hit” in Chronic Lymphocytic Leukemia: Therapeutic Strategies for Patients with 17p Deletion and TP53 Mutation
Daniel E. Ezekwudo1*, Tolulope Ifabiyi2, Angela Usim1, Ishmael Jaiyesimi1
1Department of Hematology and Oncology, William Beaumont Hospital, Oakland University William Beaumont School of Medicine, Royal Oak, MI, USA
2Oakland University William Beaumont School of Medicine, Rochester, MI USA
*Corresponding Author: Dr. Daniel Ezekwudo, Department of Hematology and Oncology, William Beaumont Hospital, 3577 W. 13 Mile Rd., Suite 202a, Royal Oak, MI, USA
Received: 14 May 2019; Accepted: 24 May 2019; Published: 28 May 2019
Article Information
Citation: Daniel E. Ezekwudo, Tolulope Ifabiyi, Angela Usim, Ishmael Jaiyesimi. “Double Hit†in Chronic Lymphocytic Leukemia: Therapeutic Strategies for Patients with 17p Deletion and TP53 Mutation. Journal of Cancer Science and Clinical Therapeutics 3 (2019): 54-69.
View / Download Pdf Share at FacebookAbstract
We aim to review the use of molecular markers for risk stratification and to describe the current standard and new targeted treatment options for the subgroup of patients with 17p deletion chronic lymphocytic leukemia (CLL) with an accompanying TP53 mutation, known as “double hit” CLL. Chromosome 17p deletion is seen in 5-9% of patients with newly diagnosed CLL; however, it represents the most common genetic aberration (≈50%) in patients with refractory/relapsed CLL. Identification of genomic and molecular markers allows risk stratification and has prognostic value. Chromosome 17p deletion has been strongly correlated with poor response to standard therapy as well as shorter overall survival. Accompanying TP53 mutation confers an even poorer response to standard therapy than 17p deletion alone. A review of current treatment options reveals the ongoing debate as to the appropriate timing of treatment initiation in this patient subpopulation. Moreover, standard treatment options still confer suboptimal benefit. Small molecule inhibitors targeting intracellular B-cell receptor signaling components such as Bruton’s tyrosine kinase (BTK), phosphatidylinositol 3-kinase (PI3K), anti BCL-2 molecules, and more recently CD19-targeted CAR T-cell therapy, have shown significant progression-free survival and overall survival advantages in patients with 17p deletion and/or TP53 mutation, while allogeneic hematopoietic cell transplant has proven clinical benefit in selected patients.
Keywords
<p>Chronic lymphocytic leukemia, 17p deletion, TP53, Ibrutinib, Idelalisib, Venetoclax, CAR T cells</p>
Article Details
1. Introduction
Chronic lymphocytic leukemia (CLL) is a lymphoproliferative disorder with the resultant clonal proliferation of mature B-lymphocytes expressing CD19, CD5 and CD23 [1]. It is the most common adult leukemia in western countries accounting for approximately 30% of all leukemias, and is associated with a highly variable clinical course [2]. An estimated 20,720 new cases of CLL will be diagnosed in the United States in 2019 [3]. Disease progression is variable as some patients may not require treatment for years and have similar survival to that of age-matched controls, while others progress rapidly with a poor prognosis despite aggressive treatment [4-6]. This variability in the progression and treatment requirement results from chromosomal abnormalities present in more than 75% of patients with CLL, including deletion (13q14), trisomy 12, deletion (11q23), and deletion (17p13), at frequencies of 45.7%, 20.8%, 18.3% and 11.9% [7]. Recent studies have shown that patients with 17p deletion have the worst clinical outcomes, and the shortest progression-free and overall survival (OS). Deletions, isochromosome formation, and unbalanced translocation have been implicated in the loss of the short arm of chromosome 17. These aberrations often result in the loss of one copy of the TP53 gene, while leaving the remaining allele mutated, resulting in resistance to DNA-damaging agents such as fludarabine and cyclophosphamide. Newer drugs targeting the B-cell receptor signaling pathway (ibrutinib, idelalisib) and BCL2 pathway (venetoclax) have shown efficacy in CLL with 17p deletion and/or TP53 mutation, with a better treatment response and longer progression-free survival; however, relapses are being reported [8]. Although still in an investigative stage, the use of chimeric antigen receptor (CAR) T cell therapy in CLL patients with 17p deletion and/or TP53 mutation has been reported to confer excellent progression free survival and overall survival benefits, even in heavily pretreated and relapsed/refractory subgroups [9]. Despite these important advances in chemotherapeutics, cure for these patients remains elusive outside of allogeneic hematopoietic cell transplant (allo-HCT).
2. Risk Stratification in CLL Using Genomic and Molecular Markers
The molecular alterations of CLL are the main reason for the heterogeneity in clinical course observed in patients with the disease. Based on recent advances, both genetic and molecular markers that aid prognostication have been established and are routinely utilized in clinical practice. For instance, the mutation status of the immunoglobulin heavy chain (IGHV) locus has been shown to have important prognostic value, with unmutated IGHV genes associated with poorer outcomes [10]. Zeta chain-associated protein (ZAP-70), commonly used as a surrogate marker for IGHV mutational status, also has prognostic implications with higher expression (>20%) associated with unfavorable clinical outcomes [11]. Furthermore, cell surface expression of CD38 characterizes an unfavorable clinical course with a more advanced disease stage, suboptimal responsiveness to chemotherapy, shorter time to initiation of the first treatment and inferior overall survival. Patients with a sole 13q deletion often express the B cell receptor (BCR) with a mutated IGHV gene, both of which have strong correlation with favorable clinical outcomes [12].
Conversely, deletions in chromosomes 11q and 17p, confirmed via conventional cytogenetics or interphase fluorescent in situ hybridization (FISH), have been categorized as high-risk markers, indicative of early disease progression [11]. Patients with trisomy 12 are categorized as intermediate risk since their clinical outcome is better than those with the 11q or 17p deletion, but worse than those without aberrations or 13q as the sole defect [11, 13]. The median survival for patients with 11q and 17p deletions usually ranges between 32-79 months, compared to those with trisomy 12q, normal karyotype, or del(13q) as the sole abnormality, with 114, 111, and 133 months median survival respectively [13]. The poor clinical outcome observed in patients with 17p deletions has been attributed in part to the loss of the tumor suppressor TP53, which is crucial for disease progression and response to chemotherapy [14]. A subset of patients with 17p deletion and complex cytogenetics (defined as more than 3 separate chromosomal abnormalities) exhibit a worse prognosis and lack of responsiveness to aggressive therapy than patients with 17p deletion alone [14].
Another chromosomal abnormality involving chromosome 17 is that of the dicentric chromosome dic(17;18) (p11.2;p11.2) resulting from an unbalanced translocation between the short (p) arms of chromosomes 17 and 18.15 Woyach et al [16], showed that dic(17;18) (p11.2;p11.2) in CLL patients is associated with early age at diagnosis, faster disease progression and tendency toward refractory disease, similar to patients with del(17p) or loss of TP53. This abnormality is also frequently associated with unmutated IGHV [17]. It has yet to be determined whether the prognosis and response to treatment of patients with dic (17;18) (p11.2;p11.2) differs from that of patients with del(17p) alone.
3. Prognostic and Predictive Value of 17p Deletion and/or TP53 Mutation in CLL
TP53 gene is involved with encoding a tumor suppressor protein containing transcriptional activation, DNA binding, and oligomerization domains. The encoded protein is responsible for a variety of functions including but not limited to the induction of cell cycle arrest, apoptosis, senescence, DNA repair, or changes in metabolism. Mutations in this gene are associated with a variety of human cancers, including hereditary cancers such as Li-Fraumeni syndrome. Also noted that across most cancers, mutation in TP53 portends to a poorer clinical outcome. Studies have shown that more than 90% of patients with del(17p) have concurrent TP53 mutations while only 40% of those with TP53 mutations have a concurrent del(17p) [18]. Approximately 5-10% of patients with CLL have TP53 mutation in the absence of del(17p), and have been shown to have clinical outcome compared to del(17p) alone [18]. Despite the fact that presence of del(17p) is correlated with inferior outcome, only 50% of those patients will become symptomatic requiring treatment within 3 years [19]. Multiple studies have shown that when these patients become symptomatic, progression-free survival achieved with first line treatment is short with both chemoimmunotherapy (CIT) and p53- independent treatment options (e.g. monoclonal antibodies and immunomodulatory agents) [18-20]. For instance, in a retrospective study involving a large cohort of patients with CLL, Strati et al. reported complete remission (CR) and nodular partial remission (nPR) of 33% and 63% of patients, respectively, following treatment with first-line therapy, however none of the patients with del(17p) achieved CR [14]. In this study a higher CR/nPR rate was associated with “lower burden” del(17p) as determined by FISH. Some investigators have contended that not all del(17p) patients have a similar response to therapy and that the number of cells with positive del(17p) by FISH may give an indirect measure of preserved p53 function; thus, patients with a lower number of cells with del(17p) may be more sensitive to therapy.
4. Management of CLL Patients with 17p Deletion and/or Aberrant TP 53 Mutation
Although CIT forms the basis for the treatment of most CLL patients, it fails to demonstrate a significant OS benefit among patients with 17p deletion and/or TP53 mutation. Fludarabine as a monotherapy or when combined with cyclophosphamide (FC) has failed to show significant efficacy among CLL patients with del(17p) and/or aberrant TP53.21,22 Furthermore, the addition of Rituximab to FC (FCR) was ineffective in same subgroup of patients; in one study, only 1 of 22 (5%) patients with del(17p) treated with FCR achieved CR, with a median progression-free survival (PFS) of 11.3 months compared with 51.8 months in the non-del(17p) cohort [21, 22].
Other CIT single agents or drug combinations have been explored in the treatment of CLL in an effort to improve outcomes in patients with del(17p) and/or aberrant TP53, with all demonstrating inferior efficacy among this subgroup of patients. Recently, promising data has been reported among patients with del(17p) and/or aberrant TP53 mutation utilizing targeted therapy agents via the B-cell receptor (BCR) signaling pathway, utilizing the Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib, the Bcl-2 inhibitor venclexta/ABT-199, and the phosphatidylinositol 3-kinase (PI3K) inhibitor idelalisib (Table 1) [23]. Most recently, the most favorable use of adoptive cell transfer utilizing anti CD19-targeted CAR T cell therapy has been reported in this patient subgroup [9, 24].
5. Targeted Therapy Via BCR-Signaling Pathway
5.1 IbrutinibBCR signaling plays an important role in CLL, with activation leading to the promotion of B cell survival and proliferation [25]. Ibrutinib (Imbruvica) was approved in 2014 by the FDA as first line treatment for patients with del(17p) due to its efficacy compared to CIT treatments [23]. It is an irreversible inhibitor of BTK, a non-receptor tyrosine kinase involved in BCR downstream signaling pathways (Figure 1) [25]. Ibrutinib has demonstrated favorable responses in relapsed or refractory CLL compared with ofatumumab [26]. In untreated CLL patients with (del)17p or mutated TP53 who received ibrutinib, an ORR of 97% was noted, with 12% attaining CR. Furthermore, the 2-year PFS was 91% [23]. This compares favorably to CIT treatment of patients with del(17p) as discussed above. In addition, the median PFS for del(17p) patients in the relapsed setting with ibrutinib monotherapy is ∼28 months; these data in the relapsed setting are much superior to those seen with CIT, even in the first-line setting. Ibrutinib also showed durable response in elderly patients (>65 yrs) with the high risk cytogenetics including (del)17p, with an overall response rate of 84% after a median follow-up of 5years [23]. Thus, ibrutinib demonstrates great potential for improving outcomes for CLL patients with (del)17p and/or TP53 mutation.
Note: ORR = overall response rate; OS = overall survival; PFS = progression free survival; R/R=relapsed/refractory; TN = treatment naïve
Table 1: Major Studies of Ibrutinib, Idelalisib and Venetoclax/ABT-199 in Chronic Lymphocytic Leukemia.
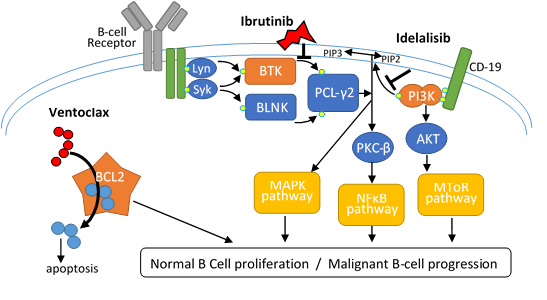
Figure 1: Pictorial representation of the B cell signaling pathway and mechanism of action of the Bruton’s tyrosine kinase (BTK) inhibitor (ibrutinib), phosphatidylinositol 3-kinase (PI3K) inhibitor (idelalisib) and BCL-2 inhibitor (venetoclax).These three novel agents inhibit different key molecules of cell activation thus interfering with BCR signaling and B cell proliferation.
In most of the studies with (del)17p patients, it was found that those with complex karyotypes as well as del(17p) performed worse than those without complex karyotypes. Patients with 17p deletions and complex cytogenetics had a shorter PFS and higher chance of developing Richter’s transformation compared to patients without complex karyotypes [14]. This crucial point was elaborated in a retrospective study using Ibrutinib-based regimens in relapsed or refractory CLL patients [27]. The study by Thomson et al. [27], showed that complex cytogenetics may be more responsible for poor response to treatment than (del)17p. Patients with 17p deletions without complex cytogenetics demonstrated favorable outcomes with ibrutinib-based therapy with a low rate of Richter transformation; those with both del(17p) and complex cytogenetics showed lower response rates and poorer outcomes. In addition, a significant association was found between the presence of complex cytogenetics and inferior event-free survival and OS in relapsed/refractory CLL patients treated with ibrutinib [27]. No similar association with del(17p) alone was found, suggesting that the presence of complex cytogenetics is an important independent prognostic indicator. Patients with del(17p) and complex cytogenetics also demonstrated poorer outcomes after allogenic stem cell transplantation than those without complex cytogenetics [28]. Farooqui et al. showed that ibrutinib is effective even in high risk patients with 17p deletion and TP53 mutation. They reported that response to ibrutinib was rapid and increased with time, and at least a 50% reduction in tumor burden was observed in the bone marrow, spleen, and lymph nodes in the majority of patients. The estimated progression-free survival for all patients at 24 months was 82%, and overall survival at 24 months was 80% [29]. In a more recent study, patients older than 65 years with untreated CLL will randomly assigned to receive bendamustine with rituximab or Ibrutinib or Ibrutinib plus Rituximab, estimated percentage of patients with progression-free survival at 2 years was 74% with bendamustine plus rituximab and was higher with ibrutinib alone (87%; hazard ratio for disease progression or death, 0.39; 95% confidence interval [CI], 0.26 to 0.58; P<0.001) and with ibrutinib plus rituximab (88%; hazard ratio, 0.38; 95% CI, 0.25 to 0.59; P<0.001). Furthermore, there was no significant difference between the ibrutinib-plus-rituximab group and the ibrutinib group with regard to progression-free survival (hazard ratio, 1.00; 95% CI, 0.62 to 1.62; P=0.49). With a median follow-up of 38 months, there was no significant difference among the three treatment groups with regard to overall survival [30].
Recently, there has been a report of resistance to ibrutinib attributed to acquired mutations in BTK and the phospholipid C-gamma 2 gene (PLCG2) [8]. Evidently, further study of more aggressive treatments in CLL patients with complex cytogenetics is warranted. Some of the recent studies on this novel therapy are summarized in Table 1.
Although ibrutinib has shown great potential among patients with poor risk cytogenetics, grade >3 neutropenia, anemia and thrombocytopenia have been reported. Other drug-related adverse events include, but are not limited to hypertension and atrial fibrillation (Table 2). It is important to note that most patients will develop lymphocytosis after initiating ibrutinib. This is an expected finding with ibrutinib and other BCR inhibitors, and generally resolves over the course of 6 to 9 months with continued ibrutinib treatment. Development of lymphocytosis does not appear to be detrimental to long-term clinical outcomes.
|
Ibrutinib |
Idelalisib |
Venetoclax |
|
|
Hematologic adverse events |
% (any grade) |
% (any grade) |
% (any grade) |
|
Neutropenia |
22-53 |
53 |
45 |
|
Anemia |
13-43 |
28 |
29 |
|
Thrombocytopenia |
43-69 |
26 |
22 |
|
Hepatotoxicity |
x |
11-18 |
x |
|
Tumor lysis syndrome |
x |
x |
12 |
|
Non- Hematologic Adverse events |
|||
|
Infection |
4-26 |
21-36 |
22 |
|
Bleeding (mostly GI) |
44-69 |
x |
x |
|
Pneumonitis |
x |
1-4 |
x |
|
Diarrhea/Colitis |
42-61 |
14-19 |
x |
|
Intestinal perforation |
x |
<1 |
x |
|
Nausea |
6-26 |
29 |
x |
|
Fatigue |
5-30 |
30 |
21 |
|
Hypertension |
14-23 |
x |
x |
|
Atrial fibrillation |
6-16 |
x |
x |
Abbreviations: x= not reported, any grade = grades 1-5
Table 2: Most common treatment-related adverse events in CLL patients treated with ibrutinib, idelalisib or venetoclax monotherapy (Any grade).
5.2 Idelalisib
Idelalisib (Zydelig), a PI3K-δ inhibitor (Figure 1), has also shown significantly improved PFS and overall survival in relapsed, refractory CLL, including subgroups with 17p deletions and/or TP 53 mutation [31]. In a randomized phase III study utilizing idelalisib plus rituximab in patients who have progressed with prior treatments, improvements in PFS were shown, including in those with 17p deletion and/or TP53 mutation [31]. Patients treated with idelalisib versus those receiving placebo had improved rates of overall response (81% vs. 13%; odds ratio, 29.92; P<0.001) and overall survival at 12 months (92% vs. 80%; hazard ratio for death, 0.28; P=0.02) [31]. Furthermore, a phase II study of idelalisib plus rituximab in treatment-naïve CLL revealed an 100% objective response (CR=33.3%, PR= 66.7%) [32]. However, despite impressive reports of the efficacy of idelalisib-based regimens, significant toxicities have been reported (Table 2). The observed toxicities have been associated with lymphocyte infiltration of tissues via an immune-mediated mechanism. The majority of the adverse drug events were noted to resolve with withdrawal of treatment and/or initiation of immunosuppression. Although not yet approved for first line treatment of patients with del(17p), the FDA has approved idelalisib for patients with relapsed/refractory CLL in combination with rituximab. A study of idelalisib in combination with rituximab as first line treatment for CLL suggested efficacy for patients with del(17p) and/or TP 53 mutation. However, increased risk for infections was also reported in this study [32]; thus, infection prophylaxis for herpes simplex virus and routine monitoring for cytomegalovirus reactivation is recommended for patients on idelalisib.
5.3 Venetoclax
The FDA in 2016 approved venetoclax (Venclexta, Venclyxto), a BCL2 inhibitor, for treatment of CLL patients with 17p deletions who had received at least one prior therapy. Constitutive elevation of BCL2 is an important mechanism by which CLL cells evade apoptosis, as resistance to apoptosis allows the accumulation of clonal lymphocytes [33]. Venetoclax is very selective for BCL2 and has demonstrated favorable results in a clinical trial with relapsed or refractory CLL, where the 15-month PFS was 69% [34]. Combinations of venetoclax with other agents are currently under pre-clinical and clinical exploration. BCL2 overexpression and BCR signaling pathways have shown to be intimately connected. In fact, a recent phase III trial showed that a combination of venetoclax and Rituximab had a progression free survival rate of about 85% versus 36.3% for bendamustine with rituximab (HR, 0.17; 95% CI, 0.11-0.25; P <0.0001) among patients with relapsed/refractory chronic lymphocytic leukemia following at least 1 prior therapy [35]. The overall response rate was 92% versus 72%, respectively. The PFS benefit extended across patients subgroups, including high- and low-risk groups. The 2-year PFS rate among patients with chromosome 17p deletion was 81.5% in the venetoclax arm versus 27.8% with bendamustine and Rituxan. The venetoclax combination is now approved for this indication in all 28 member states of the EU, as well as Iceland, Liechtenstein and Norway. An in vitro study demonstrated synergistic effects during treatment of CLL cells in peripheral blood with venetoclax and idelalisib [33]. Preclinical data have also provided evidence of synergistic activity between venetoclax and ibrutinib. Currently, a number of trials studying these novel therapies either alone or in combination are underway (Table 3).
|
Study |
Phase |
Population |
Regimen |
Status |
|
NCT02337829 |
Phase 2 |
Untreated or R/R del(17p) CLL |
Acalabrutinib |
Recruiting |
|
NCT01589302 |
Phase 2 |
R/R CLL (includes B-PLL) |
Ibrutinib |
Active, not recruiting |
|
NCT01889186 |
Phase 2 |
Untreated or R/R del(17p) CLL |
Venetoclax |
Active, not recruiting |
|
NCT01465334 |
Phase 2 |
Untreated or R/R del(17p) CLL |
Ofatumumab-high dose methylprednisolone followed by ofatumumab and alemtuzumab |
Active, not recruiting |
|
NCT02966756 |
Phase 2 |
R/R del(17p) CLL |
Venetoclax |
Recruiting |
|
NCT02756611 |
Phase 3 |
R/R including del(17p) or TP53 mutation or prior B cell receptor inhibitor therapy |
Venetoclax |
Recruiting |
|
NCT01392079 |
Phase 2 |
Untreated or R/R del(17p) or fludarabine refractory |
Subcutaneous alemtuzumab combined with oral dexamethasone, followed by alemtuzumab maintenance or allogeneic hematopoietic stem cell transplantation |
Active, not recruiting |
|
NCT02980731 |
Phase 3 |
R/R CLL including del(17p) or TP53 Mutation or prior B cell receptor Inhibitor therapy |
Venetoclax |
Recruiting |
|
NCT01500733 |
Phase 2 |
CLL in need of treatment and are older than 65 years with del(17p) |
Ibrutinib |
Active, not recruiting |
|
NCT02758665 |
Phase 2 |
Physically fit, untreated CLL with del(17p)/TP53 mutations |
Ibrutinib plus venetoclax plus obinutuzumab |
Recruiting |
|
NCT02846623 |
Phase 2 |
R/R or high-risk untreated CLL |
Atezolizumab (PD-L1 mAb) plus obinutuzumab and ibrutinib |
Recruiting |
|
NCT01659021 |
Phase 3 |
R/R CLL |
Idelalisib (GS-1101) plus ofatumumab |
Active, not recruiting |
|
NCT01675102 |
Phase 3 |
Allogeneic HCT in 17p-/p53-mutated CLL in first or second remission |
Allogeneic hematopoietic stem cell transplantation |
Recruiting |
|
NCT02044822 |
Phase 3 |
Untreated del(17p) |
Idelalisib plus rituximab |
Terminated,has results |
|
NCT02950051 |
Phase 3 |
Fit patients with previously untreated CLL without del(17p) or TP53 mutation |
(FCR/BR) versus Rituximab + venetoclax (RVe) versus obinutuzumab (GA101) + venetoclax (GVe) versus obinutuzumab + ibrutinib + venetoclax (GIVe) |
Recruiting |
Note: CLL = Chronic lymphocytic leukemia; NCT = National Clinical Trial; R/R = relapsed/refractory; B-PLL = B cell prolymphocytic leukemia
Table 3: Ongoing clinical trials of CLL with 17p deletion and/or aberrant TP-53 mutation.
5.4 Stem cell transplantation
Despite new advances in CLL treatments, no therapies have demonstrated curative properties. Allogeneic stem cell transplantation is an alternative for patients who are refractory to CIT and has been shown to provide long-term survival in high-risk patients including those with 17p deletions [42]. Recently, Schetelig et al. reported a 57% event-free survival in high risk CLL patients with an average follow up of 9.3 years [43]. Stem cell transplantation (SCT) has been proposed for many years as the only curative option for CLL patients due to the induction of a graft-versus-leukemic effect. However, the increased risk of morbidity and mortality, the older age of patients with CLL and the difficulty of achieving a minimal disease state before transplantation have limited the use of stem cell therapies. In light of these challenges, myeloablative treatments have emerged as potential curative options, especially for those healthy enough to tolerate toxicities and respond to induction therapy. Younger patients under age 60, who make up to 40% of patients with CLL, and those with earlier disease states with higher rates of chemosensitvity appear to be most ideal for stem cell transplantation [44]. Based on the 2008 International Workshop on Chronic Lymphocytic Leukemia (IWCLL) guidelines [45], allogeneic stem cell transplantation is indicated for patients with a short time to progression, those refractory to standard treatments (e.g. purine analog-based therapy) and those considered high-risk, including 17p deletion.
One of the first early trials of high-dose myeloablative therapy and bone marrow transplantation (BMT) for CLL patients was reported in a multicenter European study using allogeneic bone marrow stem cell transplantation (allo-BMT) from HLA-matched siblings in 17 resistant cases of CLL in young individuals. Engraftment was accomplished in 15 patients, however 4 toxic deaths were reported, attributed to graft versus host disease (GVHD); 2 patients relapsed following BMT, leaving 9 patients alive in complete remission at 25.6 months [46].
The first report of autologous BMT (auto-BMT) for poor risk CLL involved a pilot study reported by Rabinowe et al. [44], from the Dana Faber Cancer Center, studying both auto- and allo-BMT. All patients receiving auto-BMT achieved hematologic engraftment with similar toxicity rates to those receiving allo-BMT, including infectious (bacteremia/sepsis) and non-infectious (hemolytic uremic syndrome) sequelae. One toxic death occurred in auto-BMT due to diffuse alveolar hemorrhage syndrome. Six of the nine patients who received auto-BMT were assessed for residual disease and showed no evidence of residual CLL cells at 3 months post-transplant [44]. No significant differences in treatment outcomes between auto- and allo-BMT were observed, demonstrating the utility of auto-BMT as an option for patients, particularly those with no HLA-matched donor.
Long term follow-up of 137 patients who underwent auto-BMT and 25 who underwent allo-BMT was conducted at the Dana Faber Cancer Center [47]. PFS was significantly longer with auto-SCT compared to allo-, with a 6-year PFS at 30% versus 24% respectively. However, no significant difference in OS was observed at 6.5 years of follow-up. Additionally, the 4-year time to retreatment of autologous hematopoietic cell transplant (HCT) patients was shown to be similar to patients treated with FCR in a later trial by CLL3 German CLL Study Group [48], demonstrating the limitations of this treatment. Toxicities due to infectious (fungal) and non-infectious causes (GVHD) were similar between the two groups. Secondary malignancies occurred in 19% of patients and included basal cell carcinoma, B- and T-cell non-Hodgkin’s lymphoma and myelodysplastic syndrome (MDS). MDS was the most common secondary malignancy, all occurring in those with autologous SCT [47], making MDS an important factor to consider in treatment selection.
Non-myeloabaltive or reduced intensity transplantation has been shown to be well tolerated in older patients (over 60 years) with multiple comorbidities, while maintaining long-term remission [49, 50]. A retrospective analysis of stem cell transplantation in del(17p) patients in the European Group for Blood and Marrow Transplantation (EBMT) database, the majority of whom had reduced-intensity conditioning, demonstrated the potential for long term survival in these patients [45]. The three-year OS and PFS rates were 44% and 37%, respectively. A 42% CR rate was observed in these patients, suggesting the presence of a graft-versus-leukemic effect. Additionally, nine patients followed for over 4 years remained in remission following allo-HCT. However, acute grade 2 to 4 GVHD was seen in 48% of patients, while extensive chronic GVHD was seen in 68% [43]. The German CLL Study Group CLL3X multicenter phase II trial investigating reduced-intensity allo-SCT in poor risk patients demonstrated similar results, with 7 of 13 patients with 17p deletion surviving in CR, with a median follow up of 43 months [51]. These results demonstrate the potential of allo-HCT to overcome the poor prognosis of those patients with 17p deletion.
5.5 CD19 Chimeric antigen receptor T-Cell Therapy for del(17p) and/or TP 53 mutated CLL patients
The use of adoptive cell transfer (Figure 2) in managing advanced blood disease has garnered a lot of attention resulting in recent approvals by the FDA of CAR T cell therapy in B cell precursor-acute lymphoblastic leukemia, relapsed/refractory large B cell lymphoma, and T cell-induced severe cytokine release syndrome. In addition, several ongoing clinical trials are evaluating the efficacy of this treatment approach in other disease conditions. Recently a small, single arm phase I/II study utilizing CD19-targeted CAR T cell therapy in patients with relapsed/refractory B cell malignancies showed significant response among high-risk CLL patients including in those who were ibrutinib-refractory [9]. For the 24 CLL patients in the study, the median age was 61, with a median of 5 prior lines of therapies for CLL. Four patients had undergone stem cell transplantation. There were 19 patients who were ibrutinib-refractory and 6 venetoclax-refractory who achieved excellent overall survival and progression-free survival of 100% after a median follow-up of 6.6 months [9] Recently reported study of 26 patients with relapsed/refractory CLL who were treated with CTL019 following lymphodepleting chemotherapy achieved approximately 40% objective response rate including among patients with high risk features such as 17p deletion and/or TP53 mutation [52]. Furthermore, among those who achieved CR, non-relapsed with a median duration of response of 40 months. Preclinical studies have supported a possible synergistic effect between the CAR T cell and ibrutinib; thus, on-going studies are evaluating response rates in CLL patients treated with ibrutinib prior to and followed by concurrent anti-CD19 CAR T cell immunotherapy [53]. This remains an area of increased interest especially for high risk CLL patients with del(17p) and/or TP53 mutation.
5.6 Minimal residual disease quantification and long-term outcomes
Recent studies have shown that minimal residual disease (MRD) negativity is independently associated with significantly longer progression free survival and overall survival. In a multivariate model, mutated IGHV gene and trisomy 12 were independently associated with achievement of minimal residual disease-negative status. Using flow cytometry, Bottcher et al. evaluated MRD in patients enrolled in the GCLLSG CLL8 trial. MRD levels were characterized as low (<10−4), intermediate (≥10−4 to <10−2), or high (≥10−2), where the low-level MRD equals “MRD negativity” according to the IWCLL criteria [54]. At approximately 3 months following treatment, they reported a significantly higher proportion of patients with low-level MRD in the FCR arm compared with the FC arm (peripheral blood: 63% vs 35%, p < 0.0001; bone marrow: 44% vs 28%, p=0.0007). Their study revealed a strong correlation between low-level MRD and longer PFS, irrespective of sample type, sample timing, or therapy received. In a multivariate model, MRD was predictive for PFS and OS. Similar to the results of the CLL8 trial, the MDACC group analyzed MRD in the bone marrow of 237 patients following first line FCR therapy [55]. After course 3 and at final response assessment, they reported MRD negativity (<10−4 by 4-color flow cytometry) in 17% and 43% of patients, respectively.
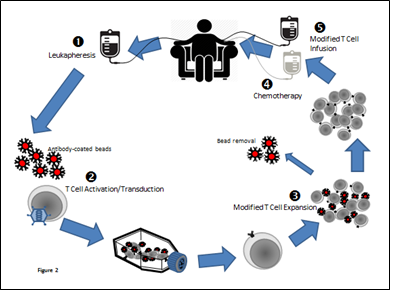
Figure 2: Pictorial representation of adoptive T cell transfer.
6. Conclusion
CLL is a chronic disease that often presents asymptomatically. Prognostic tests using FISH is recommended during management of patients with CLL [16], although the results should not be used as an indicator to initiate treatment especially in asymptomatic patients. Repeated testing may also be beneficial to help direct subsequent treatments as genetic abnormalities may change as the disease progresses. Determination of complex cytogenetics status may be helpful in determining the choice of treatment and response to treatment, as these patients tend to have very poor outcomes. Such patients may require more aggressive treatment and may possibly benefit from early stem cell transplantation [14]. Although standard therapies for CLL are not effective in patients with 17p deletions, well-tolerated therapies with TP53-independent mechanisms are available. Despite treatment-related adverse events associated with these novel agents, ibrutinib, idelalisib and venclexta have shown efficacy in the subgroup of patients with del(17p). Ongoing clinical trials are studying the use of these targeted therapies in combination with other therapies to further optimize their benefits, especially in patients with high risk features such as del(17p). It must be noted that clinical trials tend to enroll younger, low disease burden patients, whereas, in clinical practice, the average age at the time of treatment initiation is 76/77 [23], with higher comorbidity rates complicating the management of disease. As such, responses in clinical trials may not be fully representative of clinical practice. Large prospective registries like that of the Connect Chronic Lymphocytic Leukemia Disease Registry, with a heterogeneous patient population in different clinical settings, may be helpful to reference during treatment planning. Finally, the use of adoptive cell therapy has proven clinical benefit, thus a massive amount of research resources are being allocated to this area of immunotherapy design and delivery.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure Statement
The authors report no conflict of interest.
Data Availability Statement
Not applicable as no datasets were generated or analyzed during current study.
References
- Chiorazzi N, Rai KR, Ferrarini M. Chronic Lymphocytic Leukemia. N Engl J Med 352 (2005): 804-815.
- Siegel RL, Miller KD, Jemal A. Cancer Statistics. CA Cancer J Clin 67 (2017): 2017.
- Cancer Facts and Figures 2018. American Cancer Society, Atlanta, GA c2019 (2019).
- Yamamoto JF, Goodman MT. Patterns of leukemia incidence in the United States by subtype and demographic characteristics, 1997-2002. Cancer Causes Control 19 (2008): 379-390.
- Puiggros A, Blanco G, Espinet B. Genetic abnormalities in chronic lymphocytic leukemia: where we are and where we go. Blomed Res Int (2014): 435983.
- Baliakas P, Mattsson M, Stamatopoulos K, et al. Prognostic indices in chronic lymphocytic leukaemia: where do we stand how do we proceed? J Intern Med 279 (2016): 347-357.
- Van Dyke DL, Shanafelt TD, Call TG, et al. A Comprehensive Evaluation of the Prognostic Significance of 13q Deletions in Patients with B-Chronic Lymphocytic Leukemia. Br J Haematol 148 (2010): 544-550.
- Woyach J, Ruppert A, Guinn D, et al. BTKC481S-Mediated Resistance to Ibrutinib in Chronic Lymphocytic Leukemia. J of Clinl Oncol 35 (2017): 1437-1443.
- Turtle CJ, Hay KA, Hanafi LA,et al. Durable Molecular Remissions in Chronic Lymphocytic Leukemia Treated With CD19-Specific Chimeric Antigen Receptor–Modified T Cells After Failure of Ibrutinib. J Clin Oncol 35 (2017): 3010-3020.
- Parikh SA, Strati P, Tsang M, et al. Should IGHV status and FISH testing be performed in all CLL patients at diagnosis? A systematic review and meta-analysis. Blood 127 (2016): 1752-1760.
- Del Principe IM, Del Poeta G, Buccisano F, et al. Clinical significance of ZAP-70 protein expression in B-cell chronic lymphocytic leukemia. Blood 108 (2006): 853-886.
- Packham G, Krysov S, Allen A, et al.. The outcome of B-cell receptor signaling in chronic lymphocytic leukemia: proliferation or anergy. Haematologica 99 (2014): 1138-1148.
- Zenz T, Mertens D, Dohner H, et al. Importance of genetics in chronic lymphocytic leukemia. Blood Rev 25 (2011): 131-137.
- Strati P, Keating MJ, O’Brien SM, et al. Outcomes of first-line treatment for chronic lymphocytic leukemia with 17p deletion. Haematologica 99 (2014): 1350-1355.
- Döhner H, Stilgenbauer S, Benner A, et al. Genomic Aberrations and Survival in Chronic Lymphocytic Leukemia. N Engl J Med 343 (2000): 1910-1916.
- Woyach JA, Heerema NA, Zhao J, et al. Dic(17; 18) (p11.2; p11.2) is a recurring abnormality in chronic lymphocytic leukaemia associated with aggressive disease. Br J Haematol 148 (2010): 754-759.
- Gladstone DE, Blackford A, Cho E et al. The importance of IGHV Mutational Status in del (11q) and del (17p). Clin Lymphoma Myeloma Leuk 12 (2012): 132-137.
- Fenaux P, Preudhomme C, Lai JL, et al. Mutations of the p53 gene in B-cell chronic lymphocytic leukemia: a report on 39 cases with cytogenetic analysis. Leukemia 6 (1992): 246-250.
- Dohner H, Fischer K, Bentz M, et al. P53 gene deletion predicts for poor survival and non-response to therapy with purine analogs in chronic B-cell leukemias. Blood 85 (1995): 1580-1589.
- Rouby S, Thomas A, Costin D, et al. P53 gene mutation in B-cell chronic lymphocytic leukemia is associated with drug resistance and is independent of MDR1/MDR3 gene expression. Blood 82 (1993): 3452-3459.
- Eichhorst BF, Busch R, Hopfinger G, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia Blood 107 (2006): 885-891.
- Flinn IW, Neuberg DS, Grever MR, et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. J Clin Oncol 25 (2007): 793-798.
- Jain N, O’Brien S. Initial treatment of CLL: Integrating biology and functional status. Blood 126 (2015): 463-470.
- Ramos CA, Savoldo B, Torrano V, et al. Clinical responses with T lymphocytes targeting malignancy-associated κ light chains. J Clin Invest 126 (2016): 2588-2596.
- Ten Hacken E, Burger JA. Microenvironment dependency in Chronic Lymphocytic Leukemia: The basis for new targeted therapies. Pharmacol Ther 144 (2014): 338-348.
- Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus Ofatumumab in Previously Treated Chronic Lymphoid Leukemia. N Engl J Med 371 (2014): 213-223.
- Thompson PA, O’Brien SM, Wierda WG, et al. Complex karyotype is a stronger predictor than del(17p) for inferior outcome in relapsed or refractory cll patients treated with ibrutinib-based regimens. Cancer 121 (2015): 3612-3621.
- Jaglowski SM, Ruppert AS, Heerema NA, et al. Complex karyotype predicts for inferior outcomes following reduced-intensity conditioning allogeneic transplant for chronic lymphocytic leukaemia. Br J Haematol 159 (2012): 82-87.
- Farooqui MZ, Valdez J, Martyr S, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. Lancet Oncol 16 (2015): 169-176.
- Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N Engl J Med 379 (2018): 2517-2528.
- Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and Rituximab in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med 370 (2014): 997-1007.
- O’Brien SM, Lamanna N, Kipps TJ, et al. A phase 2 study of idelalisib plus rituximab in treatment-naïve older patients with chronic lymphocytic leukemia. Blood 126 (2015): 2686-2694.
- Itchaki G, Brown RJ. The potential of venetoclax (ABT-199) in chronic lymphocytic leukaemia. Ther Adv Hematol 7 (2016): 270-287.
- Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med 374 (2016): 311-322.
- Seymour JF, Kipps TJ, Eichhorst B, Hillmen P, D’Rozario J, Assouline S et al. Venetoclax–Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N Engl J Med 378 (2018): 1107-1120.
- Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med 373 (2015): 2425-2437.
- O’Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol 15 (2014): 48-58.
- O’Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol 17 (2016): 1409-1418.
- Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ Inhibition by Idelalisib in Patients with Relapsed Indolent Lymphoma. N Engl J Med 370 (2014): 1008-1018.
- Stilgenbauer, S, Eichhorst B, Schetelig J, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol 17 (2016): 768-778.
- Jones JA, Wierda WG, Choi MY, et al. Venetoclax activity in CLL patients who have relapsed after or are refractory to ibrutinib or idelalisib. Poster session presented at: American Society of Clinical Oncology (ASCO) Annual Meeting (2016): 3-7.
- Seymour, JF, Ma S, Brander DM, et al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. Lancet Oncol 2 (2017): 230-240.
- Schetelig J, van Biezen A, Brand R, et al. Allogeneic hematopoietic stem-cell transplantation for chronic lymphocytic leukemia with 17p deletion: a retrospective European Group for Blood and Marrow Transplantation analysis. J Clin Oncol 26 (2008): 5094-5100,
- Rabinowe BSN, Soiffer RJ, Gribben JG, et al. Autologous and allogeneic bone marrow transplantation for poor prognosis patients with B-cell chronic lymphocytic leukemia. Blood 82 (1993): 1366-1376,
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute – Working Group 1996 guidelines. Blood 111 (2008): 5446-5456.
- Michallet M, Corront B, Hollard D, et al: Allogeneic bone marrow transplantation in chronic lymphocytic leukemia: 17 cases. Report from the EBMTG. Bone Marrow Transplant 7 (1991): 275-279.
- Gribben JG, Zahrieh D, Stephans S, et al. Autologous and allogeneic stem cell transplantations for poor-risk chronic lymphocytic leukemia. Blood 106 (2005): 4389-4396.
- Dreger P, Dohner H, McClanahan F, et al. Early autologous stem cell transplantation for chronic lymphocytic leukemia: long-term follow-up of the German CLL Study Group CLL3 trial. Blood 119 (2012): 4851-4859.
- Schetelig J, Thiede C, Bornhäuser M, et al. Evidence of a graft-versus-leukemia effect in chronic lymphocytic leukemia after reduced-intensity conditioning and allogeneic stem-cell transplantation: The Cooperative German Transplant Study Group. J Clin Oncol 21 (2003): 2747-2753.
- Sorror M, Maris M, Sandmaier B, et al. Hematopoietic cell transplantation after nonmyeloablative conditioning for advanced chronic lymphocytic leukemia. J Clin Oncol 23 (2005): 3819-3829.
- Dreger P, Dohner H, Ritgen M, et al. Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood 116 (2010): 2438-2447.
- Porter DL, Frey NV, Melenhorst JJ, et al. Randomized, phase II dose optimization study of chimeric antigen receptor modified T cells directed against CD19 (CTL019) in patients with relapsed, refractory CLL [abstract]. Blood 124 (2014).
- Fraietta JA, Beckwith KA, Patel PR, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood 127 (2016): 1117-1127.
- Bottcher S, Ritgen M, Fischer K, et al. Minimal Residual Disease Quantification Is an Independent Predictor of Progression-Free and Overall Survival in Chronic Lymphocytic Leukemia: A Multivariate Analysis From the Randomized GCLLSG CLL8 Trial. J Clin Oncol 30 (2012): 980-988.
- Strati P, Keating MJ, O’Brien SM, et al. Eradication of bone marrow minimal residual disease may prompt early treatment discontinuation in CLL. Blood 123 (2014): 3727-3732.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 74.74%
Acceptance Rate: 74.74%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 