Pharmacological Reinvention: Drug Repurposing Strategies to Tackle Aggressive High Grade Serous Ovarian Carcinoma (HGSOC)
Faisal Iqbal*
Department of Pharmaceutical Sciences, Herbert M.and Carol H.Retzky College of Pharmacy, University of Illinois Chicago (UIC), USA
*Corresponding Author: Faisal Iqbal. Department of Pharmaceutical Sciences, Herbert M.and Carol H.Retzky College of Pharmacy, University of Illinois Chicago (UIC), USA
Received: 25 September 2025; Accepted: 17 October 2025; Published: 14 November 2025
Article Information
Citation: Faisal Iqbal Pharmacological Reinvention: Drug Repurposing Strategies to Tackle Aggressive High Grade Serous Ovarian Carcinoma (HGSOC). Journal of Cancer Science and Clinical Therapeutics. 9 (2025): 189-198.
View / Download Pdf Share at FacebookAbstract
The poor prognosis and limited treatment option make high grade serous ovarian carcinoma (HGSOC) is an aggressive malignancy. Drug repurposing provides a cost effective and fast strategy to recognize novel treatment possibilities. In this study, investigated the individual and joint therapeutic potential of three repurposed drugs like auranofin, metformin and chlorpromazine (CPZ) in HGSOC. A potent effect for all three drugs was revealed, while triple combination of drugs screening is strongest reduction in cell proliferation. The triple combination of drug synergistic effect was more noticeable than single or double drug treatment. The findings recommend that the combination of auranofin, CPZ and metformin are helpful against HGSOC of OVCAR3 cell line. The hemocytometer and clonogenic assay were performed to evaluate the counting and survival cells in colonies form, further ANOVA and Tukey’s post-hoc test was run for statistical analysis and validated the significance of drug repurposing effects while after polymerase chain reaction (PCR) the gel electrophoresis was performed for molecular analysis. The study defines a novel pharmacological approach that repurposes existing drugs to achieve a better anti-cancer effectiveness in HGSOC.
Keywords
<p>HGSOC; OVCAR3; Auranofin; Metformin; CPZ</p>
Article Details
1. Introduction
The predominant and aggressive subtype of epithelial ovarian cancer is high grade serous ovarian carcinoma (HGSOC), it contains around 70% of cases and most cause of cancer death in ovarian cancer. Despite early response for chemotherapy and chemoresistance, the 40% of the women survival rate is only 5 years. The alternative and quick therapeutics are required with innovative strategies like drug repurposing to recognize the new anti-cancer drugs [1-6]. The drug repurposing based cost effective and time saving tactic to cancer therapy mainly when specific combined drugs targeted cancer cells. HGSOC is categorized by redox imbalance, genomic instability and metabolic reprogramming and can be defined through drug grouping. In this perspective, the three drugs are repurposing like metformin, an anti-diabetic drug known to disrupt cancer metabolism; chlorpromazine (CPZ), a typical antipsychotic drugs and auranofin it is a rheumatoid arthritis drug, targets redox homeostasis and can induce cell death in chemoresistant ovarian cancer cells [7-13]. Preclinical studies have shown that these drugs possess individual anti-cancer properties, but their combinatorial potential in HGSOC remains largely unexplored. To hypothesize that the simultaneous mitochondrial function, metabolic signaling and disruption of redox balance through a triple drug combination will induce synergistic cytotoxicity in HGSOC cells. This study goals to evaluate auranofin, CPZ and metformin for therapeutic effectiveness individually and grouping based using in vitro model of HGSOC and explain the molecular mechanism. The findings provide strong foundation for drugs repurposing to innovative therapeutic strategies against aggressive ovarian cancer possibly overcoming limitations of current treatment regimens [14-20]. The outcomes validate that auranofin, metformin and CPZ significantly reduce the number of cells counting and colonies in OVCAR3 cells, a typical model of high grade serous ovarian carcinoma (HGSOC). The hemocytometer and clonogenic assays confirmed that triple drugs were more effective than single or double drugs to suppress the colony formation, further statistical analysis performed like ANOVA and Tukey’s post-hoc test validated the significance of these drugs effects. After PCR, gel electrophoresis analysis provides mechanistic insight, displayed the drug resistance pathways and revealed a potential molecular basis of observed synergistic activity. So, the outcomes present a novel therapeutic approach that repurposes clinically available drugs to improve the anticancer activity in HGSOC, offering a promising pathway for improved treatment approaches.
2. Materials and Methods
2.1 Cell Line and Culture Condition
The cell line OVCAR3 of human high grade serous ovarian carcinoma (HGSOC) was obtained from the American Type Culture Collection (ATCC). Cells were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin and incubated at 37°C in a humidified 5% CO2 atmosphere. STR profiling was used for cell line authentication and all experiments were conducted on mycoplasma free cells within 10 passages.
2.2 Drug Treatment and Experimental Design
Cells were seeded in 6 well plates. Metformin, CPZ and auranofin were purchased from Sigma-Aldrich. CPZ, auranofin and metformin stock solutions were prepared and stored at -20°C. While final concentrations were prepared freshly in complete media. Control, single, double and triple treatment each has three wells in 6 well plates for cell counting and colonies forming. First day, during cell suspension, remove the supernatant while cell pellet remains in the falcon tube then 0.5ml added RPMI media and gently mix it. After that RPMI media 1ml put in each well of the 6 well plate then add 0.5ml from the falcon tube resuspended cells, mix it vigorously and observed on the fluorescent microscope and keep the 6 well plate in the incubator. Day two first remove all yesterday media and put 2ml RPMI media in each well, then start adding drugs, one well was control (untreated) while in other wells added drugs like 0.5 µM auranofin of 10 µl, 1 µM metformin of 10 µl, CPZ 2 µM of 10 µl, other was combo (auranofin (0.5 µM of 10 µl) + metformin (1 µM of 10 µl)), (auranofin (0.5 µM of 10 µl) + CPZ (2 µM of 10 µl)), (CPZ (2 µM of 10 µl) + metformin (1 µM of 10 µl)) and last one was triple drugs (auranofin (0.5 µM of 10 µl) + metformin (1 µM of 10 µl) + CPZ (2 µM of 10 µl)), after that check the each well plate on the fluorescent microscope and keep the 6-well plates in the incubator for 2 days. The day four remove the media then add PBS three time and wash out each well, 1ml trypsin added then incubate it for 5 minutes in the incubator, check the cells condition if cells are floating then add 2ml media and mix it gently in the falcon tubes, took P100 cell plates for each well separately and put 7ml RPMI media and added cell suspension 3 ml (1 ml trypsin + 2 ml RPMI) already in the falcon tubes and put in the P100 cell plates total volume was 10ml, observed the P100 cell plates on the fluorescent microscope and 48 hours keep it back into the incubator.
2.3 Hemocytometer
Cell counting technique hemocytometer was performed, first prepared a cell suspension then diluted the sample 1:1 with trypan blue dye to evaluate the viable and non-viable cells. Cell suspension sample 10 µl and trypan blue 10 µl was mixed, total volume 20 µl. Load 10 µl into the hemocytometer chamber and carefully pipetting at the hemocytometer socket. To fill the chamber without air bubbles and keep the hemocytometer under fluorescent microscope and focus on grid lines to count the cells in four large corner squares. The blue stain cells are non-viable while viable cells usually appear in uniform.
2.4 Clonogenic Assay Protocol for Colony Counting
Treat the cells with drugs and incubate for 48 hours according to the cells condition. After that rinse gently with PBS and fix the cells using methanol (100%) for 10 minutes then use the 0.5% crystal violet to stain the colonies and incubate at room temperature for 15 minutes, rinse gently and remove stain. Allow plates to dry completely then count the colonies manually under fluorescent microscope, only ≥ 50 cells count per colony.
2.5 ANOVA and Tukey’s Post-Hoc Test
Analysis of variance (ANOVA) is a statistical technique used to define the significant differences among the means of multiple groups. In this study, one way ANOVA was used to compare colony numbers across different treatment conditions (control (untreated), single drug and drug combinations) to evaluate whether drug treatments had a measureable effects on clonogenic survival. Though, ANOVA indicates at least one group differs from other and it does not specify which group are significantly different. To address this, Tukey’s post-hoc test was performed to allow for pairwise comparisons between all treatment groups. This strategy gives a clear identification of specific drug that reduced the number of colony formation compared with control, single, double and triple drug while ensuring the reliability and robustness of statistical interpretation.
2.6 RNA Extraction
RNA was isolated from ovarian cancer cells followed the QIAGEN RNeasy kit according to the manufacturer instructions. The RNA purity was verified on Nano Drop. For gene expression, the amount of RNA was reverse transcribed into complementary DNA (cDNA). The cDNA was stored at -20°C until use in subsequent PCR amplification.
2.7 Primer Designing
The TP53 gene was used to design the primers for polymerase chain reaction (PCR) for OVCAR3 cell line. The forward primer (GAAGAACAACTGTCTCACCGC) and reverse primer (TTAAAGAAGCCGAGACGGGC) was used for PCR amplification.
2.8 Polymerase Chain Reaction (PCR) and Gel Electrophoresis
The conventional PCR 20 µl was performed. The 10x PCR buffer 2 µl but final concentration 1x, MgCl2 (25mM) 1.2 µl while final concentration 1.5 µl, dNTP mix (10mM each) 0.4 µl however final concentration 200 µM each, forward primer (10 µM) 0.4 µl though final concentration 0.2 µM, reverse primer (10 µM) 0.4 µl and final concentration 0.2 µM, 5U/ µl taq DNA polymerase 0.2 µl and its final concentration 0.5 U, DNA sample 1 µl and nuclease free water remaining add to complete 20 µl. Thermal cycling initial denaturation 95°C for 2 minutes, 30 cycles run, denaturation 94°C for 30 second, annealing 54°C for 30 second, extension 72°C for 30 seconds and final extension at 72°C for 2 minutes.
Prepared a 2% agarose gel for PCR products, agarose was dissolved with 50ml 1x TAE buffer, heat on microwave until fully melted, after cooling at 60°C, add 2 µl DNA stain (ethidium bromide). The solution was poured into gel tray and comb inserted to make a well. After 20 minutes gel solidified to transferred into the electrophoresis tank and covered the gel with running buffer 1x TAE to fully submerged. PCR products 5 µl with 1 µl loading dye loaded in wells and 100bp DNA ladder was loaded in another well for molecular size marker. The electrophoresis was run 30 minutes at 120 v, finally check the result at gel imaging system.
3. Results and Discussion
3.1 Drug Repurposing Strategy in HGSOC Cells
Initial screening demonstrated that auranofin, metformin and CPZ each drug reduced cell viability in OVCAR3 cell line. The hemocytometer was used before cell seeding and after drug treatment to check the drug impact on ovarian cancer cells.
Table 1: Hemocytometer data of OVCAR3 cell line.
|
Condition/Treatment |
Time point |
Replicate values (×104 cells) |
n (number of replicates) |
Mean ± SD |
Reported answer (×104) |
|
Control (untreated) well 1- before cell seeding |
Day 1 |
7, 5, 8, 9 |
4 |
14.50 ± 3.42 |
29 |
|
Control (untreated) well 1- After 48 hours of media changed |
Day 4 |
9, 5, 6, 8 |
4 |
14.00 ± 3.66 |
28 |
|
Control (untreated) well 2- before cell seeding |
Day 1 |
5, 7, 9, 9 |
4 |
15.00 ± 3.82 |
30 |
|
Control (untreated) well 2- After 48 hours of media changed |
Day 4 |
7, 6, 8, 7 |
4 |
14.00 ± 1.64 |
28 |
|
Control (untreated) well 3- before cell seeding |
Day 1 |
3, 9, 9, 8 |
4 |
14.50 ± 5.74 |
29 |
|
Control (untreated) well 3- After 48 hours of media changed |
Day 4 |
2, 8, 8, 9 |
4 |
13.50 ± 6.40 |
27 |
|
Auranofin (0.5 µM) well 1- before cell seeding |
Day 1 |
9, 7, 5, 6 |
4 |
13.50 ± 3.42 |
27 |
|
Auranofin (0.5 µM) well 1- after treatment |
Day 4 |
4, 5, 7, 6 |
4 |
11.00 ± 2.58 |
22 |
|
Auranofin (0.5 µM) well 2- before cell seeding |
Day 1 |
9, 5, 7, 8 |
4 |
14.50 ± 3.42 |
29 |
|
Auranofin (0.5 µM) well 2- after treatment |
Day 4 |
7, 4, 5, 6 |
4 |
11.00 ± 2.58 |
22 |
|
Auranofin (0.5 µM) well 3- before cell seeding |
Day 1 |
7, 6, 7, 8 |
4 |
14.00 ± 1.64 |
28 |
|
Auranofin (0.5 µM) well 3- after treatment |
Day 4 |
7, 7, 8, 1 |
4 |
11.50 ± 6.40 |
23 |
|
Metformin (1 µM) well 1- before cell seeding |
Day 1 |
9, 9, 2, 9 |
4 |
14.50 ± 7.00 |
29 |
|
Metformin (1 µM) well 1- after treatment |
Day 4 |
2, 7, 8, 7 |
4 |
12.00 ± 5.42 |
24 |
|
Metformin (1 µM) well 2- before cell seeding |
Day 1 |
6, 8, 7, 9 |
4 |
15.00 ± 2.58 |
30 |
|
Metformin (1 µM) well 2- after treatment |
Day 4 |
6, 7, 5, 6 |
4 |
12.00 ± 1.64 |
24 |
|
Metformin (1 µM) well 3- before cell seeding |
Day 1 |
5, 5, 9, 9 |
4 |
14.00 ± 4.62 |
28 |
|
Metformin (1 µM) well 3- after treatment |
Day 4 |
8, 9, 4, 3 |
4 |
12.00 ± 5.88 |
24 |
|
CPZ (2 µM) well 1- before cell seeding |
Day 1 |
7, 5, 7, 9 |
4 |
14.00 ± 3.26 |
28 |
|
CPZ (2 µM) well 1- after treatment |
Day 4 |
5, 5, 5, 7 |
4 |
11.00 ± 2.00 |
22 |
|
CPZ (2 µM) well 2- before cell seeding |
Day 1 |
5, 4, 7, 9 |
4 |
12.50 ± 4.44 |
25 |
|
CPZ (2 µM) well 2- after treatment |
Day 4 |
4, 3, 8, 6 |
4 |
10.50 ± 4.44 |
21 |
|
CPZ (2 µM) well 3- before cell seeding |
Day 1 |
6, 7, 5, 9 |
4 |
13.50 ± 3.42 |
27 |
|
CPZ (2 µM) well 3- after treatment |
Day 4 |
5, 6, 5, 7 |
4 |
11.50 ± 1.92 |
23 |
|
Combo (auranofin + metformin) well 1- before cell seeding |
Day 1 |
7, 8, 9, 5 |
4 |
14.50 ± 3.42 |
29 |
|
Combo (auranofin + metformin) well 1- after treatment |
Day 4 |
5, 4, 7, 3 |
4 |
9.50 ± 3.42 |
19 |
|
Combo (auranofin + metformin) well 2- before cell seeding |
Day 1 |
8, 7, 5, 9 |
4 |
14.50 ± 3.42 |
29 |
|
Combo (auranofin + metformin) well 2- after treatment |
Day 4 |
3, 5, 3, 7 |
4 |
9.00 ± 3.84 |
18 |
|
Combo (auranofin + metformin) well 3- before cell seeding |
Day 1 |
6, 6, 7, 8 |
4 |
13.50 ± 1.92 |
27 |
|
Combo (auranofin + metformin) well 3- after treatment |
Day 4 |
4, 4, 6, 5 |
4 |
9.50 ± 1.92 |
19 |
|
Combo (auranofin + CPZ) well 1- before cell seeding |
Day 1 |
6, 7, 8, 9 |
4 |
15.00 ± 2.58 |
30 |
|
Combo (auranofin + CPZ) well 1- after treatment |
Day 4 |
4, 4, 4, 6 |
4 |
9.00 ± 2.00 |
18 |
|
Combo (auranofin + CPZ) well 2- before cell seeding |
Day 1 |
9, 8, 7, 4 |
4 |
14.00 ± 4.32 |
28 |
|
Combo (auranofin + CPZ) well 2- after treatment |
Day 4 |
5, 6, 5, 3 |
4 |
9.50 ± 2.52 |
19 |
|
Combo (auranofin + CPZ) well 3- before cell seeding |
Day 1 |
7, 7, 5, 9 |
4 |
14.00 ± 3.26 |
28 |
|
Combo (auranofin + CPZ) well 3- after treatment |
Day 4 |
4, 4, 3, 6 |
4 |
8.50 ± 2.52 |
17 |
|
Combo (CPZ + metformin) well 1- before cell seeding |
Day 1 |
5, 5, 8, 9 |
4 |
13.50 ± 4.12 |
27 |
|
Combo (CPZ + metformin) well 1- after treatment |
Day 4 |
7, 6, 3, 2 |
4 |
9.00 ± 4.76 |
18 |
|
Combo (CPZ + metformin) well 2- before cell seeding |
Day 1 |
5, 5, 7, 9 |
4 |
13.00 ± 3.84 |
26 |
|
Combo (CPZ + metformin) well 2- after treatment |
Day 4 |
6, 5, 3, 4 |
4 |
9.00 ± 2.58 |
18 |
|
Combo (CPZ + metformin) well 3- before cell seeding |
Day 1 |
6, 7, 9, 8 |
4 |
15.00 ± 2.58 |
30 |
|
Combo (CPZ + metformin) well 3- after treatment |
Day 4 |
5, 5, 6, 4 |
4 |
10.00 ± 1.64 |
20 |
|
Combo (auranofin + metformin + CPZ) well 1- before cell seeding |
Day 1 |
6, 5, 9, 9 |
4 |
14.50 ± 4.12 |
29 |
|
Combo (auranofin + metformin + CPZ) well 1- after treatment |
Day 4 |
4, 4, 3, 3 |
4 |
7.00 ± 1.16 |
14 |
|
Combo (auranofin + metformin + CPZ) well 2- before cell seeding |
Day 1 |
7, 6, 5, 9 |
4 |
13.50 ± 3.42 |
27 |
|
Combo (auranofin + metformin + CPZ) well 2- after treatment |
Day 4 |
4, 2, 5, 4 |
4 |
7.50 ± 2.52 |
15 |
|
Combo (auranofin + metformin + CPZ) well 3- before cell seeding |
Day 1 |
5, 5, 8, 9 |
4 |
13.50 ± 4.12 |
27 |
|
Combo (auranofin + metformin + CPZ) well 3- after treatment |
Day 4 |
4, 4, 5, 2 |
4 |
7.50 ± 2.52 |
15 |
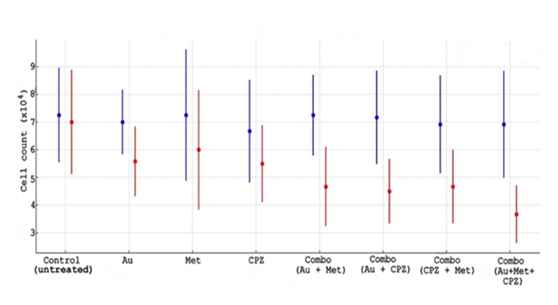
Hemocytometer based cell counts were performed for OVCAR3 cells to evaluate the effects of single and combinatorial (double and triple) drug treatments. Control (untreated) wells remain stable, before seeding (day 1) and after media change (day 4) in all wells. In contrast, the auranofin (0.5 µM), metformin (1 µM) and CPZ (2 µM) individually have modest reduction in OVCAR3 cancer cell counts, while double drug combo was more cell reduction as compared to individual drugs in all wells. The combo (triple drugs) has strong inhibitory effect. The outcomes present that triple drug treatment synergistically enhances growth suppression in OVCAR3 cells.
Figure 1: OVCAR3 cell line of hemocytometer data used for replicating scatter plot with error bars for each condition. The scatter plot displays the mean cell counts with error bars for each treatment condition before and after treatment. The blue points representing the before treatment and red points representing the after treatment. Each error bar reflects the standard deviation across replicates. The Au is presenting auranofin while Met is metformin.
3.2 Initial Drug Treatment and Immediate Effects
After exposure to the drug combination, some of the HGSOC cells died and some shown growth inhibition and these effects were observed on fluorescent microscope. The clonogenic assay was designed for cell surviving assays for colonies having 50 or more cells. In the situation of the three drug treatments even a tiny surviving cell fraction may form colonies. The colony formation of late kinetics indicates that the surviving population is not completely eradicated but rather inter in slow proliferation which may be due to stress. When counting the number and evaluating the size and morphology of colonies to define both plating efficiency and surviving fraction. The control (untreated) compared with low number of colonies to confirm the anticancer effects of drugs where small or less dense colonies shown slow proliferation.
3.3 Biological and Therapeutic Implications
The observation that cells can form colonies at a slower rate after treatment with auranofin, metformin and CPZ. The drugs were effective and results shown significance of clonogenic assay for therapy development and disclose the risk of recurrence may inspire to design the supplementary treatment and expected to these slow cell proliferation survival eradicate or block their regrowth.
The clonogenic assay is a helpful for the HGSOC long term reproductive viability using the three drugs (auranofin, metformin and CPZ). These drugs have significant anti-cancer effects and the clonogenic assay assist to detect survival cells in colonies form. So, colony formation observation, even delay or alteration in colony morphology provide critical insight into treatment efficacy, drug resistance etc. The inclusive understanding can guide more therapeutic approaches to ensure complete eradication of cancer cells.
Table 2: The outcomes of clonogenic assay after drug treatment.
|
Treatment Group |
Set 1 colonies |
Set 2 colonies |
Set 3 colonies |
Colony Size |
Interpretation |
|
Control (untreated) |
80 |
84 |
86 |
Large, dense |
Cells were healthy, great proliferative capacity |
|
Auranofin |
60 |
61 |
64 |
Slightly smaller |
Auranofin inhibits redox pathways, moderately impairs survival |
|
Metformin |
57 |
58 |
61 |
Smaller colonies |
Metabolic stress slows growth |
|
CPZ |
54 |
55 |
58 |
Some faint or irregular colonies |
Disrupts autophagy and signaling, cytotoxic |
|
Combo (auranofin + metformin) |
20 |
23 |
25 |
Smaller, fewer dense colonies |
Synergistic stress on mitochondria + redox system |
|
Combo (auranofin + CPZ) |
17 |
19 |
22 |
Small, irregular colonies |
Enhanced ROS + autophagy inhibition = synergy |
|
Combo (CPZ + metformin) |
15 |
16 |
19 |
Scattered, faint colonies |
Combined stress on energy metabolism and autophagy |
|
Combo (auranofin + metformin + CPZ) |
5 |
6 |
9 |
Tiny colonies |
Triple stress (ROS, metabolism, signaling); cells die or fail to replicate |
In high grad serous ovarian carcinoma (HGSOC), the clonogenic assay play a critical role in evaluating the effects of anticancer treatment, when different drugs are used individually or combine define the strong anti-cancer effects. Though drugs shown slow proliferation of HGSOC cells and the survival cells were detectable in colonies form.
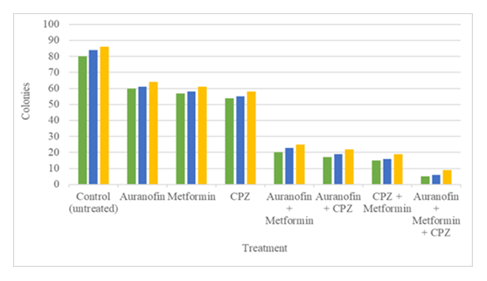
Figure 2: The clonogenic assay results. The graphical bar data was taken after implementing the auranofin, metformin and CPZ on OVCAR3 cell line. The control (untreated) colonies were 80, 84 and 86 while cells were large and healthy. Auranofin colonies were 60, 61 and 64 but slightly smaller in size. Metformin colonies were 57, 58 and 61 with small in size. CPZ colonies were 54, 55 and 58 have faint and irregular in size. Auranofin + metformin colonies were 20, 23 and 25 with smaller, fewer and dense forms. Auranofin + CPZ colonies were 17, 19 and 22 while size was small and irregular. CPZ + metformin colonies were 15, 16 and 19 with scattered and faint cell forms. The last one was auranofin + metformin + CPZ colonies were 5, 6 and 9, tiny but strong cell reduction. The single drugs inhibit cells proliferation but allow some cell survival while double combo have more effective and triple combo shows strong inhibition. The set 1 is green color bar, set 2 is blue color bar and set 3 is presenting yellow color bar.
These outcomes have potential using the drug repurposing approaches against HGSOC of OVCAR3 cell line. The auranofin, metformin and CPZ exploit key vulnerabilities. This approach are highly effective in reducing the risk of resistance. All three drugs are either approved from FDA or tested clinically for non-cancer, offering a practical route to fast clinical translation. In vivo authentication will include in the future studies with drug repurposing to genomic profiling for HGSOC.
3.4 Plating Efficiency (PE) and Surviving Fraction (SF) of OVCAR3 Cell Line Data Interpretation
The colony formation assay is also known as clonogenic assay, technique is highly sensitive and keeps ability to grow from single cell to colony through nonstop proliferation. Specifically, it is helpful in cancer research and has skills for cancer cells to divide, recover and form colonies. The process start at low density seeding the cells in culture dishes and confirms that each cell grow into separate colony without touching, after that allow the cells to attach and treated with agents of interest and media is changed either or left depend on the treatment period. The cells are incubated for few days according to the experimental situation and survival cells proliferate and come out in colonies form. While after incubation period, the colonies are fixed using methanol and stained with crystal violet or similar dyes to visualize them, 50 or more cells counted as a colony and indicate successful remain for proliferation. Plating efficiency (PE) and surviving fraction (SF) are separately calculated based on the number of colonies formed relative to the number of cells initially plated. The clonogenic assay is ideal for evaluating synergy between treatments. The assay required accurate handling and is sensitive like cell density, media change and physical disturbance. Though this methodology remains gold standard for oncology and drug synergy studies. The plating efficiency (PE) was 80%, 84% and 86% respectively for control set 1, 2 and 3. The surviving fraction (SF) for auranofin 75%, 72.6%, 74.4%, metformin 71.2%, 69.0%, 70.9%, CPZ 67.5%, 65.4%, 67.4%, combo (auranofin + metformin) 25%, 27.3%, 29.0%, combo (auranofin + CPZ) 21.2%, 22.6%, 25.5%, combo (CPZ + metformin) 18.7%, 19.0%, 22.0%, combo (auranofin + metformin + CPZ) 6.2%, 7.1%, 10.4%. The auranofin, metformin and CPZ individually retain 65.4-75% survival. The double combination of SF drops significantly to 18.7-29.0%. While triple combo (auranofin + metformin + CPZ) shows strongest impact of 6.2-10.4% survival, indicating a synergistic effect. Cells were seeded at low density (100 cells/ cell culture plate). The colonies were stained and counted while SF was calculated with untreated control (PE). These findings suggest that combo drug repurpose significantly enhances anti-proliferative effects in OVCAR3 cells compared to individual treatment.
3.5 ANOVA (Analysis of Variance)
It is a statistical test used to compare the meanings of three groups. The clonogenic assay data was used for ANOVA and Tukey post-hoc.
Table 3: Clonogenic assay data for ANOVA.
|
Treatment |
Colonies Group 1 |
Colonies Group 2 |
Colonies Group 3 |
|
Control (untreated) |
80 |
84 |
86 |
|
Auranofin |
60 |
61 |
64 |
|
Metformin |
57 |
58 |
61 |
|
CPZ |
54 |
55 |
58 |
|
Combo (auranofin + metformin) |
20 |
23 |
25 |
|
Combo (auranofin + CPZ) |
17 |
19 |
22 |
|
Combo (CPZ + metformin) |
15 |
16 |
19 |
|
Combo (auranofin + metformin + CPZ) |
5 |
6 |
9 |
After clonogenic assay data was used further for analysis of variance or ANOVA.
Table 4: One way ANOVA table.
|
Source |
Sum of Square |
df |
F |
p-value |
|
Treatment |
15964.5 |
7 |
417.83 |
6.73x10-17 |
|
Residual |
87.33 |
16 |
- |
- |
The effect of treatments was highly significant and confirmed strong differences among drug group while F value 417.83 is very high and indicate that treatment related variance tremendously surpasses with group variance while p value was 6.73 x 10-17. The outcomes indicate that variation in colony counts are due to influenced of different drug treatments. The consistent control group showed highest colony formation, while single drug treatment with CPZ, auranofin and metformin produced meaningful reduction in clonogenic survival. The double drug was more effective to decrease the colony number as compared to single and show the potential of synergistic effects. The triple drug combination (auranofin + metformin + CPZ) was very effective for the reduction of colony formation. Overall, the ANOVA outcomes confirmed that drug treatment especially triple drug shows significant inhibitory effects on ovarian cancer cell clonogenicity.
3.6 Tukey’s HSD Post-Hoc Test
The main purpose of Tukey’s HSD post-hoc test is to find out which specific group are significantly different from each other.
Table 5: Tukey’s HSD post-hoc comparison table.
|
Comparison |
Mean difference |
p-value |
Significance |
|
Control vs auranofin |
23.33 |
<0.001 |
Yes |
|
Control vs metformin |
26.67 |
<0.001 |
Yes |
|
Control vs CPZ |
30 |
<0.001 |
Yes |
|
Control vs auranofin + metformin |
63.67 |
<0.001 |
Yes |
|
Control vs auranofin + CPZ |
68 |
<0.001 |
Yes |
|
Control vs CPZ + metformin |
70.67 |
<0.001 |
Yes |
|
Control vs triple combo |
78.33 |
<0.001 |
Yes |
|
Auranofin vs metformin |
3.33 |
0.42 |
No |
|
Auranofin vs CPZ |
6.67 |
0.15 |
No |
|
Auranofin vs auranofin + metformin |
40.33 |
<0.001 |
Yes |
|
Auranofin vs auranofin + CPZ |
44.67 |
<0.001 |
Yes |
|
Auranofin vs CPZ + metformin |
47.33 |
<0.001 |
Yes |
|
Auranofin vs triple combo |
55 |
<0.001 |
Yes |
|
Metformin vs CPZ |
3.33 |
0.42 |
No |
|
Metformin vs auranofin + metformin |
37 |
<0.001 |
Yes |
|
Metformin vs auranofin + CPZ |
41.33 |
<0.001 |
Yes |
|
Metformin vs CPZ + metformin |
44 |
<0.001 |
Yes |
|
Metformin vs triple combo |
51.67 |
<0.001 |
Yes |
|
CPZ vs auranofin + metformin |
33.67 |
<0.001 |
Yes |
|
CPZ vs auranofin + CPZ |
38 |
<0.001 |
Yes |
|
CPZ vs CPZ + metformin |
40.67 |
<0.001 |
Yes |
|
CPZ vs triple combo |
48.33 |
<0.001 |
Yes |
|
Auranofin + metformin vs auranofin + CPZ |
4.33 |
0.38 |
No |
|
Auranofin + metformin vs CPZ + metformin |
7 |
0.14 |
No |
|
Auranofin + metformin vs triple combo |
14.67 |
<0.01 |
Yes |
|
Auranofin + CPZ vs CPZ + metformin |
2.67 |
0.56 |
No |
|
Auranofin + CPZ vs triple combo |
10.33 |
<0.05 |
Yes |
|
CPZ + metformin vs triple combo |
7.67 |
0.09 |
No |
The No present the not significant (p > 0.05), p < 0.05 (significant), p < 0.01 (more significant), p < 0.001 (very significant) and p < 0.0001 (extremely significant). Tukey’s several comparison analysis observed significant differences. The control (untreated) group was significantly different from all treatments and have higher colonies counts, while single drug treatments were not highly different from each other and comparisons among single drugs shown no significant difference and indicate individual potencies were broadly similar, double drug was more effective as compared to single, while triple combo show more significance reduction compared to single and double drug. Some combinations were not significant.
In contrast, combine treatment demonstrated more suppression of colonies formation than single drug, while pairwise comparison revealed that auranofin + metformin, auranofin + CPZ and CPZ + metformin were each significantly more effective than any single drug. While triple drug (auranofin + metformin + CPZ) was more effective in reducing the clonogenic growth and was significantly more effective compared to all other treatments including double drug treatments. The Tukey analysis highlight a clear efficacy, all treatments reduced colony formation compared with control, the triple drugs treatment was more effective as compared to single and double drug treatments. These outcomes provide robust statistical evidence that combine drug strategies presented superior anti clonogenic effects in ovarian cancer cells.
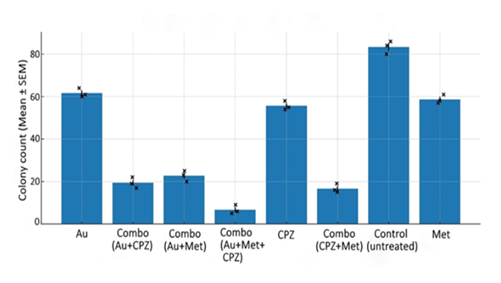
Figure 3: The mean colony counts (Mean ± SEM) from clonogenic assay presents bar chart across all treatment groups. The control (untreated) group displayed robust colony formation and confirmed the high clonogenic potential of untreated ovarian cancer cells. Single drug treatments with auranofin (Au), metformin (Met) or CPZ remain moderate but consistent decrease in colony number compared with control, the double drug synergistic auranofin + metformin, auranofin + CPZ and CPZ + metformin was more effective as compared to single treatments. While most striking observation was the effect of triple drug (auranofin + metformin + CPZ) which destroyed the colony formation and it was highly potent as compared to single and double drug treatments. The error bars and replicate demonstrate the reproducibility of these findings and showing minimum overlap between control and combine treatment groups. Overall, the figure highlight a clear trend in which single and double drug reduced the number of colonies formation while triple drug was more effective. The mean ± SEM stands for mean ± standard error of the mean.
3.7 Molecular Analysis
After polymerase chain reaction (PCR) the gel electrophoresis was performed and got the expected result. In initial phase the drugs shown some impact but later after transferred the cells from 6 well plates to P100 plates the cells grow normally.
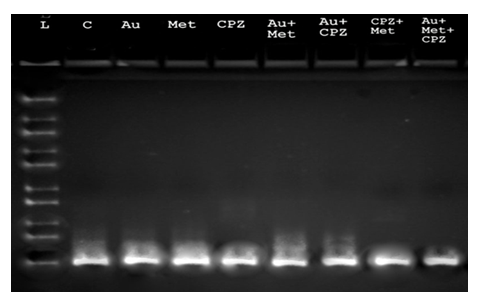
Figure 4: OVCAR3 cell line of gel electrophoresis analysis for molecular pathway affected by the drug repurposing. L is a ladder, C is a control, AU is auranofin, Met is metformin and CPZ is chlorpromazine. Combo (auranofin + metformin, auranofin + CPZ, CPZ + metformin) shown modest cancer cells reduction. While auranofin + metformin + CPZ is a triple drug combo and has a strong anticancer effects. Though, after 48 hours of drug treatment, ovarian cancer cells start proliferating normally and develop resistance against drugs. While at the end gel electrophoresis gave expected marker size.
4. Conclusion
The study defines the drug repurposing of auranofin, metformin and CPZ apply significant anticancer effects against HGSOC of OVCAR3 cell line in initial stage. These drugs effects collectively reduced cancer cell proliferation. The findings suggest that repurposed drug combination is highly effective to resist the aggressive HGSOC. Hemocytometer and clonogenic assay with ANOVA and Tukey test were used for statistical analysis evidence to evaluate the drug repurpose of OVCAR3 cell line data. The PCR and gel electrophoresis was performed for molecular analysis. In short, this study supports drug repurpose as an innovative and cost effective approach for developing new treatment for ovarian cancer.
Competing Interest
The author declares no competing interest.
Funding:
This research was conducted without any funding support.
Author Contributions:
F.I: Conceptualization, methodology, investigation, visualization, writing original draft, resource, review and editing.
References
- Satish K, Ramraj S P, Elayapillai R C, et al. Novel ovarian cancer maintenance therapy targeted at mortalin and mutant p53. International Journal of Cancer 147 (2020): 1086-1097.
- Petr H, Viktor H, Karolína Š, et al. Targeted DNA sequencing of high-grade serous ovarian carcinoma reveals association of TP53 mutations with platinum resistance when combined with gene expression. International Journal of Cancer 155 (2024): 104-116.
- Zhao R, Ning X, and Lu H. XTP8 Promotes Ovarian Cancer Progression by Activating AKT/AMPK/mTOR Pathway to Regulate EMT. Cell Biochem Biophys 82 (2024): 945-957.
- Yuan Q, Lv N, Chen Q. Application of single cell sequencing technology in ovarian cancer research (review). Funct Integr Genomics 24 (2024): 144.
- Junfen X, Weiguo L, Xinyi W, et al. Single-cell transcriptomics reveals the aggressive landscape of high-grade serous carcinoma and therapeutic targets in tumor microenvironment. Cancer Letters 593 (2024): 216928.
- Manead K, Lydia J D, Daniel D, et al. Aulosirazole Stimulates FOXO3a Nuclear Translocation to Regulate Apoptosis and Cell-Cycle Progression in High-Grade Serous Ovarian Cancer (HGSOC) Cells. Molecular Pharmacology 106 (2024): 145-154.
- Giudice E, Huang T, Nair J R. The CHK1 inhibitor prexasertib in BRCA wild-type platinum-resistant recurrent high-grade serous ovarian carcinoma: a phase 2 trial. Nature Communication 15 (2024): 2805.
- Domcke S, Sinha R, Levine D A, et al. Evaluating cell lines as tumour models by comparison of genomic profiles. Nature Communications 4 (2023): 21-26.
- Beaufort C M, Wilting SM, van der Heijden AG, et al. Ovarian cancer cell line panel (OCCP): Clinical importance of in vitro morphological subtypes. PLOS ONE 9 (2014): e103988.
- Zhang X, Zhang J, Zhang L, et al. Comprehensive genomic profiling of high-grade serous ovarian carcinoma from Chinese patients identifies co-occurring mutations in the Ras/Raf pathway with TP53. Cancer Medicine 8 (2019): 2281-2290.
- Zhao Z, Zhang Y, Zhang X. Genomic analysis of genetic heterogeneity and evolution in high-grade serous ovarian carcinoma. Oncogene 40 (2021): 493-503.
- Kong X, Zhang Y. Characterization of ovarian cancer cell line NIH-OVCAR3 and implications of genomic, transcriptomic, proteomic and functional DNA damage response biomarkers for therapeutic targeting. Cancers 12 (2020): 19-39.
- Ereku LT, Mackay RE, Craw P, et al. RPA using a multiplexed cartridge for low cost point of care diagnostics in the field. Analytical Biochemistry 547 (2018): 84-88.
- Iqbal F, Asif MS, Qureshi AG, et al. RPA-Based colorimetric detection of SARS-Cov-2 (Covid-19) and its physiological effects. International Journal of Health Sciences 6 (2023): 6804-6818.
- Naveed AM, Royaidar J, Wadie YRR, et al. Epidemiology and Resistance Pattern in Microbial Pneumonia: A Review: Epidemiology and Resistance Pattern in Microbial Pneumonia. Pakistan Journal of Health Sciences 3 (2022): 27-31.
- Iqbal F. Enhancing the effectiveness of Chimeric Antigen Receptor (CAR) T cells against tumors through CRISPR/Cas9-mediated PD-1 disruption. International Journal of Health Sciences 7 (2023): 1836-1850.
- Iqbal F. CRISPR/Cas9-based manipulation of oncogenic chromosomal changes in vivo and drug impact on blood pressure. International Journal of Health Sciences 7 (2023): 2130-2139.
- Gockley A, Melamed A, Bregar AJ, et al. Outcomes of Women with High-Grade and Low-Grade Advanced-Stage Serous Epithelial Ovarian Cancer. Obstet. Gynecol 129 (2017): 439-447.
- Marica G, Erika C, Vincenzo C, et al. Identification of Novel Somatic TP53 Mutations in Patients with High-Grade Serous Ovarian Cancer (HGSOC) Using Next-Generation Sequencing (NGS). Int. J. Mol. Sci 19 (2018): 1510.
- Iqbal F. Bridging clinical insight and laboratory model in high-grade serous ovarian carcinoma (HGSOC) using DNA sequencing-based profiling of TP53. Oncoscience. 12(2025): 168-174.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 74.74%
Acceptance Rate: 74.74%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 