Photoinduced Erythema Multiforme and Vandetanib: Report of Two Cases
Soto-García D1,2*, Suh-Oh HJ1,2, Álvarez C1,3, Hernández Cancela RM1,3, Vega-Rodríguez P4, Flórez A1,2
1DIPO Research Group, Galicia Sur Health Research Institute (IIS Galicia Sur), SERGAS-UVIGO, Spain
2Department of Dermatology, University Hospital of Pontevedra, Spain
3Department of Pathology, University Hospital of Pontevedra, Spain
4Department of Oncology, University Hospital of Pontevedra, Spain
*Corresponding Authors: Diego Soto-García, DIPO Research Group, Galicia Sur Health Research Institute (IIS Galicia Sur), SERGAS-UVIGO. Department of Dermatology, Av. Montecelo, 0, 36164 Casas Novas, Pontevedra, Spain, Tel: +34986800269; Fax: +65 6536 5503
Received: 16 March 2021; Accepted: 30 March 2021; Published: 02 April 2021
Article Information
Citation: Soto-GarcÃa D, Suh-Oh HJ, Ãlvarez C, Hernández Cancela RM, Vega-RodrÃguez P, Flórez A. Photoinduced Erythema Multiforme and Vandetanib: Report of Two Cases. Journal of Cancer Science and Clinical Therapeutics 5 (2021): 197-200.
View / Download Pdf Share at FacebookKeywords
<p>Erythema multiforme; Adverse reaction; Phototoxicity; Vandetanib; Medullary thyroid cancer</p>
Article Details
Abbreviations:
PEM: photo-induced erythema multiforme; RET: rearranged during transfection
1. Introduction
Vandetanib is an oral multikinase inhibitor approved for patients with progressive medullary thyroid cancer. The association of vandetanib with cutaneous toxicities is well known, being phototoxicity the most characteristic [1]. Photo-induced erythema multiforme (PEM) is a rare entity described in relation to several drugs [2]. We report two cases of PEM induced by vandetanib. To our knowledge there is only one previous case reported [3].
2. Case Presentation
2.1 Case 1
A 52-year-old woman presented with a pruritic exanthema 14 days after starting treatment with vandetanib for metastatic medullary thyroid cancer. On examination, she presented confluent rounded erythematous papules forming plaques located in the frontal region of the scalp, upper back, presternal area and in the upper extremities (Figure 1). Some of the lesions had a circular appearance and an atypical target. There was no mucosal involvement. The patient denied recent infections, including oral herpes, or other changes in her medication, and she had no fever or systemic symptoms. Porphyrins, ANA, antiDNA, antiRo and antiLa antibodies were negative. Histopathological examination revealed focal vacuolar degeneration of the basal layer and apoptotic keratinocyte bodies without vasculitic changes (Figure 2). The patient was treated with oral and topical corticosteroids with a good evolution of the lesions after one month of treatment. Vandetanib treatment was stopped and switched for cabozatinib without recurrence of skin lesions.
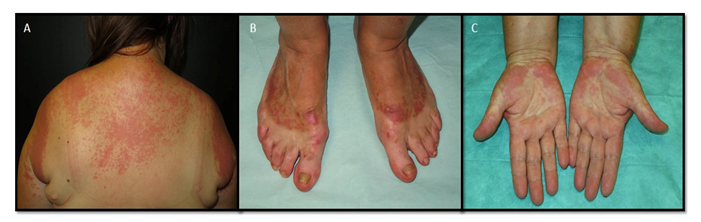
Figure 1: Photo induced erythema multiforme. (A) and (B) Sharp demarcation between photo-exposed and non photo-exposed skin; (C) Acute onset of rounded erythematous papules, plaques of polycyclic borders and some atypical targets.
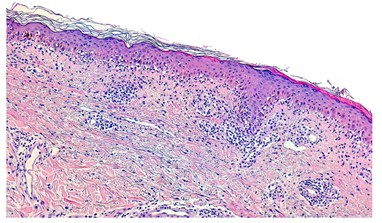
Figure 2: Hematoxylin-eosin stain; original magnification x200. Superficial perivascular lymphocytic infiltrate, interface dermatitis with basal layer liquefaction and necrotic keratynocites. There is no fibrinoid necrosis with liquefactive degeneration at the dermoepidermal junction or atrophy of the epidermis.
2.2 Case 2
A 72-year-old woman referred from the Oncology Department, due to a skin rash 21 days after starting vandetanib. She presented erythematous, edematous plaques on the facial area, upper part of the trunk and extremities. Some of the lesions had a pseudovesicular appearance and atypical targets. Non photo-exposed areas such as the upper eyelids and submental region were spared. The patient denied recent infectious processes or introduction of any other drug. There was no mucosal involvement or systemic symptoms. Histopathological examination showed a lymphocytic infiltrate in the superficial dermis with lichenoid dermatitis, vacuolar degeneration of the basal layer and abundant necrotic keratinocytes. The patient was treated with topical corticosteroids and extreme photoprotective measures with disappearance of the lesions after three weeks, no changes were made in her oncological treatment.
Vandetanib is a multikinase inhibitor that targets RET (REarranged during Transfection) tyrosine kinase, vascular endothelial growth factor receptor, and epidermal growth factor receptor. It currently is approved by the US Food and Drug Administration for the treatment of progressive medullary thyroid cancer, and it is being used for non-small cell lung cancer with RET mutation [1]. Frequently reported adverse events include QT prolongation, diarrhea, and rash [4]. Reported mucocutaneous complications included photosensitivity, acneiform eruption, dry skin, hand-foot reaction, and Stevens-Johnson syndrome [5]. In a meta-analysis of cutaneous toxicity, the incidence of all grade skin rashes due to vandetanib was 46%, and it was concluded that van-detanib has the highest association of cutaneous reactions among the anti–vascular endothelial growth factor tyrosine kinase inhibitors. The most common reasons for dose decrease or cessation of vandentanib were diarrhea and rash (1% and 1.3%, respectively) [1, 6].
Photosensitivity is the most characteristic vandetanib´s cutaneous reaction. It has been observed in up to 37% of patients [1]. It can manifest as exaggerated sunburn, erythematous or lichenoid eruption, and less frequently as photo-induced subacute cutaneous lupus erythematosus or PEM [3].
PEM is a rare, and probably underdiagnosed dermatosis, that has been linked to drugs and reactivations of herpes simplex. Rodriguez-Pazos et al. reviewed the ten drug-induced PEM cases reported at the moment in the literature [2]. In their review, the only chemotherapeutic drug involved was paclitaxel. Although it is usually recommended to complete the diagnosis with a photopatch test, in the cases of oncological treatment drugs this study was not performed [2]. In our cases, given the high clinical suspicion due to the photodistributed localization of the lesions, clear temporal relationship to drug introduction and characteristic histology; the diagnose of PEM was made. Up until now, there was only one previous case reported of PEM related with vandetanib [3].
We present two cases of PEM due to vandetanib. Vandetanib is the first-choice treatment for advanced medullary thyroid cancer and it is also used for non-small cell lung cancer [1]. We want to highlight the importance of informing patients receiving vandetanib about the need for strict photoprotection to avoid phototoxicity reactions and prevent dose reduction or drug cessation.
Disclosures
All authors state that no conflicts of interest or funding support exist. All authors drafted the work and substantively revised it. All authors read and approved the final manuscript. Patients gave written consent to use their images.
References
- Doan HQ, Hu MI, Goldstein J, et al. Vandetanib photoinduced cutaneous toxicities. Cutis 103 (2019): E24-E29.
- Rodríguez-Pazos L, Gómez-Bernal S, Rodríguez-Granados MT, et al. Photodistributed erythema multiforme. Actas Dermo-Sifiliograficas 104 (2013): 645-653.
- Caro-Gutiérrez D, Floristán Muruzábal MU, Gómez de la Fuente E, et al. Photo-induced erythema multiforme associated with vandetanib administration. Journal of the American Academy of Dermatology 71 (2014): E142-144.
- Campbell MJ, Seib CD, Gosnell J. Vandetanib and the management of advanced medullary thyroid cancer. Current Opinion in Oncology 25 (2013): 39-43.
- Yoon J, Oh CW, Kim CY. Stevens-Johnson syndrome induced by vandetanib. Annals of Dermatology 23 (2011): S343-345.
- Rosen AC, Wu S, Damse A, et al. Risk of rash in cancer patients treated with vandetanib: systematic review and meta-analysis. Journal of Clinical Endocrinology and Metabolism 97 (2012): 1125-1133.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 74.74%
Acceptance Rate: 74.74%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 