Revisiting the Role of Immunotherapy for Colorectal Cancer Treatment in Patients with Constitutional Mismatch Repair Deficiency
Ellis L Eikenboom1,2, Erna M C Michiels3, Dirk J Grünhagen4, Marianne de Vries5, Anja Wagner2*, Manon CW Spaander1*#
1Erasmus MC Cancer Institute, University Medical Center Rotterdam, Department of Gastroenterology and Hepatology, 3000 CA Rotterdam, The Netherlands
2Erasmus MC Cancer Institute, University Medical Center Rotterdam, Department of Clinical Genetics, 3000 CA Rotterdam, The Netherlands
3Princess Máxima Center for Pediatric Oncology, 3584 CS Utrecht, The Netherlands
4Erasmus MC Cancer Institute, University Medical Center Rotterdam, Department of Surgical Oncology and Gastrointestinal Surgery; 3000 CA Rotterdam, The Netherlands
5Erasmus MC Cancer Institute, University Medical Center Rotterdam, Department of Radiology; 3000 CA Rotterdam, The Netherlands
*Authors contributed equally
*Corresponding Author: Manon CW Spaander, Erasmus MC Cancer Institute, University Medical Center Rotterdam, Department of Gastroenterology and Hepatology, 3000 CA Rotterdam, The Netherlands.
Received: 14 July 2022; Accepted: 25 July 2022; Published: 15 December 2022
Article Information
Citation: Ellis L Eikenboom, Erna M C Michiels, Dirk J Grünhagen, Marianne de Vries, Anja Wagner, Manon CW Spaander. Revisiting the Role of Immunotherapy for Colorectal Cancer Treatment in Patients with Constitutional Mismatch Repair Deficiency. Journal of Cancer Science and Clinical Therapeutics 6 (2022): 428-430.
View / Download Pdf Share at FacebookAbstract
Patients with constitutional mismatch repair deficiency syndrome (CMMRD) usually develop multiple tumors at a young age. Chemotherapy was previously shown to be less effective in these tumors. Recent reports suggest immunotherapeutic treatment in CMMRD-associated tumors. Here, we present a CMMRD patient, successfully treated with immunotherapy for metastasized colorectal cancer (CRC). A young adolescent male was diagnosed with T2N2M1 cecum carcinoma with liver metastases. He received four initial doses of nivolumab (3 mg/kg) with ipilimumab (1 ml/ kg) every three weeks, followed by 41 doses of nivolumab alone every two weeks. A complete response was achieved; also pathological assessment of removed liver metastases was indicative of a complete response. At the time of writing, 14 months after end of treatment, the CRC had not recurred. Immunotherapeutic treatment resulted in a complete response of the primary CRC and metastases. Immunotherapy as first line treatment should be strongly considered in treatment of CMMRD-associated CRCs.
Keywords
<p>Colorectal Cancer; Constitutional Mismatch Repair Deficiency Syndrome; Immunotherapy; MSI</p>
Article Details
1. Introduction
Constitutional Mismatch Repair Deficiency Syndrome (CMMRD) is a rare syndrome, caused by biallelic, pathogenic germline variants in one of the DNA mismatch repair (MMR) genes MLH1, MSH2, MSH6 or PMS2. As a consequence, the DNA MMR is hampered, causing CMMRD carriers to be at high risk to develop multiple malignancies, especially brain tumors, hematological malignancies, and colorectal cancer (CRC). In contrast to Lynch syndrome, where one pathogenic MMR germline variant is involved, predominantly causing tumors of the large intestine and gynecological tract at adult age, CMMRD-associated tumors usually present in early childhood or adolescence. Indications for diagnostics of CMMRD are among others the development of malignancies under the age of 25 and at least two hyper pigmented (café-au-lait) macules without molecular diagnosis of Neurofibromatosis type I (NF1), as stated by the European Consortium Care for CMMRD (C4CMMRD) [1]. The rarity of the disease and the overlap in clinical features with NF1 does not always make clinicians consider CMMRD. Additionally, family history is not always positive for LS-associated tumors, which can cause delay in CMMRD diagnosis. The high cancer incidence stresses the importance of early and regular surveillance upon diagnosis, usually consisting of regular examination by the pediatrician, as well as regular MRI of the brain, colonoscopy, and assessment of the stomach, small intestine, urine, and gynecological tract [2]. In case of tumor development, the standard treatment consists of systemic chemotherapy. However, it has been shown that CMMRD-related tumors are usually less responsive to several types of chemotherapy. Therefore, it has been recently suggested to treat CMMRD-related tumors with immunotherapy. Here, we present a CMMRD-patient, whose metastasised CRC was successfully treated with immunotherapeutic compounds nivolumab and ipilimumab: a complete response of the primary tumor, regional lymphnode metastases, and liver metastases was still visible 14 months after end of treatment.
2. Case Presentation
A young adolescent male was diagnosed with cecal CRC at semiannual colonoscopic surveillance, performed in the context of CMMRD. At the age of 15, he had presented with rectal bleeding caused by an advanced adenoma and mild axillar freckling. Consequently, CMMRD was diagnosed, based on two pathogenic germline variants in DNA MMR gene MSH6 (c.651dupT, p.Lys218* and c.3957dupA, p.Ala1320fs). His medical history also included a stage III T-cell lymphoblastic non-Hodgkin lymphoma at the age of 16 years, for which he was curatively treated with prednisone, doxorubicin, 6-mercaptopurine, and vincristine. Besides a sister, who died due to Burkitt’s lymphoma at the age of 12 years, no other tumors were diagnosed in first-degree relatives. After radiological examination, the tumor was staged as a T2N2M1 cecal cancer, with multiple liver metastases (Figure 1). Pathological assessment showed a poorly differentiated CRC, with a microsatellite instability-high (MSI-H) and ultra-hypermutated phenotype (≥100 mutations/Mb). Immunohistochemical assessment revealed heterogeneous staining for MSH6. Next-generation sequencing identified both pathogenic MSH6 germline variants in the tumor, as well as somatic variants in MAP2K1, FBXW7, TP53, and APC genes.
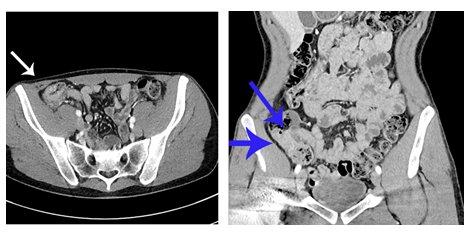
Figure 1: Before immunotherapeutic treatment: abdominal Computed Tomography (CT) scans, axial (left) and coronal (right) view. Arrows depict cecal tumor.
After a multidisciplinary meeting and international consultation, it was decided to start immunotherapeutic treatment with nivolumab and ipilimumab. Our patient received four doses of nivolumab (3 mg/kg) with ipilimumab (1 ml/kg, every three weeks), followed by 41 doses of nivolumab alone (3 mg/kg, every two weeks). Overall, he tolerated the immunotherapy very well: the only noticed side effects were problems with the nails and non-progressive fatigue. Of note, the latter already existed before induction of immunotherapy. A complete response of both the primary tumor and regional nodal metastases was achieved after two years of immunotherapeutic treatment (Figure 2A, 2B). As the widespread liver metastases were still noted on the Computed Tomography (CT) scan and no uptake on the Positron Emission Tomography (PET) CT scan was found, a diagnostic laparoscopic wedge resection was performed of an easily accessible lesion. Pathological assessment showed necrosis, calcifications, fibrosis, and an inflammatory infiltrate, indicative for a complete response (Figure 2C, 2D). The remaining liver metastases were not surgically removed but were assumed to be in complete response. At the time of writing, the CRC had not recurred, representing a complete response of 14 months after end of treatment. Unfortunately, two weeks after the wedge resection, the patient was diagnosed with a grade III anaplastic astrocytoma, for which he is currently being treated. The development of this tumor also fits into the context of CMMRD.
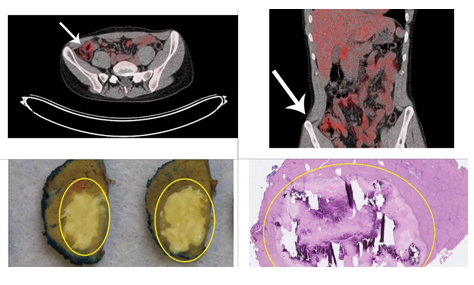
Figure 2: After immunotherapeutic treatment. Upper row: abdominal Fluorodeoxyglucose (FDG)-Positron Emission Tomography (PET) CT scans, axial (upper left) and coronal (upper right) view. Arrows depict location where tumor was previously located. Lower row: wedge resection of liver segment 3, containing two metastases. Pathological assessment showed complete response upon immunotherapeutic treatment. Macroscopic (down left) and microscopic (down right) view.
3. Discussion
3.1 Treatment for CRC: Systemic Chemotherapy
The standard treatment for metastatic CRC despite age of onset and molecular characteristics is systemic chemotherapy. However, assessment of the MSI-state of the tumor is key for defining more optimal treatment strategies. Not only do MSI-H tumors usually have a better prognosis, when compared to their microsatellite stable counterparts, chemotherapeutic agents such as cisplatin, carboplatin, and 5-fluorouracil (5-FU) are thought to somehow affect the MMR machinery. As a consequence, MSI-H tumor cells, having no properly functioning MMR machinery, are usually less responsive to these types of chemotherapeutic agents [3]. Accordingly, no increased survival in patients with MSI-H CRC treated with 5-FU-based chemotherapy was shown [4]. As most of the CMMRD-associated tumors are MSI-H, effect of systemic chemotherapy on response and survival is expected to be limited. This, together with its high toxicity, makes systemic chemotherapy less suitable for CRC treatment in CMMRD-related tumors.
3.2 Rationale for Immunotherapy Treatment in CMMRD Patients with CRC
MSI-H tumors are usually ultra-hypermutated and form neoantigens, enabling the tumor to be recognized by the immune system. Immunotherapy is therefore a promising new treatment modality for patients with MSI-H tumors. Recently, the use of immunotherapy in CMMRD-related tumors has been suggested, with most studies focusing on brain tumors [5]. Immunotherapy, however, could potentially also be applied in treating other LS or CMMRD-related tumors, as these tumors also display an MSI-H phenotype [6]. This belief was strengthened as a significantly longer progression-free survival was shown in advanced MSI-H CRC patients treated with immunotherapy (pembrolizumab), compared to chemotherapy [7]. Additionally, when administered preoperatively, immunotherapy was found to induce a pathological response in surgically removed early-stage MMR deficient tumors [8]. Our patient was treated with PD1 receptor blocker nivolumab and CTLA4 blocker ipilimumab; this combination is currently approved for treatment of metastasized melanoma and renal cell carcinoma. Recently published results of the Checkmate-142 study demonstrated a high response rate in metastatic MSI-H CRC upon treatment with nivolumab and ipilimumab [9]. In line with previous research, our patient had a complete response to immunotherapy with very mild side effects. So far, immunotherapy is thought to have fewer side effects than chemotherapy: this is particularly important to CMMRD patients, as they usually develop multiple tumors at a young age, for which chemotherapy is often the standard-of-care. A large disadvantage of chemotherapy is the high morbidity. This was also the reason that our patient refused chemotherapy after suffering from major side-effects during earlier chemotherapeutic treatment for non-Hodgkin lymphoma. Another disadvantage of chemotherapy is the chance to develop treatment-induced tumors. For example, the use of temozolomide in CMMRD-related glioblastoma multiforme is discouraged, as it was shown to induce somatic mutations in patients, with an increased risk of secondary malignancies as a consequence [10]. One could hypothesize that also other types of chemotherapy may further increase the risk of future tumor development. As emphasized, this increased risk comes on top of the a priori high chances of tumor development, due to the already present pathogenic biallelic MMR germline variants.
References
- Wimmer K, Kratz CP, Vasen HF, et al. Diagnostic criteria for constitutional mismatch repair deficiency syndrome: suggestions of the European consortium 'care for CMMRD' (C4CMMRD). J Med Genet 51 (2014): 355-365.
- Tabori U, Hansford JR, Achatz MI, et al. Clinical Management and Tumor Surveillance Recommendations of Inherited Mismatch Repair Deficiency in Childhood. Clin Cancer Res 23 (2017): e32-e37.
- Tougeron D, Sueur B, Zaanan A, et al. Prognosis and chemosensitivity of deficient MMR phenotype in patients with metastatic colorectal cancer: An AGEO retrospective multicenter study. International Journal of Cancer 147 (2020): 285-296.
- Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 28 (2010): 3219-3226.
- AlHarbi M, Ali Mobark N, AlMubarak L, et al. Durable Response to Nivolumab in a Pediatric Patient with Refractory Glioblastoma and Constitutional Biallelic Mismatch Repair Deficiency. Oncologist 23 (2018): 1401-1406.
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357 (2017): 409-413.
- André T, Shiu KK, Kim TW, J, et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med 383 (2020): 2207-2218.
- Chalabi M, Fanchi LF, Dijkstra KK, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med 26 (2020): 566-576.
- Overman MJ, Lonardi S, Wong KYM, et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol 36 (2018): 773-779.
- Hunter C, Smith R, Cahill DP, et al. A hypermutation phenotype and somatic MSH6 mutations in recurrent human malignant gliomas after alkylator chemotherapy. Cancer Res 66 (2006): 3987-3991.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 74.74%
Acceptance Rate: 74.74%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 