A Methionine PET Targeting for Gamma Knife Radiosurgery used to Treat Recurrent Malignant Gliomas at Inoperable Stage
Elly Chaskis1, Stéphane Simon2, Diana Rodriguez Garcia2, Corentin Martens3, Gaetan Van Simaeys3, Nilou Sadeghi4, Philippe Martinive5, Olivier De Witte1, Serge Goldman3, Florence Lefranc1*
1Department of Neurosurgery, Université Libre de Bruxelles (ULB), Brussels, Belgium
2Department of Medical Physics, Institut Jules Bordet, Université Libre de Bruxelles (ULB), Brussels, Belgium
3Department of Nuclear Medicine, Hôpital Erasme, Université Libre de Bruxelles (ULB), Brussels, Belgium
4 Department of Radiology, Hôpital Erasme, Université Libre de Bruxelles (ULB), Brussels, Belgium
5 Department of Radiotherapy, Institut Jules Bordet, Université Libre de Bruxelles (ULB), Brussels, Belgium
*Corresponding Author: Florence Lefranc, MD, Service de Neurochirurgie – Hôpital Erasme- Université Libre de Bruxelles, Route de Lennik, 808 - 1070 Brussels - Belgium
Received: 26 November 2020; Accepted: 03 December 2020; Published: 15 December 2020
Article Information
Citation:
Elly Chaskis, Stéphane Simon, Diana Rodriguez Garcia, Corentin Martens, Gaetan Van Simaeys, Nilou Sadeghi, Philippe Martinive, Olivier De Witte, Serge Goldman, Florence Lefranc. A Methionine PET Targeting for Gamma Knife Radiosurgery used to Treat Recurrent Malignant Gliomas at Inoperable Stage. Journal of Surgery and Research 3 (2020): 457-471.
View / Download Pdf Share at FacebookAbstract
Purpose: The current study investigates the usefulness of metabolic positron emission tomography (PET) imaging (in particular with 11C-methionine (MET)), for target definition during gamma knife radiosurgery (GKRS) of locally multirecurrent malignant glioma at inoperable stage. Patients and
Methods: We retrospectively evaluated the results of GKRS with MET-PET targeting for 24 adult focally recurrent inoperable malignant gliomas treated at the Erasme Gamma Knife Center between 2007 and 2018. We evaluated the type of tumour progression (local vs remote), progression-free survival (PFS), overall survival (OS) and toxicity after MET-PET targeting of GKRS for these 24 patients.
Results: The median PFS after GKRS for the 24 patients with malignant gliomas was 5.5 (2-46) months and 4.5 (3-10), 4.5 (2-8) and 13.5 (2-46) months for glioblastoma, anaplastic glioma and grade II glioma patients, respectively. The median OS from the GKRS procedure for patients with recurrent glioblastomas (n=12) was 18 (6-45) months, 8 (6-184) months for anaplastic gliomas (n=6), and 22 (16-65) months for grade II gliomas (n=6). All patients with grade III and II multirecurrent gliomas (n=12) showed an early favourable local metabolic response, while this response was observed only in 6/12 of the glioblastomas. However, the majority (9/12=75%) of these grade II/III glioma patients further developed new lesions. GKRS treatment was associated with diverse chemotherapies in more than 50% (13/24) of cases. Post-GKRS radio-necrosis was observed in only one patient.
Conclusion: Based on a limited series of 24 patients, our study shows, for the first time, the GKRS-induced metabolic response in focally recurrent inoperable malignant gliomas. GKRS could be part of the multidisciplinary approach for multirecurrent malignant gliomas that cannot be anymore treated
Keywords
<p>Gamma knife radiosurgery; Gliomas; Methionine PET; Recurrence; Inoperable</p>
Article Details
1. Introduction
Malignant gliomas are infiltrating tumours, which makes complete surgical resection elusive, and tumour recurrence is more than frequent. Glioma recurrence around the resection cavity occurs in almost all patients, and the management options for evolutive malignant gliomas are limited despite advances in surgical, chemotherapeutic and radiotherapeutic techniques [1]. Moreover, the remaining glioma cells that migrate after debulking are resistant to conventional treatments [2].
Stereotactic radiosurgery (SRS), including gamma knife radiosurgery (GKRS), is often beneficial, resulting in improved survival for patients [3-10]. Nevertheless, SRS remains underutilized as part of the multimodality management of recurrent malignant gliomas [3-10]. SRS treatments can be refined with the use of various imaging approaches to improve tumour targeting. Among the different approaches, metabolic imaging using positron emission tomography (PET) in addition to conventional magnetic resonance imaging (MRI) can provide relevant information on tumour metabolism, which allows for more accurate diagnoses and treatments [11, 12]. Radiolabelled amino acids have been used in neuro-oncological practice since 1983 [13]. The largest experience with this class of PET tracers for brain tumour imaging has been gained with 11C- methionine (MET), and MET is an essential amino acid labelled with the positron-emitting isotope carbon-11, which is associated with a 20-min half-life [14, 15]. The value of PET imaging with MET (MET-PET) has been evaluated in glioma patients in terms of tumour delineation [16, 17], prognostication, histological grade and molecular IDH1 mutation [18, 19], differentiation of tumour recurrence from radiation injury [20-22], response assessment to alkylating agents [23-25] and target definition in radiotherapy planning [26-30], including re-irradiation [31]. Our group has shown for example that the addition of MET-PET data for the resection guidance of anaplastic gliomas and glioblastomas provides a target contour substantially different from that obtained by contrast-enhanced MRI alone in approximately 80% of cases and that a complete resection of the tumour area with increased methionine uptake resulted in significantly longer survival of patients [32, 33].
Gamma knife radiosurgery (GKRS) has the highest requirements in terms of imaging accuracy, as the treatment is applied in a single high-dose session with no spatial control other than medical imaging. However, discrepancies between lesion distributions on MET-PET and magnetic resonance imaging (MRI) in patients with malignant gliomas have been evident for more than 15 years [34]. Miwa et al. [34] demonstrated that biologic imaging helps to detect tumour infiltration in brain areas with a non-specific MRI appearance in a significant number of patients. In the current study, we retrospectively evaluated GKRS, with the inclusion of a MET-PET target, as a treatment option after standard therapies for adult multirecurrent inoperable malignant gliomas. This study also aims to assess the level and timing of the MET-PET response after GKRS, local and distant tumour progression, progression-free survival, overall survival from the GKRS procedure and toxicity. GKRS was performed as part of the multimodality management of patients with recurring malignant gliomas at inoperable stage and was combined with other therapies.
2. Patients and Methods
2.1 Patients
All patients included since 2000 in the glioma database of our Gamma Knife Unit (Erasme Hospital/Brussels, Belgium) were retrospectively reviewed. All patients underwent standard multimodal treatments: surgery(- ies), radio- and/or chemotherapies, including the Stupp temozolomide-based protocol for glioblastomas [35] (Table 1). Radiosurgery treatment was planned on a Leksell GammaPlan platform to which MRI and MET- PET data files were imported. T1, T2, FLAIR, and T1 with contrast MRI sequences were acquired on Philips, ACSNT1.5, Intera 1.5 and Achieva 15 scans, and PET data were acquired on Philips. Images were collected 20 minutes after the injection of 555 MBq of MET. For treatment planning, all areas with either contrast enhancement on T1 or increased MET uptake within an area of increased FLAIR signals were considered targets. On MET-PET images, lesions were visually delineated using colour thresholding derived from a previous study [36].
Patients who were lost to follow-up or who had missing data were excluded from the study. This study was approved by the local ethics committee review board (P2019/199) of the Erasme Hospital. Since 2000, 72 adult patients (age 25-64) with malignant gliomas have been treated in our unit by GKRS using PET data. Our series included 50 grade IV glioblastomas, 12 grade III anaplastic gliomas and 10 grade II gliomas. We excluded 38 glioblastomas from this series for the following reasons: 22 patients did not receive the Stupp protocol after surgery because they underwent surgery before 2005; 14 patients had incomplete clinical follow- up; one patient was targeted by means of FDG-PET instead of MET-PET, and the remaining patient was treated with GKRS as a boost during radiotherapy. Six patients with anaplastic gliomas and 4 patients with grade II gliomas were also excluded from our final analysis because of missing metabolic data. Our final study group thus includes 24 adult patients with recurrent malignant gliomas, including 12 glioblastomas, 6 anaplastic gliomas and 6 grade II gliomas. These patients were all treated between May 2007 and September 2018.
For each patient, the following data were collected: sex; age at diagnosis; previous treatment including surgical procedures, radiotherapy and chemotherapies; functional status prior to GKRS (assessed using the Karnofsky Performance Status (KPS)); post- radiosurgical complications; adjuvant therapies associated with GKRS; follow-up data; time interval for the local response evaluated by MET-PET; time for the local and at-distance recurrence (progression-free survival (PFS)); and overall survival (OS) after GKRS. The molecular characteristics of the gliomas included only the status of the 1p19q co-deletion. Other molecular characteristics (isocitrate dehydrogenase status and O(6) methylguanine methyltransferase methylation status) were not evaluated because data were missing for the majority of the patients.
3. Results
3.1 Demographics
The patient demographics are summarized in Table 1 and detailed in Table S1 (supplementary data). Thirteen patients (54%) received second- or third-line chemotherapy associated with GKRS (Table S1). Seven glioblastoma patients received either temozolomide (TMZ) (3), lomustine (Lom) (3) or carboplatine (Platine) (1). Three grade III glioma patients received either TMZ (2) or the combination of procarbazine, Lom and vincristine (PCV) (1), and 3 grade II glioma patients received Lom (1), TMZ (1) or PCV (1) (Table S1).
3.2 Outcome
The PFS after GKRS and the OS from the GKRS procedure are summarized in Table 2 and detailed in Table S1 (supplementary data). Of the 12 glioblastoma patients locally treated by GKRS at recurrence, 6 patients had an early unfavourable response (5 with local evolution and one at-distance of the treated area) at the first MET-PET follow-up (3 months), 6 patients had a long-term metabolic response for >= 6 months, 4 patients developed local evolution of the disease and 2 patients developed multiple new distant lesions after 6 months (Table S1). Figures 1 and 2 illustrate the representative GKRS treatment plan and the metabolic follow-up for the glioblastoma patients. All 6 grade III GKRS-treated gliomas showed a local, early metabolic response by MET-PET (between 2 and 4 months), but
all 6 had developed either new lesions at the first follow-up (2-5 mo) (N=4) or local recurrence at the second follow-up (6-8 mo) (N=2) (Tables 2 and S1 for details). All 6 grade II GKRS-treated gliomas showed a local, early metabolic response by MET-PET (at 2 to 6 months posttreatment). Two patients showed remote, early (2-4 mo) new lesions (patients 20 and 23 in Table S1) without local recurrence. Systemic treatment was indicated, and the prognosis was good (OS 33 and 25). Two patients showed stable disease for more than 1 year without treatment (patients 21 and 22). Two patients showed stable disease for more than 1 year with chemotherapy associated with GKRS (patients 19 and 24 in Table S1). Only one patient with local recurrence after a response of more than one year was treated by surgery (patient 22). Two patients with pluri-focal evolution were treated by radiotherapy. One patient that had been previously irradiated on the operative field developed radio-necrosis 7 months after GKRS treatment (patient 23). Figure 3 illustrates a patient with a long-term metabolic response.
Post-GKRS radio-necrosis was observed in only one patient (patient number 23 in Table S1) with a history of previous external brain radiotherapy at a dose of 50.4 Gy for an oligodendroglioma grade II 5 years earlier. Radiologically, the patient presented with cerebral oedema 7 months after GKRS (20 Gy), and radio- necrosis was confirmed by histological analysis. Salvage palliative surgery was performed after GKRS in 6 cases (2 glioblastomas, 1 grade III and 3 grade II gliomas) in 5 patients due to local progression on MRI (using the RANO criteria) confirmed by metabolic PET- MET; imaging signs were associated with neurological symptoms (focal neurological deficit or seizures) in 2 patients and with symptomatic radio-necrosis in one patient.
|
Number of patients |
All gliomas |
Grade IV gliomas |
Grade III gliomas |
Grade II gliomas |
|
24 |
12 |
6 |
6 |
|
|
Sex |
10 M (42%); 14 F (58%) |
9 M (75%); 3 F (25%) |
3 M (50%); 3 F (50%) |
4 M (67%); 2 F (33%) |
|
Median Age (years) |
49.5 (extremes 25-64) |
53 (extremes 36-64) |
42.5 (extremes 37-57) |
45.5 (extremes 25-63) |
|
Histological characteristics |
- |
10 primary IV 2 IV2 |
4 OIII 2 AIII |
3 OII 3 AII |
|
conventional RT before GKRS |
21 |
All 12 |
All 6 |
3 |
|
Median KPS before GKRS |
70 (extremes 60-100) |
75 (extremes 60-100) |
70 (extremes 60-90) |
75 (extremes 70-100) |
|
Average time from histological diagnosis to GKRS (mo) |
58 (extremes 0-252) |
19.5 (extremes 5-170) |
46 (extremes 0-252) |
125 (extremes 96-208) |
|
Median GKRS dose (Gy) |
15 (extremes 15-24) |
16 (extremes 15-20) |
15 (extremes 15-20) |
18 (extremes 15-24) |
|
Median GKRS targeted volume (mm3) |
6517 (extremes 587-28383) |
5565,5 (extremes 2932-18193) |
7967 (extremes 1013-28383) |
4394,5 (extremes 587-9228) |
Abbreviations: AIII: anaplastic astrocytoma; AII: astrocytoma; F: female; GKRS: gamma knife radiosurgery; KPS: Karnofsky Performance Status; M: male; mo: months; OIII: anaplastic oligodendroglioma; OII: oligodendroglioma; RT: radiotherapy; IV: glioblastoma; IV2: secondary glioblastoma
Table 1: Patient demographics.
|
Grade IV gliomas (n=12) |
Grade III gliomas (n=6) |
Grade II gliomas (n=6) |
|
|
Median PFS (mo) |
4.5 (extremes 3-10) |
4.5 (extremes 2-8) |
13.5 (extremes 2-46) |
|
Median OS (mo) |
17.5 (extremes 6-45) |
8 (extremes 6-184) |
21.5 (extremes 16-65) |
|
Early local metabolic response |
6/12 (3 mo) |
All 6/6 (2-4 mo) |
All 6/6 (2-6 mo) |
|
Long term metabolic response |
6/12 (6 mo) |
0 (6-8 mo) |
4 (> 12 mo) |
Abbreviations: GKRS: gamma knife radiosurgery; mo: months; OS: overall survival; PFS: progression free survival
Table 2: Outcome.
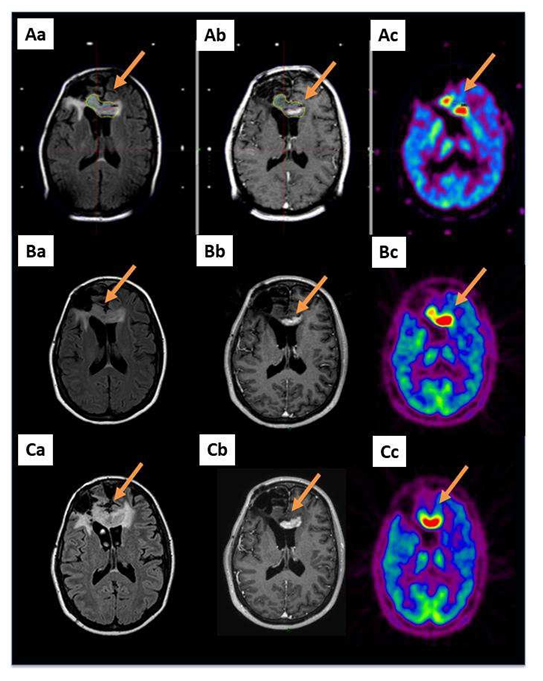
Figure 1: A 36-year-old patient with a glioblastoma (patient 6 in Table S1) already treated by the Stupp protocol after a cerebral biopsy presented with a radiological progression located in the corpus callosum 2 years after her diagnosis (arrows). A GKRS treatment, 15 Gy on a volume of 9716 mm3 including the MET-PET target, was performed. Aa: MRI reformatted axial FLAIR; Ab: reformatted axial T1-weighted with contrast and Ac: MET-PET study performed on the day of the GKRS treatment; B and C show the same sequences 3 and 6 months after GKRS, respectively. Three months after GKRS, MET-PET (Bc) indicated suspected local failure. Six months after the GKRS treatment, all exams confirmed the progression (C: same sequences).
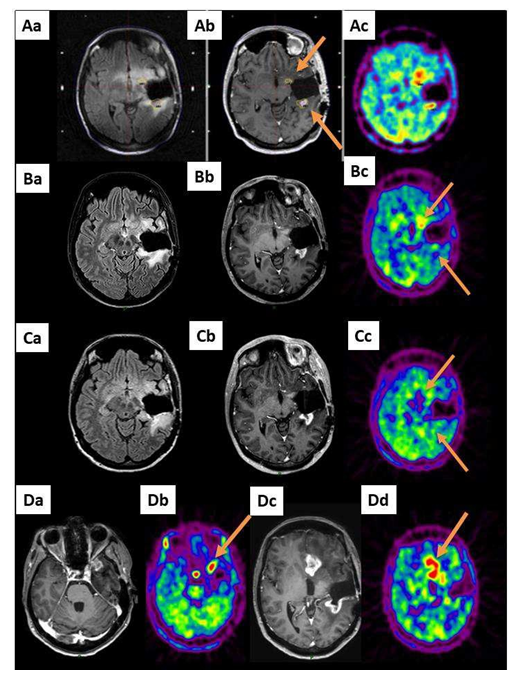
Figure 2: A 42-year-old patient (patient 7 in Table S1) already treated by the Stupp protocol for left temporal glioblastoma after large surgery presented with a radiological progression located in the operative cavity wall 6 months after her diagnosis (2 spots, arrows). A GKRS treatment, 15 Gy covering a total volume of 2932 mm3 including the 2 MET-PET targets, was performed. Aa: MRI reformatted axial FLAIR, Ab: reformatted axial T1- weighted with contrast and Ac: MET-PET study on the day of the GKRS treatment (2 targets, arrows). B and C show the same sequences 3 and 6 months after GKRS, respectively. Three months (B) and 6 months (C) after GKRS, the metabolic exams revealed a local response. However, six months after the treatment, the MRI and MET PET exams revealed remote progression (Da and Dc: axial T1-weighted with contrast and Db and Dd: MET-PET).
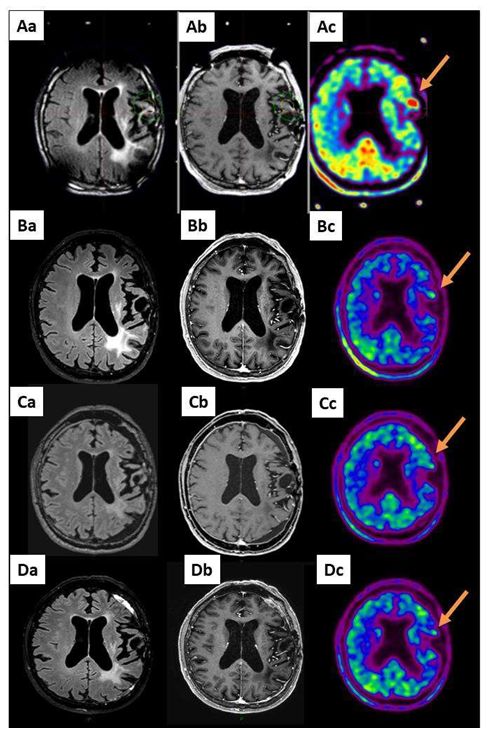
Figure 3: A 63-year-old patient with a grade II evolutive astrocytoma (AII) (patient 19 in Table S1) already treated by PCV chemotherapy, radiotherapy and TMZ chemotherapy after large surgical resection received GKRS treatment 20 Gy on a volume of 2312 mm3 including the MET-PET target (arrow). Aa: MRI reformatted axial FLAIR, Ab: reformatted axial T1-weighted with contrast and Ac: MET-PET study performed on the day of the GKRS treatment. B, C and D show the same sequences 3, 6 and 12 months after GKRS: long-term local response.
4. Discussion
The management of multirecurrent malignant gliomas is challenging due to their spatial and temporal heterogeneity, ability to infiltrate the surrounding brain tissue and interaction with the tumour microenvironment [37, 38]. Numerous recent clinical trials using second-line chemotherapy [39, 40] or immunotherapy [41, 42] have failed to improve survival. The efficacy of GKRS at recurrence can also be of limited efficacy mainly due to the highly infiltrative nature of the gliomas. GKRS limited efficacy can also be linked to the difficult interpretation of the morphological changes demonstrated on classical neuroimaging after primary treatment of gliomas, leading to a high level of incertitude in the definition of recurrent lesions as targets for GKRS treatment. The discrepancy between lesion distributions on MET-PET and MRI in patients with malignant gliomas has been stressed in the literature [34] and is well demonstrated in a preliminary account of our experience with MET- PET integration in GKRS planning [43]. A study on 79 glioblastoma patients revealed that the metabolically active tumour volume on metabolic images prior to histological diagnosis is considerably larger than the volume of contrast enhancement (median volumes 23.8 ml versus 13.5 ml) [44]. We therefore took the option to integrate metabolic images by means of MET-PET to the GKRS planning of multirecurrent inoperable malignant gliomas and to follow the metabolic response after treatment. Such integration has already been proven useful for classical or conformal radiation therapy and carbon ion therapy of gliomas [26, 28, 30, 31, 45]. To the best of our knowledge, this is the first study evaluating the metabolic response of GKRS for recurrent grade II and III gliomas.
In our current retrospective study based on 24 adult multirecurrent malignant glioma patients, we observed that all patients with grades III and II recurrent gliomas demonstrated an early favourable local metabolic response to GKRS, while this outcome was only the case for 50% of the glioblastoma patients (Table 2). However, due to the infiltrative nature of these tumours, the majority (75%) of these grade II and III glioma patients developed new lesions. Several recent publications have studied the indication, efficacy and radio-necrosis risk of GKRS for recurrent grade IV glioblastomas [3-7, 46-49]. In our small series we observed only one case of radio-necrosis in a grade II glioma after previous external brain radiotherapy.
In our study including 12 recurrent glioblastomas, we observed that half of them demonstrated long-term metabolic control for more than 6 months. However, the other half presented early evolution after 3 months with either local failure or distant lesions. The median PFS for this glioblastoma population is 4.5 months. In the study by Frischer et al. on 42 recurrent glioblastomas, the time to radiological progression after GKRS was 4.4 months and mainly occurred beyond the GKRS- irradiated area [6]. In our series, the marginal dose was always equal to or greater than 15 Gy. In the retrospective large study of Niranjan et al. on 297 patients with recurrent or residual glioblastomas treated by GKRS, the important prognostic variables included tumour volume <14 cm3, marginal radiation dose of >= 15 Gy and younger age (<60 years) of the patients [3]. In this series, adverse radiation effects were noted in 23% of cases and were mainly controlled with corticosteroids [3]. The study by Imber et al. (2017) on 174 recurrent glioblastomas also confirmed that young age, small recurrence, and high prescription dose were prognostic variables for a lower risk of symptomatic secondary effects [7]. It was recently shown in a small cohort of 9 patients with recurrent glioblastoma that a single dose of bevacizumab prior to GKRS permitted safe prescription dose escalation up to 22 Gy [47].
Because of the infiltrative nature of the disease, 54% of our patients received second- or thirdline chemotherapy associated with GKRS as we frequently performed after a surgery. Our series is therefore heterogeneous and it is difficult to ascertain if the limited response that we have is because of the GKRS or chemotherapy, or both. A single centre prospective phase II study is currently assessing the safety and efficacy of the addition of early stereotactic GKRS for residual tumours after surgery (and the standard treatment) of newly diagnosed glioblastoma [50]. All patients will receive GKRS with 15 Gy (prescribed to the 50% isodose enclosing all residual tumour areas) early (within 24-72 h) after surgery.
5. Conclusion
Our study aims to highlight the potential of using metabolically targeted GKRS in the multimodality management of inoperable malignant glioma local recurrence. Based on a limited series of 24 patients, the current study demonstrates the GKRS-induced metabolic response in multirecurrent malignant gliomas. Because GKRS could be part of the multidisciplinary approach for multirecurrent malignant gliomas that cannot be anymore treated by surgery, we will evaluate the actual benefits of GKRS in patients with malignant glioma local recurrence in a prospective controlled clinical trial.
Declarations
Not applicable.
Funding
This study was made possible thanks to a grant from
Dominique Jonckheere, Tom, a member of the Beatles’
Empire. This work was supported by the Fonds Erasme (Brussels, Belgium) and the Association Vinçotte Nuclear (AVN, Brussels, Belgium).
References
Supplementary data
Abbreviations: AIII: anaplastic astrocytoma; AII: astrocytoma; Bi: biopsy; F: female; GKRS: gamma knife radiosurgery; KPS: Karnofsky Performance Status; lom: lomustine; M: male; mo: months; OIII: anaplastic oligodendroglioma; OII: oligodendroglioma; OS: overall survival; PCV: procarbazine lomustine vincristine; PFS: progression free survival; Res: surgical resection; RT: radiotherapy; TMZ: temozolomide; IV: glioblastoma; IV2: secondary glioblastoma
Table S1: Patient demographics.
- Weller M, van den Bent M, Tonn JC, et al. European Association for Neuro-Oncology (EANO) Task Force on European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol 18 (2017): 315-329.
- Lefranc F, Le Rhun E, Kiss R, et Glioblastoma quo vadis: Will migration and invasiveness reemerge as therapeutic targets? Cancer Treat Rev 68 (2018): 145-154.
- Niranjan A, Monaco EA III, Kano H, et al. Stereotactic Radiosurgery in the Multimodality Management of Residual or Recurrent Glioblastoma Multiforme. Prog Neurol Surg 31 (2018): 48-61.
- Sutera PA, Bernard ME, Gill BS, et al. Salvage stereotactic radiosurgery for recurrent gliomas with prior radiation therapy. Future Oncol 13 (2017): 2681-2690.
- Holt DE, Bernard ME, Quan K, et al. Salvage stereotactic radiosurgery for recurrent glioblastoma multiforme with prior radiation J Cancer Res Ther 12 (2016): 1243- 1248.
- Frischer JM, Marosi C, Woehrer A, et Gamma Knife Radiosurgery in Recurrent Glioblastoma. Stereotact Funct Neurosurg 94 (2016): 265-272.
- Imber BS, Kanungo I, Braunstein S, et Indications and Efficacy of Gamma Knife Stereotactic Radiosurgery for Recurrent Glioblastoma: 2 Decades of Institutional Experience. Neurosurgery 80 (2017): 129-139.
- Sadik ZHA, Hanssens PEJ, Verheul JB, et al. Gamma Knife Radiosurgery for Recurrent J Neurooncol 140 (2018): 615-622.
- Elaimy AL, Mackay AR, Lamoreaux WT, et Clinical Outcomes of Gamma Knife Radiosurgery in the Salvage Treatment of Patients With Recurrent High-Grade Glioma. World Neurosurg 80 (2013): 872-878.
- Morris SL, Zhu P, Rao M, et al. Gamma Knife Stereotactic Radiosurgery in Combination With Bevacizumab for Recurrent Glioblastoma World Neurosurg 127 (2019): 523-533.
- Goldman S, Pirotte BJ. Brain tumors. Methods Mol Biol 727 (2011): 291-315.
- Galldiks N, Law I, Pope WB, et al. The use of amino acid PET and conventional MRI for monitoring of brain tumor Neuroimage Clin 13 (2016): 386-394.
- Bergström M, Collins VP, Ehrin E, et Discrepancies in brain tumor extent as shown by computed tomography and positron emission tomography using [68Ga]EDTA, [11C]glucose, and [11C]methionine. J Comput Assist Tomogr 7 (1983): 1062-1066.
- Galldiks N, Langen KJ, Pope WB. From the clinician's point of view - What is the status quo of positron emission tomography in patients with brain tumors? Neuro Oncol 17 (2015):1434-1444.
- Herholz K, Langen KJ, Schiepers C, et Brain tumors. Semin Nucl Med 42 (2012): 356-370.
- Kamson DO, Juhász C, Buth A, et Tryptophan PET in pretreatment delineation of newly-diagnosed gliomas: MRI and histopathologic correlates. J Neurooncol 112 (2013): 121-132.
- Venneti S, Dunphy MP, Zhang H, et Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo. Sci Transl Med 7 (2015): 274.
- Kamson DO, Mittal S, Robinette NL, et Increased tryptophan uptake on PET has strong independent prognostic value in patients with a previously treated high-grade glioma. Neuro Oncol 16 (2014): 1373-1383.
- Lopci E, Riva M, Olivari L, et al. Prognostic value of molecular and imaging biomarkers in patients with supratentorial glioma. Eur J Nucl Med Mol Imaging 44 (2017): 1155-1164.
- Alkonyi B, Barger GR, Mittal S, et Accurate differentiation of recurrent gliomas from radiation injury by kinetic analysis of α- 11C-methyl-L-tryptophan PET. J Nucl Med 53 (2012): 1058-1064.
- Terakawa Y, Tsuyuguchi N, Iwai Y, et Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med 49 (2008): 694-699.
- Tsuyuguchi N, Takami T, Sunada I, et Methionine positron emission tomography for differentiation of recurrent brain tumor and radiation necrosis after stereotactic radiosurgery--in malignant glioma. Ann Nucl Med 18 (2004): 291-296.
- Galldiks N, Kracht LW, Burghaus L, et al. Use of 11C-methionine PET to monitor the effects of temozolomide chemotherapy in malignant Eur J Nucl Med Mol Imaging 33 (2006): 516-524.
- Galldiks N, Kracht LW, Burghaus L, et Patient-tailored, imaging-guided, long-term temozolomide chemotherapy in patients with glioblastoma. Mol Imaging 9 (2010): 40-46.
- Galldiks N, Langen KJ. Amino Acid PET - An Imaging Option to Identify Treatment Response, Posttherapeutic Effects, and Tumor Recurrence? Front Neurol 7 (2016):
- Grosu AL, Weber WA, Riedel E, et L- (methyl-11C) methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int J Radiat Oncol Biol Phys 63 (2005a): 64-74.
- Matsuo M, Miwa K, Tanaka O, et al. Impact of [11C]methionine positron emission tomography for target definition of glioblastoma multiforme in radiation therapy Int J Radiat Oncol Biol Phys 82 (2012): 83-89.
- Iuchi T, Hatano K, Uchino Y, et al. Methionine Uptake and Required Radiation Dose to Control Glioblastoma. Int J Radiat Oncol Biol Phys 93 (2015): 133-140.
- Navarria P, Reggiori G, Pessina F, et Investigation on the role of integrated PET/MRI for target volume definition and radiotherapy planning in patients with high grade glioma. Radiother Oncol 112 (2014): 425-429.
- Mahasittiwat P, Mizoe JE, Hasegawa A, et al. l-[METHYL-(11)C] methionine positron emission tomography for target delineation in malignant gliomas: impact on results of carbon ion radiotherapy. Int J Radiat Oncol Biol Phys 70 (2008): 515-522.
- Miwa K, Matsuo M, Ogawa S, et Re- irradiation of recurrent glioblastoma multiforme using 11C-methionine PET/CT/MRI image fusion for hypofractionated stereotactic radiotherapy by intensity modulated radiation therapy. Radiat Oncol 9 (2014): 181.
- Pirotte B, Goldman S, Dewitte O, et Integrated positron emission tomography and magnetic resonance imaging-guided resection of brain tumors: a report of 103 consecutive procedures. J Neurosurg 104 (2006): 238-253.
- Pirotte BJ, Levivier M, Goldman S, et Positron emission tomography-guided volumetric resection of supratentorial high- grade gliomas: a survival analysis in 66 consecutive patients. Neurosurgery 64 (2009): 471-481.
- Miwa K, Shinoda J, Yano H, et al. Discrepancy between lesion distributions on methionine PET and MR images in patients with glioblastoma multiforme: insight from a PET and MR fusion image J Neurol Neurosurg Psychiatry 75 (2004): 1457-1462.
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC Lancet Oncol 10 (2009): 459-466.
- Tang BN, Van Simaeys G, Devriendt D, et al. Three-dimensional Gaussian model to define brain metastasis limits on 11C-methionine Radiother Oncol 89 (2008): 270-277.
- Seystahl K, Wick W, Weller M. Therapeutic options in recurrent glioblastoma-An Crit Rev Oncol Hematol 99 (2016): 389-408.
- Poon CC, Sarkar S, Yong VW, et Glioblastoma-associated Microglia and Macrophages: Targets for Therapies to Improve Prognosis Brain 140 (2017): 1548- 1560.
- Kim MM, Umemura Y, Leung Bevacizumab and Glioblastoma: Past, Present, and Future Directions Cancer J 24 (2018): 180- 186.
- Wick W, Gorlia T, Bendszus M, et Lomustine and Bevacizumab in Progressive Glioblastoma N Engl J Med 377 (2017): 1954- 1963.
- Reardon DA, Lassman AB, Van Den Bent M, et al. Efficacy and safety results of ABT-414 in combination with radiation and temozolomide in newly diagnosed glioblastoma. Neuro Oncol 19 (2017): 965-975.
- Jackson CM, Choi J, Lim M. Mechanisms of Immunotherapy Resistance: Lessons From Nat Immunol 20 (2019): 1100- 1109.
- Levivier M, Massager N, Wikler D, et al. Use of stereotactic PET images in dosimetry planning of radiosurgery for brain tumors: clinical experience and proposed classification. J Nucl Med 45 (2004): 1146-1154.
- Suchorska B, Jansen NL, Linn J, et al. German Glioma Network. Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology 84 (2015): 710-719.
- Grosu AL, Weber WA, Franz M, et Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys 63 (2005b): 511-519.
- Niranjan A, Kano H, Iyer A, et al. Role of adjuvant or salvage radiosurgery in the management of unresected residual or progressive glioblastoma multiforme in the pre-bevacizumab era. J Neurosurg 122 (2015): 757-765.
- Abbassy M, Missios S, Barnett GH, et Phase I Trial of Radiosurgery Dose Escalation Plus Bevacizumab in Patients With Recurrent/Progressive Glioblastoma. Neurosurgery 83 (2018): 385-392.
- Shah JL, Li G, Shaffer JL, et al. Stereotactic Radiosurgery and Hypofractionated Radiotherapy for Glioblastoma. Neurosurgery 82 (2018): 24-34.
- Redmond KJ, Mehta Stereotactic Radiosurgery for Glioblastoma. Cureus 7 (2015): e413.
- Brehmer S, Grimm MA, Förster A, et al. Study Protocol: Early Stereotactic Gamma Knife Radiosurgery to Residual Tumor After Surgery of Newly Diagnosed Glioblastoma (Gamma- GBM). Neurosurgery 84 (2019): 1133-1137.
- This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license 4.0


 Impact Factor: * 4.2
Impact Factor: * 4.2 Acceptance Rate: 72.62%
Acceptance Rate: 72.62%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 