The Effects of Intravenous Diuretics on the use of Mechanical Ventilation on Covid 19 Patients requiring Supplemental Oxygen: A Propensity Score Matched Observational Study
Kwang Jin Choi*, Rachel Baccile¶, Jackie Soo MPH¶, David Meltzer
Department of Medicine, The University of Chicago Medical Center, Chicago, Illinois, United States
¶These authors contributed equally to this work
*Corresponding Author: Kwang Jin Choi, Department of Medicine, The University of Chicago Medical Center, Chicago, Illinois, United States
Received: 29 March 2024; Accepted: 05 April 2024; Published: 18 April 2024
Article Information
Citation: Kwang Jin Choi, Rachel Baccile, Jackie Soo MPH, David Meltzer. The Effects of Intravenous Diuretics on the use of Mechanical Ventilation on Covid 19 Patients requiring Supplemental Oxygen: A Propensity Score Matched Observational Study. Journal of Surgery and Research. 7 (2024): 141-150.
View / Download Pdf Share at FacebookAbstract
Objective: To examine the effects of intravenous diuresis on the use of mechanical ventilation in Covid patients requiring supplemental oxygen. Methods: 983 hospitalized adult patients with Covid 19 infections needing supplemental oxygen between March 2020 to February 2022 were included in the study. The primary outcome was the use of mechanical ventilation. We compared the outcome in patients who were exposed to intravenous furosemide before mechanical ventilation to those patients who were not exposed to intravenous furosemide during hospitalization using propensity-score matching analysis.
Results: Among the 983 patients who received intravenous furosemide, 186 (18.9%) patients required mechanical ventilation. Of the 491 patients who did not receive intravenous furosemide during hospitalization, 102 (20.7%) required mechanical ventilation. There was a significant negative association between patients exposed to intravenous furosemide who required mechanical ventilation than those who did not receive intravenous furosemide who required mechanical ventilation. (Odds Ratio of 0.37; 95% Confidence Interval 0.21 to 0.66; P value < 0.01)
Conclusions: In this retrospective cohort study involving a moderately sized sample of hospitalized Covid 19 patients who required supplemental oxygen, intravenous diuresis was significantly associated with lower use of mechanical ventilation compared to those that were not exposed to intravenous diuresis (adjusted Odds Ratio 0.37; 95% Confidence Interval 0.21 to 0.66; P value < 0.01).
Keywords
<p>Covid 19, Diuretics, Mechanical ventilation, Critical care</p>
Article Details
Introduction
Infections from the Coronavirus 2 (SARS-CoV-2), the virus that causes Coronavirus disease in 2019 to 2023 now number more than 104 million in the United States [1]. A subset of these patients developed a more severe form of the disease characterized by acute respiratory distress syndrome and respiratory failure [2]. Subsequently, mechanical ventilation was common and has been associated with high rates of morality in Covid 19 patients [3]. The COVID-19 epidemic revealed the vulnerability of healthcare systems and how they can rapidly be overloaded in excess of the available ICU beds and ventilator capacity. The number of ventilators and critical care beds had reach maximum capacity, which strained resources and may have ultimately cost patient lives. Nearly eight in ten hospitalized Covid patients required supplemental oxygen and as many as one third of these patients were mechanically ventilated [4-7]. High intensive care unit occupancy has been linked to worse patient outcomes, including mortality [4,8,9]. Ultimately, the emergence of the coronavirus disease in 2019 (COVID-19) led to high demand for intensive care services worldwide.
The use of diuretics has been studied in critical ill patients, specifically in acute respiratory distress syndrome. Recent literature advocates for a conservative fluid strategy in acute respiratory distress syndrome patients. However, little data exists on the Covid 19 patients who develop acute respiratory distress syndrome and the effects of diuresis on mechanical ventilation. To our knowledge, no or few studies have evaluated the association between intravenous diuretics and the use of mechanical ventilation in moderate to severe Covid 19 patients.
We therefore undertook this study to evaluate the effects of intravenous diuretics on the use of mechanical ventilation in moderate to severe Covid 19 patients. We designed a retrospective cohort study to examine the association between intravenous diuretics and the use of mechanical ventilation on hospitalized COVID-19 patients requiring supplemental oxygen.
Materials and Methods
Setting and data
We conducted this retrospective cohort study at The University of Chicago Medical Center in Chicago, Illinois. The study protocol was approved by the University of Chicago Biological Sciences Division/The University of Chicago Medical Center’s Institutional Review Board (IRB) Committee. Patient informed consent was waived by the University of Chicago Biological Sciences Division/The University of Chicago Medical Center’s IRB because the data was analyzed anonymously. All research was performed in accordance with relevant guidelines and regulations by the University of Chicago Biological Sciences Division/The University of Chicago Medical Center. The data was obtained from the COVID Datamart maintained by the Center for Research Informatics at The University of Chicago Medical Center. The COVID Datamart contains all clinical data available on all inpatient and outpatient visits to the University of Chicago Medical Center facilities [10-16]. No data were manually abstracted from the electronic medical record or charts. The data obtained included patients’ demographics (age, gender, and race/ethnicity), vital signs, laboratory test results, imaging test results, medication administration data, historical and current medication lists, historical and current diagnoses, hospital discharge and procedure diagnoses. All personal health-identifying information was de-identified, and data were extracted using an electronic coding system to maintain confidentiality. International Classification of Diseases (ICD)-9 and ICD-10 codes were pulled from the index hospitalization to construct comorbidity variables using the Elixhauser Comorbidity Software.
Study sample
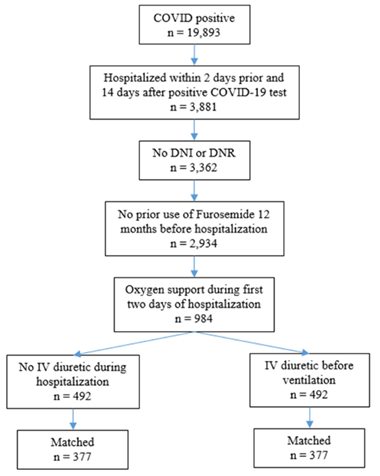
Study sample included adult patients age 18 or above who had a positive test result for SARS-CoV-2 from March 2020 to February 15, 2022, were hospitalized within 2 days before and 14 days after their positive test, and who received supplemental oxygen support (including nasal cannula mask, venturi mask, non-rebreather mask or high flow nasal cannula mask) during first two days of hospitalization. Patients were excluded if they were “do not intubate” or “do not resuscitate” or had prior diuretics (Furosemide) use within 12 months prior to COVID-19 hospitalization admission date. Patients were defined as receiving intravenous diuretic (Furosemide) if they received the medication before the start of ventilation versus those who were not exposed to intravenous diuretic at all during hospitalization [17-25]. The study’s design is illustrated in figure 1.
End Points: The primary endpoint was mechanical ventilation, defined as the patient requiring either noninvasive or invasive mechanical ventilation at any point during the hospitalization.
Statistical analysis
We performed all statistical using Stata M16 and R version 4.1.1 statistical software. Baseline patient characteristics are summarized using mean and standard deviation for continuous variables and as numbers and percentages for categorical variables. Chi squared tests were conducted to detect differences in baseline characteristics. Fisher’s exact test was used in cases of cell sizes < 5.
A propensity score method was used to account for confounders using R’s MatchIt package. The variables included in the propensity score calculation were chosen in based on association with intravenous diuretic exposure. A nearest neighbor match without replacement and a caliper of 0.2 was used.
We assessed ventilation status between the intravenous diuretic and no intravenous diuretic groups using unadjusted and adjusted logistic regression. Adjusted models included covariates for demographics, comorbidities, COVID-19 specific therapies (Dexamethasone, Remdesivir, and Tocilizumab), length of stay, and ICU admission [26-33].
We conducted multiple sensitivity analyses including a smaller, 0.1 Caliper in the Propensity Score Matching Analysis, Adjusted Multivariable Logistic Regression Analysis and a Competing Risk Regression Analysis, where death was the competing risk.
Results
Cohort Characteristics
There were 981 patients included in our sample. Of these, 491 (50%) received intravenous diuretic prior to ventilation and 490 did not received any intravenous diuretic exposure during hospitalization. The cohort characteristics are presented in table 1.
|
No IV diuretic use at all during hospitalization (N=491) |
IV diuretic use during hospitalization before ventilation (N=492) |
p-value |
|||
|
N |
% |
N |
% |
||
|
Age (mean) |
52.2 |
59.3 |
0 |
||
|
Female |
243 |
49.5 |
237 |
48.2 |
0.679 |
|
Race |
|||||
|
Black |
431 |
87.8 |
404 |
82.1 |
0.035 |
|
White |
35 |
7.1 |
46 |
9.3 |
|
|
Other |
25 |
5.1 |
42 |
8.5 |
|
|
Hispanic |
23 |
4.7 |
37 |
7.5 |
0.063 |
|
Comorbidities |
|||||
|
CHF |
29 |
5.9 |
25 |
5.1 |
0.57 |
|
Cirrhosis |
6 |
1.2 |
3 |
0.6 |
0.341 |
|
Sepsis |
7 |
1.4 |
4 |
0.8 |
0.385 |
|
Renal Insufficiency |
4 |
0.8 |
1 |
0.2 |
0.217 |
|
CKD |
28 |
5.7 |
21 |
4.3 |
0.302 |
|
ESRD |
30 |
6.1 |
7 |
1.4 |
0 |
|
Hypertension |
100 |
20.4 |
100 |
20.3 |
0.987 |
|
Diabetes |
60 |
12.2 |
70 |
14.2 |
0.353 |
|
Obesity |
291 |
59.3 |
329 |
66.9 |
0.014 |
|
COPD |
30 |
6.1 |
21 |
4.3 |
0.193 |
|
Asthma |
41 |
8.4 |
20 |
4.1 |
0.005 |
|
Hypernatremia |
3 |
0.6 |
0 |
0 |
0.124 |
|
Hyponatremia |
7 |
1.4 |
6 |
1.2 |
0.777 |
|
Hyperkalemia |
17 |
3.5 |
7 |
1.4 |
0.038 |
|
Hypokalemia |
22 |
4.5 |
8 |
1.6 |
0.009 |
|
Kidney Failure |
21 |
4.3 |
17 |
3.5 |
0.504 |
|
Length of Stay (mean) |
7.4 |
11 |
0 |
||
|
ICU admission |
95 |
19.3 |
182 |
37 |
0 |
|
Drug Exposure |
|||||
|
Previous Diuretic |
31 |
6.3 |
35 |
7.1 |
0.616 |
|
Previous Vasopressor |
50 |
10.2 |
56 |
11.4 |
0.53 |
|
Tocilizumab during hospitalization |
19 |
3.9 |
65 |
13.2 |
0 |
|
Dexamethasone during hospitalization |
189 |
38.5 |
281 |
57.1 |
0 |
|
Remdesivir during hospitalization |
239 |
48.7 |
369 |
75 |
0 |
Table 1: Baseline characteristics of the overall cohort
Propensity score matched analysis
After the propensity score matching for the receipt of intravenous furosemide, our sample included 754 patients. The propensity score matching was successful with no statistically significant baseline differences between the groups [34-43]. The mean age in our cohort was 58.9 years, was predominantly Black (97.3%), and 11.8% had received at least one dose of COVID-19 vaccination before their positive COVID-19 test. The baseline cohort characteristics before and after the propensity score matching are presented in tables 2 and 3 reespectively.
|
No IV diuretic use at all during hospitalization (N=490) |
IV diuretic use during hospitalization before ventilation (N=491) |
p-value |
|||
|
N |
% |
N |
% |
||
|
Demographics |
|||||
|
Age (mean) |
|||||
|
Female |
243 |
49.6 |
237 |
48.3 |
0.67 |
|
Race |
|||||
|
Black |
430 |
87.8 |
404 |
82.4 |
0.05 |
|
White |
35 |
7.1 |
46 |
9.4 |
|
|
Other |
25 |
5.1 |
21 |
4.3 |
|
|
Comorbidities |
|||||
|
Congestive Heart Failure |
29 |
5.9 |
25 |
5.1 |
0.57 |
|
Cirrhosis |
6 |
1.2 |
3 |
0.6 |
0.34 |
|
Acute Kidney Injury |
4 |
0.8 |
1 |
0.2 |
0.19 |
|
Chronic Kidney Disease |
28 |
5.7 |
21 |
4.3 |
0.3 |
|
Hypertension |
100 |
20.4 |
100 |
20.4 |
0.99 |
|
Diabetes |
60 |
12.2 |
70 |
14.3 |
0.35 |
|
COPD |
30 |
6.1 |
21 |
4.3 |
0.19 |
|
Labs |
|||||
|
Elevated Serum Creatinine/BUN |
1 |
0.2 |
1 |
0.2 |
1 |
|
Hyponatremia |
3 |
0.6 |
2 |
0.4 |
0.69 |
|
Hyperkalemia |
6 |
1.2 |
3 |
0.6 |
0.34 |
|
Physical Exam |
0 |
||||
|
Weight (mean) |
85.4 kg |
100.7 kg |
0 |
||
|
O2 dependence |
4 |
0.8 |
1 |
0.2 |
0.22 |
|
Leg Edema |
1 |
0.2 |
2 |
0.4 |
1 |
|
Pulmonary Edema |
0 |
0 |
0 |
0 |
1 |
|
Dyspnea |
5 |
1 |
2 |
0.4 |
0.29 |
|
Fluid Balance |
0 |
0 |
0 |
0 |
1 |
|
Systolic/ |
128 |
132 |
0.01 |
||
|
Diastolic BP (mean) |
78 |
80 |
0.06 |
||
|
Drug Exposure |
|||||
|
Previous Diuretic |
31 |
6.3 |
35 |
7.1 |
0.62 |
|
Previous ACE Inhibitors |
31 |
6.3 |
36 |
7.3 |
0.53 |
|
Previous Beta Blocker |
39 |
8 |
48 |
9.8 |
0.32 |
|
COVID Vaccination |
63 |
12.9 |
49 |
10 |
0.16 |
Table 2: Baseline Cohort Characteristics Before Propensity Score Matching
Mechanical ventilation
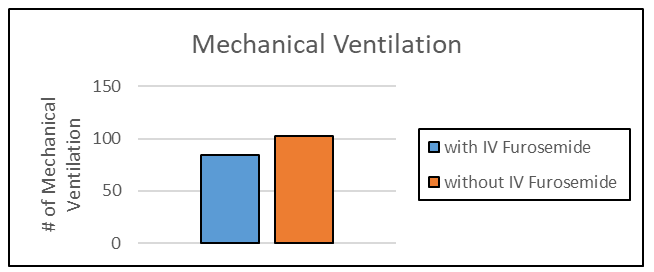
Among the 754 patients included in the analysis, 122 patients (16.2%) required mechanical ventilation in their index COVID hospitalization [44-51]. Figure 2 illustrates the number of mechanical ventilations in those patients exposed to intravenous furosemide compared to those patients not exposed to intravenous furosemide. In the unadjusted analysis, there was no significant association between patients who had received intravenous diuretic and the primary endpoint compared to those who did not receive intravenous diuretic (unadjusted odds ratio 1.08, 95% CI 0.73-1.59) [52-56]. In the multivariable regression analysis adjusting for demographic variables, comorbidities, medication history, ICU admission, LOS, and COVID-19 vaccination, there was a significant lower odds ratio associated with intravenous diuretic and mechanical ventilation compared to those who did not receive intravenous diuretic (adjusted OR 0.37, 95% CI 0.21-0.66) (p-value = 0.001) as shown in table 4.
|
Adjusted analyses: OR (95% CI) |
||||
|
Unadjusted OR (95% CI) |
Adjusted for demographic variables1 |
Adjusted for demographic variables and comorbidities2 |
Adjusted for demographic variables, comorbidities, medication history, ICU, LOS, vaccination3 |
|
|
Ventilation during index COVID hospitalization |
1.08 (0.73, 1.59) |
1.08 (0.73, 1.60) |
1.13 (0.76, 1.68) |
0.37 (0.21, 0.66)** |
*p < .05; **p < .01; ***p < .001
1Sex, age, race, ethnicity
2Sex, age, race, ethnicity, CHF, cirrhosis, sepsis, CKD, ESRD, hypertension, diabetes, obesity, COPD, asthma
3Sex, age, race, ethnicity, CHF, cirrhosis, sepsis, CKD, ESRD, hypertension, diabetes, obesity, COPD, asthma, prior diuretic use, prior vasopressor use, remdesivir use, dexamethasone use, tocilizumab use, length of stay, ICU admission, COVID-19 vaccination
Table 4: Mechanical Ventilation during index COVID Hospitalization
Sensitivity analyses
A sensitivity analysis using a smaller caliper for propensity score matching (0.1) found similar results (unadjusted OR 1.04, 95% CI 95% 0.69-1.58, p-value=0.833; adjusted OR 0.29, 95% CI 0.15-0.58, p-value<0.001) is presented in table 5. The second sensitivity analysis using adjusted multivariable regression analysis showed similar results (adjusted OR 0.48, 95% CI 0.30-0.76) (p-value < 0.05)) as presented in table 6. The third sensitivity analysis using a risk competing regression analysis, where death was the competing risk, found no significant association between the competing risk and the primary outcome in the adjusted analysis (Odds Ratio 1.50 95% CI 0.93, 2.40) is shown in table 7. In the competing risk regression analysis, individuals were excluded if they were mechanically ventilated or discharged on the same day as their admission date (no time at risk).
|
Adjusted analyses: OR (95% CI) |
||||
|
Unadjusted OR (95% CI) |
Adjusted for demographic variables1 |
Adjusted for demographic variables and comorbidities2 |
Adjusted for demographic variables, comorbidities, medication history, ICU, LOS, vaccination3 |
|
|
Ventilation during index COVID hospitalization |
1.04 (0.70, 1.58) |
1.04 (0.70, 1.60) |
1.07 (0.70, 1.64) |
0.29 (0.15, 0.57)*** |
N=710
*p < .05; **p < .01; ***p < .001
1Sex, age, race, ethnicity
2Sex, age, race, ethnicity, CHF, cirrhosis, sepsis, CKD, ESRD, hypertension, diabetes, obesity, COPD, asthma
3Sex, age, race, ethnicity, CHF, cirrhosis, sepsis, CKD, ESRD, hypertension, diabetes, obesity, COPD, asthma, prior diuretic use, prior vasopressor use, remdesivir use, dexamethasone use, tocilizumab use, length of stay, ICU admission, COVID-19 vaccination
Table 5: Sensitivity Analysis #1: Ventilation during index COVID hospitalization, using Matching Caliper 0.1
|
Adjusted analyses: OR (95% CI) |
||||
|
Unadjusted OR (95% CI) |
Adjusted for demographic variables1 |
Adjusted for demographic variables and comorbidities2 |
Adjusted for demographic variables, comorbidities, medication history, ICU, LOS3 |
|
|
Ventilation during index COVID hospitalization |
0.79 (0.57, 1.08) |
0.94 (0.67, 1.32) |
1.05 (0.74, 1.50) |
0.48 (0.30, 0.76)* |
* p < .05; ** p < .01; *** p < .001
1Sex, age, race
2 Sex, age, race, CHF, cirrhosis, sepsis, acute renal insufficiency, CKD, ESRD, hypertension, diabetes, obesity, COPD, asthma
3 Sex, age, race, CHF, cirrhosis, sepsis, acute renal insufficiency, CKD, ESRD, hypertension, diabetes, obesity, COPD, asthma, prior diuretic use, prior vasopressor use, remdesivir use, dexamethasone use, tocilizumab use, length of stay, ICU admission
Table 6: Sensitivity Analysis #2: Adjusted Multivariable Regression Analysis
Mechanical ventilation during index COVID hospitalization
|
Adjusted analyses: OR (95% CI) |
|||
|
Unadjusted OR (95% CI) |
Adjusted for demographic variables1 |
Adjusted for demographic variables and comorbidities2 |
|
|
Ventilation during Hospitalization (Death as Competing Risk) |
1.43 (0.91, 2.24) |
1.41 (0.90, 2.21) |
1.50 (0.93, 2.40) |
*p < .05; **p < .01; ***p < .035
1Sex, age, race
2Sex, age, race, CHF, cirrhosis, sepsis, acute renal insufficiency, CKD, ESRD, hypertension, diabetes, obesity, COPD, asthma
Some individuals were excluded if they were ventilated or discharged on the same day as their admission date (no time at risk
Table 7: Sensitivity Analysis #3: Competing Risk Regression Analysis between the Primary Outcome and Death
Discussion
Our research showed that among a moderately sized sample of patients who were hospitalized with Covid 19 requiring supplemental oxygen, patients exposed to intravenous furosemide was significantly associated with a lower use of mechanical ventilation compared to those that were not exposed to intravenous furosemide (adjusted Odds Ratio 0.37, 95% CI 0.21-0.66 p-value <0.05). However, given the observational design of the study and the relatively wide confidence interval, the results of the study should not be taken to mean all Covid 19 patients should receive intravenous diuresis.
It appears the overall pathophysiology and clinical course of COVID-19-related lung injury suggests that it is broadly similar to other forms of virally-mediated acute respiratory distress syndrome. The likely mechanism involved decreasing pulmonary edema with diuresis in Covid 19 patients who developed acute respiratory distress syndrome, resulting in improved gas exchange and oxygenation. This is similar to other strategies such as “keeping the lungs dry” and “maintaining a slightly negative fluid balance,” typically seen in the management of acute respiratory distress syndrome in non-Covid patients [13].
Our results support the use of intravenous diuresis as tolerated in hospitalized Covid 19 patients requiring supplemental oxygen to decrease the risk of mechanical ventilation. Noteworthy, one of the clinical practices during the Covid 19 pandemic was to intubate Covid 19 patients who showed acute respiratory failure or distress, early. Despite this, our study showed intravenous diuresis was still effective in reducing the use of mechanical ventilation in severe Covid 19 patients. More importantly, our results could have implications in the acute management of possible future viral outbreaks whose disease course is complicated by severe respiratory injury.
Reynolds, who was the first to report on anti-hypertensive agents such as hydrochlorothiazide in COVID-19 patients, found no statistically significant association between their use and adverse effects or the severity of Covid 19 disease [57]. In our study, we specifically focused on only diuretics and mechanical ventilation. In another study, Tsolaki suggested cautious use of diuretics in mechanically ventilated COVID-19 patients and hypothesized detrimental effects by exacerbating heart-lung interactions, especially when a strategy of increased PEEP was applied [58]. However, the paper focused on COIVD- 19 patients already on mechanical ventilation, while our study examined patients before the initiation of mechanical ventilation (58). Another study showed that among patients hospitalized with COVID-19, the baseline use of diuretics did not have a significant impact on the mortality or severity of the illness [56]. The study only look at oral diuretics [56] while in our research chose to study intravenous diuretics because they were more likely to achieve effective volume removal when compared to oral diuretics.
The risk of unmeasured confounding cannot be fully dismissed in any observational study. One interpretation of our results is that selection bias likely existed in which patients the clinician(s) decided to give intravenous diuretics to. The patient population who received intravenous diuretics were likely to be different compared to the population of patients whose clinician(s) decided not to give intravenous diuretics. With that said, propensity score matching whose aim is to create an overall balance between comparison groups, was used to better account for this potential bias for the receipt of intravenous furosemide. In our propensity score matching analysis, an overall 76.6% of the patients (754 of the original 984) were paired. In particular, comorbidities, along with medications and laboratory values were all well matched between the comparison groups. Notably, the acute severity of the illness, characterized in one way by the use of low flow Nasal Cannula versus high flow Nasal Cannula, was not included in the propensity score analysis because it had already been accounted for in the study. All of the patients included in this study were either those on low flow Nasal Cannula, Venturi Mask, non-Rebreather Mask or high flow Nasal Cannula during the hospitalization. It should be noted, despite propensity score matching analysis, there may still be residual unmeasured confounder(s) affecting the results.
The study adjusted for likely confounders such as age, race, obesity, chronic kidney disease, diabetes, hypertension, chronic lung disease and congestive heart failure. In addition, medication administration, specifically Covid 19-specific therapies used during hospitalization were adjusted for because Remdesivir (an antiviral agent), Dexamethasone (a steroid) and Tocilizumab (an Interleukin-6 Receptor Antibody) have all been shown to decrease lung inflammation, and theoretically could effect the need for mechanical ventilation. The patient’s Covid Vaccination Status was also included in the analysis for similar reasons. Confidence in the results is supported by the multiple sensitivity analyses performed, all of which showed unchanged conclusions. The consistency of the results across the sensitivity analyses is reassuring.
One limitation in our study was it only examined intravenous furosemide and not other types of diuretics such as bumetanide or oral diuretics. The single center design may limit generalizability of these results. Most of the studied cohort was of a single race (Black 82.6%) which could limit generalizability. We did not use objective variables, such as PaO2:Fio2 ratio to define hypoxia. One possible limitation is the potential bias introduced by the study’s definition of intravenous diuretic exposure, which was defined as receiving intravenous furosemide at any point prior to mechanical ventilation initiation. If mechanical ventilation was initiated earlier in a patient's stay, they therefore, may have had less time to be exposed to intravenous diuretics, which could have resulted in biased results. With that said, at the hospital this study was carried out at, during most of the pandemic intravenous diuretics was recommended to be given as tolerated to Covid 19 patients who were hypoxic and thus likely would have helped mitigated this potential bias. Also, due to small sample sizes, some covariates in adjusted models do not have observations in all levels of the coviarate. In this case, the covariate is dropped from the model. Finally, due to the observational nature of this study, a randomized clinical trial is the best approach to determine whether any benefit from intravenous diuresis can be attributed to Covid 19 patients.
Further research should be aimed at examining the effects on mortality. One question that is raised by our study: if intravenous diuresis decreases mechanical ventilation, does it also decrease the severity of Covid 19 disease? Specifically, the progression to acute respiratory distress syndrome? Other research could focus on if acute dialysis shows similar results. Studying the effect of diuresis on the viral load of Covid 19 is interesting, as a case report from China showed the isolation of SARS-CoV-2 from the urine of a Covid 19 patient [29]. Finally, investigating the effects of diuresis on the long-term complications of Covid 19 such as “long Covid” is another potential area of research.
In conclusion, we identified that intravenous diuresis decreases the use of mechanical ventilation in Covid 19 patients requiring supplemental oxygen.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available because the authors were not using primary data, but rather secondary data owned by the University of Chicago Biologic Sciences Division/The University of Chicago Medical Center which is governed by an Institutional Review Broad, but are available from the corresponding author on reasonable request and with permission of the University of Chicago Biologic Sciences Division/The University of Chicago Medical Center’s Institutional Review Board.
References
- Centers for Disease Control. United States COVID-19 cases and deaths by state (https://covid.cdc.gov/covid-data-tracker/)
- Kazory A, Ronco C, McCullough PA. SARS-CoV-2 (COVID-19) and intravascular volume management strategies in the critically ill. Proc (Bayl Univ Med Cent) 10 (2020): 1-6.
- Patrick WZ, Stephanie JS, Timothy B, et al. Mortality associated with intubation and mechanical ventilation in patients with COVID-19 medRxiv 2020.08.13.20174524
- Douin, David J. ICU bed utilization during the coronavirus disease 2019 Pandemic in a Multistate Analysis- March to June 2020. Critical Care Explorations 3 (2021): e0361.
- Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 180 (2020): 934-943.
- Guo, Tao. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiology 27 (2020): 12.
- Zhou, Fei. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. The Lancet 395 (2020): 10229.
- Kim SH, Chan CW, Olivares M, et al. ICU admission control: An empirical study of capacity allocation and its implication for patient outcomes. Manag Sci 61 (2015): 19-38.
- Wilcox, M. Elizabeth, et al. Higher ICU Capacity Strain Is Associated with Increased Acute Mortality in Closed ICUs*. Critical Care Medicine 48 (2020): 709-716.
- Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. New England Journal of Medicine 3 (2021): 31.
- Guan WJ, Ni ZY, Clinical Characteristics of Covid-19 in China. New England Journal of Medicine 27 (2020): 63.
- Gandhi RT, Lynch JB, Del Rio C. Mild or Moderate Covid-19. Solomon CG, ed. New England Journal of Medicine 24 (2020): 31
- Casey JD, Semler MW, Rice TW. Fluid Management in Acute Respiratory Distress Syndrome. Seminars in Respiratory and Critical Care Medicine 40 (2019): 57-65.
- Sibbald WJ, Short AK, Warshawski FJ, et al. Thermal Dye Measurements of Extravascular Lung Water in Critically III Patients. Chest 87 (1985): 585-592.
- Torres Acosta MA, Singer BD. Pathogenesis of COVID-19-induced ARDS: implications for an ageing population. The European Respiratory Journal 56 (2020): 82.
- Batah SS, Fabro AT. Pulmonary pathology of ARDS in COVID-19: A pathological review for clinicians. Respiratory Medicine 176 (2021): 106239.
- Bellani G. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive Care units in 50 countries. J. Am. Med. Assoc 315 (2016): 788-800.
- Menter T. Post-Mortem Examination of COVID19 Patients Reveals Diffuse Alveolar Damage with Severe Capillary Congestion and Variegated Findings of Lungs and Other Organs Suggesting Vascular Dysfunction. Histopathology 77 (2020): 14134.
- Aziz, Shadman. Managing ICU Surge during the COVID-19 Crisis: Rapid Guidelines. Intensive Care Medicine 8 (2020): 1-23.
- Ackermann, Maximilian. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. New England Journal of Medicine 383 (2020): 5432.
- Grasselli, Giacomo. Risk Factors Associated with Mortality among Patients with COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Internal Medicine 180 (2020): 1345.
- Rosenbaum, Lisa. Facing Covid-19 in Italy- Ethics, Logistics, and Therapeutics on the Epidemic’s Front Line. New England Journal of Medicine 18 (2020): 562.
- Armstrong RA. Outcomes from Intensive Care in Patients with COVID-19: A Systematic Review and Meta-Analysis of Observational Studies. Anaesthesia 30 (2020): 201.
- Clarke AL. Coping with COVID-19: Ventilator Splitting with Differential Driving Pressures Using Standard Hospital Equipment. Anaesthesia 9 (2020): 61.
- King, William P. Emergency Ventilator for COVID-19. Plos one 15 (2020): e0244963.
- Alhazzani, Waleed. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Medicine 28 (2020): 81.
- Matthay, Michael A. Treatment for Severe Acute Respiratory Distress Syndrome from COVID-19. The Lancet Respiratory Medicine 20 (2020): 3012.
- Sprung, Charles L. Adult ICU Triage during the Coronavirus Disease 2019 Pandemic: Who Will Live and Who Will Die? Recommendations to Improve Survival*. Critical Care Medicine 48 (2020): 1196-1202.
- Sun, Jing et al. Isolation of Infectious SARS-CoV-2 from Urine of a COVID-19 Patient. Emerging Microbes & Infections 9 (2020): 991-993.
- Vohra, Adam S. Intensive Care Unit Admission with Community-Acquired Pneumonia. The American Journal of the Medical Sciences 350 (2015): 380-386.
- Ruhnke, Gregory W. The Drivers of Discretionary Utilization. Academic Medicine 92 (2017): 703-708.
- Weir, Ronald E. The Relative Ability of Comorbidity Ascertainment Methodologies to Predict In-Hospital Mortality among Hospitalized Community-Acquired Pneumonia Patients. Medical Care 56 (2018): 950-955.
- Li X, Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS?. Critical Care 24 (2020): 123.
- Comparison of Two Fluid-Management Strategies in Acute Lung Injury. New England Journal of Medicine. 354 (2006): 2564-2575.
- Wiedemann HP. A perspective on the fluids and catheters treatment trial (FACTT). Fluid restriction is superior in acute lung injury and ARDS. Cleveland Clinic Journal of Medicine 75 (2008): 42-48.
- Tang X, Du R, Wang R, et al. Comparison of Hospitalized Patients with Acute Respiratory Distress Syndrome Caused by COVID-19 and H1N1. Chest 11 (2020): 91.
- Heresi GA, Arroliga AC, Wiedemann HP, et al. Pulmonary Artery Catheter and Fluid Management in Acute Lung Injury and the Acute Respiratory Distress Syndrome. Clinics in Chest Medicine 27 (2006): 627-635.
- Pulmonary-Artery versus Central Venous Catheter to Guide Treatment of Acute Lung Injury. New England Journal of Medicine 354 (2006): 2213-2224.
- Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. New England Journal of Medicine 21 (2020): 36.
- Kox M, Waalders NJB, Kooistra EJ, et al. Cytokine Levels in Critically Ill Patients With COVID-19 and Other Conditions. JAMA 3 (2020): 639.
- Prandota J. Furosemide: Progress in Understanding Its Diuretic, Anti-inflammatory, and Bronchodilating Mechanism of Action, and Use in the Treatment of Respiratory Tract Diseases. American Journal of Therapeutics. 2002;9(4):317-328.
- Karmouty-Quintana H, Thandavarayan RA, Keller SP, et al. Emerging Mechanisms of Pulmonary Vasoconstriction in SARS-CoV-2-Induced Acute Respiratory Distress Syndrome (ARDS) and Potential Therapeutic Targets. International Journal of Molecular Sciences 21 (2021): 8081.
- Garvin MR, Alvarez C, Miller JI, et al. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. eLife. 2020;9.
- Van de Veerdonk FL, Netea MG, Van Deuren M, et al. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. eLife 9 (2020): 555.
- Song JW, Zhang C, Fan X, et al. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nature Communications 11 (2020): 382
- Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. The Lancet Respiratory Medicine 11 (2020): 405.
- McElvaney OJ, McEvoy NL, McElvaney OF, et al. Characterization of the Inflammatory Response to Severe COVID-19 Illness. American Journal of Respiratory and Critical Care Medicine 202 (2020): 812-821.
- Zeng Z, Yu H, Chen H, et al. Longitudinal changes of inflammatory parameters and their correlation with disease severity and outcomes in patients with COVID-19 from Wuhan, China. Critical Care 24 (2020): 111.
- Jain A, Chaurasia R, Sengar NS, et al. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Scientific Reports 10 (2020): 598
- Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduction and Targeted Therapy 5 (2020): 4132
- Papamanoli A, Yoo J, Grewal P, et al. High-dose methylprednisolone in nonintubated patients with severe COVID-19 pneumonia. European Journal of Clinical Investigation 51 (2020): 231.
- Meltzer DO, Best TJ, Zhang H, et al. Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. JAMA Network Open 3 (2020): e2019722.
- Lopez Bernal, Jamie, et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. New England Journal of Medicine 21 (2021): 891
- Geleris, Joshua, et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. New England Journal of Medicine 7 (2020): 2410.
- Guragai N, Vasudev R, Hosein K, et al. Does Baseline Diuretics Use Affect Prognosis in Patients With COVID-19? Cureus 13 (2021): e15573.
- Reynolds HR, Adhikari S, Pulgarin C, et al. Renin–angiotensin–aldosterone system inhibitors and risk of Covid-19. N Engl J Med 11 (2018): 365.
- Tsolaki V, Zakynthinos GE, Mantzarlis K, et al. Increased mortality among hypertensive COVID-19 patients: Pay a closer look on diuretics in mechanically ventilated patients. Heart Lung 49 (2020): 894-895.
- Swenson KE, Swenson ER. Pathophysiology of Acute Respiratory Distress Syndrome and COVID-19 Lung Injury. Crit Care Clin 37 (2021): 749-776.
- Ahmed A, Young JB, Love TE, et al. A propensity-matched study of the effects of chronic diuretic therapy on mortality and hospitalization in older adults with heart failure. Int J Cardiol 125 (2008): 246-253.


 Impact Factor: * 4.2
Impact Factor: * 4.2 Acceptance Rate: 72.62%
Acceptance Rate: 72.62%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 