Robot-assisted Inguinal Lymphadenectomy: Evolution of the technique with the Alexis® Wound Protector/Retractor
Gennaro Musi1,2, Gabriele Cozzi1, Sara Coppola3, Stefano Luzzago1,2, Matteo Ferro1, Gian Marco Orsolini3, Mattia Luca Piccinelli1, Ettore Di Trapani1, Francesco Verrecchia3, Giovanni Cordima1, Ottavio de Cobelli1,2, Elisabetta Pennacchioli3, Francesco Alessandro Mistretta1,2*
1Division of Urology. European Institute of Oncology, IRCCS. Via Ripamonti 435, 20141 Milan, Italy.
2Department of Oncology and Hematology-Oncology. Università degli Studi di Milano, Milan, Italy.
3Division of Melanoma and Sarcomas. European Institute of Oncology, IRCCS. Via Ripamonti 435, 20141 Milan, Italy.
*Corresponding Author: Francesco Alessandro Mistretta, Division of Urology. European Institute of Oncology, IRCCS. Via Ripamonti 435, 20141 Milan, Italy.
Received: 10 August 2023; Accepted: 18 August 2023; Published: 22 February 2024
Article Information
Citation:
Gennaro Musi, Gabriele Cozzi, Sara Coppola, Stefano Luzzago, Matteo Ferro, Gian Marco Orsolini, Mattia Luca Piccinelli, Ettore Di Trapani, Francesco Verrecchia, Giovanni Cordima, Ottavio de Cobelli, Elisabetta Pennacchioli, Francesco Alessandro Mistretta. Robot-assisted Inguinal Lymphadenectomy: Evolution of the technique with the Alexis® Wound Protector/ Retractor. Journal of Surgery and Research. 7 (2024): 66-71.
View / Download Pdf Share at FacebookAbstract
Introduction: Robot-assisted inguinal lymphadenectomy (RAIL) has been described as a reliable alternative to traditional open surgery. We reported the evolution of our technique for RAIL after introducing the Alexis® wound protector/retractor (AWPR).
Materials and Methods: We reviewed records of six patients who underwent RAIL with the use of the AWPR for penile or integumental tumors. We recorded clinical, surgical and oncologic data. Findings were compared with our robotic historic series, but no statistical analyses could be conducted due to the limited size of population.
Results: Overall median age was 65 years old, and 63% of patients were men. Median operative time for RAIL with AWPR was 225 minutes (interquartile range [IQR] 198-243), lower than that observed in simple RAIL (279; 210- 300). Estimated blood loss was clinically not significant in both groups. Median RAIL with AWPR LN yield was similar to simple RAIL (9 [7-17] vs. 11[8-16], respectively). Similar hospital stay was recorded (3 [3-3] vs. 4 [3-5] days), as well as similar median time to inguinal drain removal 37 vs 32 days (AWPR vs. simple RAIL patients). Neither open conversions, nor intraoperative major complications were reported. In RAIL with AWPR cohort two patients (33.3%) reported a postoperative complication (one skin necrosis and one infected haematoma), similar to those who received simple RAIL. No locoregional relapses or deaths were reported during follow-up.
Conclusions: RAIL with AWPR was confirmed safe, feasible, with adequate lymph nodes yield and comparable complication rate relative to our historic robotic series.
Keywords
<p>Inguinal lymphadenectomy, Robotics, Penile cancer, Melanoma, Merkel cell carcinoma</p>
Article Details
Abbreviations
AWPR: Alexis® wound protector/retractor.
BMI: body mass index.
CCI: Charlson Comorbidity Index.
EBL: estimated blood loss
ILND: inguinal lymph node dissection.
IQR: interquartile range
RAIL: robot-assisted inguinal lymphadenectomy.
RAPLND: robot-assisted pelvic lymph nodes dissection.
VEIL: video endoscopic inguinal lymphadenectomy.
Introduction
Inguinal lymph node dissection (ILND) is a crucial part of the surgical treatment of several malignancies, such as penile carcinoma, melanoma, Merkel cell carcinoma, squamous cell carcinoma, dermal duct tumor [1,2]. Open ILND is traditionally burdened by relevant complications such as seroma, lymphedema, infection, and skin flap complications [3]; morbidity ranges from 19% to 77% [2]. Many efforts have been spent to reduce these complications without jeopardizing oncologic outcomes [4]. Videoendoscopic ILND (VEIL) has been reported the first time in 1998 for melanoma [5]. Anyway, the spread of VEIL has been limited by equipment, ergonomy and long learning curve [6]. Robot-assisted inguinal lymphadenectomy (RAIL), first described in 2009 [7], was reported as a reliable alternative due to three-dimensional view, reduced surgical trauma, movements’ precision and use of wristed instruments that improve dexterity and facilitate suturing [8]. In 2020 we reported our preliminary experience with RAIL [1] performed according to the technique reported by Sotelo et al. [9]. Since then, we tried to improve our technique aiming to obtain the best possible trocar positioning, standardize the surgical technique, limit the gas leakage and reduce operative time. We report the evolution of our surgical technique with the introduction of the Alexis® wound protector/retractor (AWPR) (Applied Medical, Santa Margarita, CA, USA).
Material and Methods
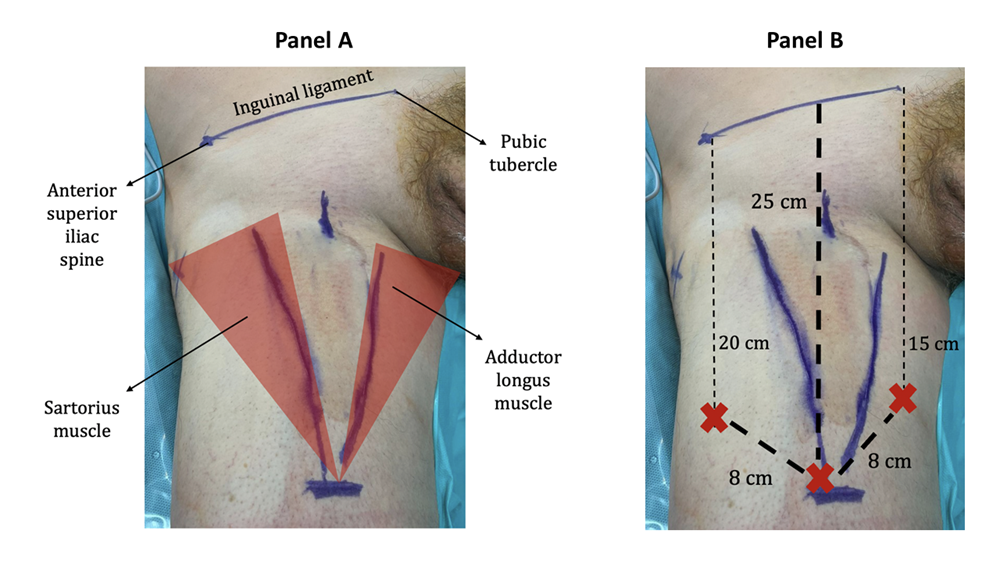
This study was conducted according to the ethical guidelines of the Declaration of Helsinki. A specific informed consent from all patients was obtained. We reviewed the records of the patients who underwent ILND since the introduction of RAIL at our Institution in December 2016 and identified patients who underwent RAIL with the use of the AWPR. If indicated based on current guidelines, patients also underwent a robot-assisted pelvic lymph nodes dissection (RAPLND). Both RAIL and RAPLND were performed using a DaVinci Xi Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA). For RAIL, patients were placed in supine position. After general anesthesia, legs were positioned on stirrups in frog leg position with external rotation and abduction. With a dermographic pen, the main landmarks were marked: the anterior superior iliac spine, the pubic tubercle, the medial border of the sartorius muscle laterally and the medial border of the adductor longus muscle medially. Any palpable lymph nodes were marked too (figure 1). A line was drawn from to the anterior superior iliac spine to the pubic tubercle. From the middle of this line, a 28 cm line was drawn downwards to the inferior aspect of the femoral triangle (figure 1).
Figure 1: Panel A: The main landmarks: the anterior superior iliac spine, the pubic tubercle, the medial border of the sartorius muscle laterally and the medial border of the adductor longus muscle medially. Panel B: A line is drawn from to the anterior superior iliac spine to the pubic tubercle. From the middle of this line, a 28 cm line is drawn downwards to the inferior aspect of the femoral triangle. The red “X”s represents the robotic trocars.
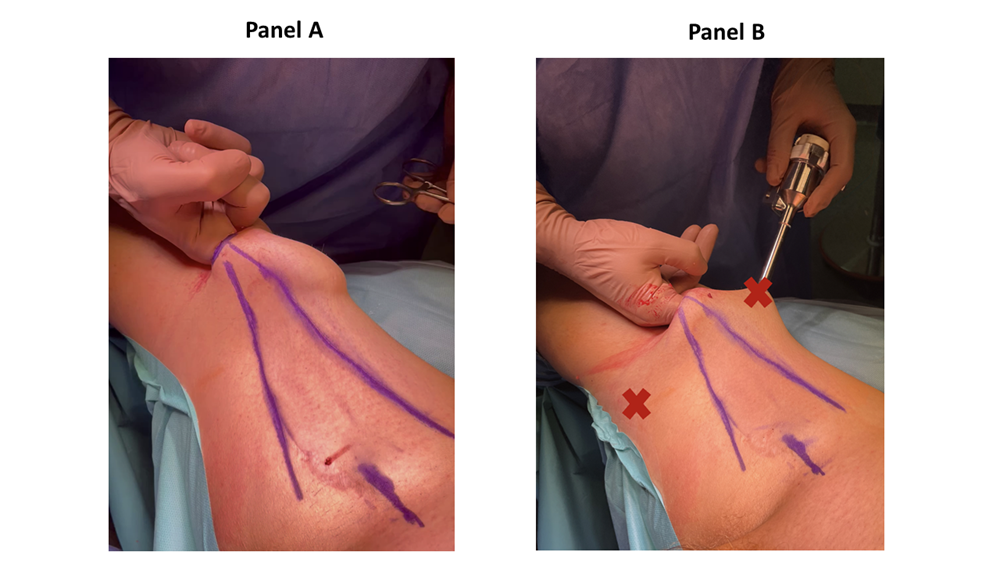
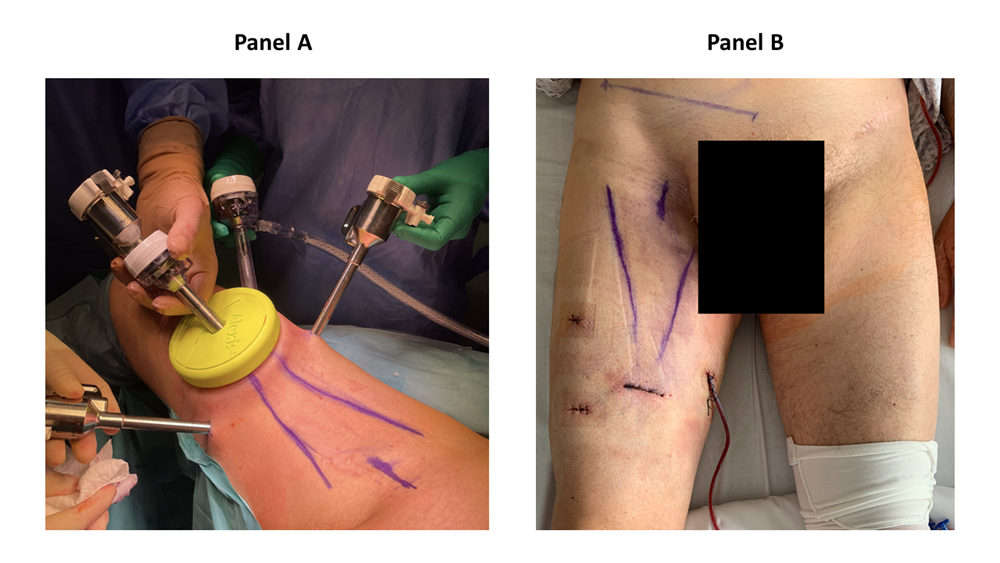
There, a 3 cm horizontal incision was performed. The Scarpa’s fascia was identified, and a subcutaneous space is gained by a blunt finger dissection (figure 2). Two 8 mm robotic trocars were placed 8 cm and 45° cranially from first incision (figure 2). The AWPR with the camera port was placed in the first incision and the space was inflated at a pressure of 15 mmHg. A 12 mm trocar for the assistant was placed between the AWPR and left robotic trocar (figure 3). The DaVinci Xi Surgical System could be alternatively placed on the left or right side of the patient, for both right and left inguinal dissection, depending on the spatial operating room necessities. Thirty-degree camera, monopolar scissors and bipolar Maryland forceps were used. Surgical technique has been already described in our previous publication [1]. Briefly, the dissection was extended from the adductor longus muscle medially, the sartorius muscle laterally and inguinal ligament cranially. Small vessels were clipped and divided, while care was taken to spare the saphenous vein as it contributes to lymphatic drainage [10]. Lymph nodes were dissected from the fascia lata and, encountering the fossa ovalis, dissected away at their superolateral and superomedial limits. After the completion of the dissection, a 10 Ch Jackson-Pratt drain with suction was placed. The specimen was removed in an endobag and extracted from the AWRP (Supplementary figure 1). The Scarpa’s fascia was sutured with polyglactin 2/0 and skin with poliglecaprone 4/0 (figure 3). RAPLND was then performed, where indicated, redocking the robot for a transperitoneal approach as previously reported [1]. The pelvic drainage tube was removed in the first postoperative day. The inguinal drainage tube was removed when the drainage volume was less than 50 ml within 72 hours. For every patient, we recorded age, sex, body mass index (BMI), Charlson Comorbidity Index (CCI) [11], primary tumor, operative time, hospital stay, lymph nodes yield, complications according to the Clavien-Dindo classification [12], time to inguinal drain removal, and follow-up. Data were reported as median and interquartile range or as percentages.
Results
From December 2016 to July 2023, 19 patients underwent RAIL. The last six patients (4 men and 2 women) underwent RAIL with the use of the AWPR and were included in the present analysis (table 1). Median age was 67 years (interquartile range [IQR] 61-71 years). Median BMI was 25,4 kg/m2 (16,3-30,1). Median CCI was 2 (0-3). Three patients harbored Melanoma (50%), two patients had Merkel cell carcinoma (33.3%), one patient had penile cancer (22.2%). In all patients with integumental disease an ipsilateral RAPLND was performed, while the patient with penile cancer received a bilateral RAIL. Median operative time was 225 minutes (198-243). Estimated blood loss (EBL) was not clinically significant in all patient. Median lymph nodes yield was 9 (7-17) for monolateral RAIL. All patients with integumental cancer had nodal invasion at RAIL, but not the patient harboring penile cancer. No intraoperative complications were reported. Median hospital stay was 3 days (3-3). Median time to inguinal drain removal was 37 days (29-50). No procedure was converted to open traditional surgery. One patient (16.7%) experienced a post-operative grade I complication (one small skin necrosis treated conservatively), while one (16.7%) patient had a grade II complication (local infection treated with oral antibiotics). All patients underwent adjuvant treatments after the surgery, according to the guidelines of each specific disease. No locoregional relapses or deaths were reported.
Discussion
According to the current guidelines for penile cancer, bilateral modified ILND or dynamic sentinel lymph node biopsy should be performed in intermediate or high-risk patients with no palpable nodes, while patients with palpable nodes (cN1/cN2) should undergo radical ILND [13,14]. Limit ILND only to patients with palpable nodes would expose many patients with occult metastasis to risk of spread and potentially worse oncologic outcomes [15,16]. Furthermore, there is still a subset of patients with stage III melanoma and skin cancers for whom ILND represents the best therapeutic option [2]. Thus, it is of outmost importance to develop an oncological adequate surgical technique to perform this surgery minimizing its complications. Due to the increased ergonomic and three-dimensional vision, robotic approach properly replicates the open technique, maintaining the oncologic principles [17]. In consequence, since the first report of RAIL in 2009, this approach appeared to be feasible, safe, and oncologically effective [8], with the only absolute contraindications are severe cardiac or respiratory failure [2]. RAIL series reported lower rates of skin necrosis, severe lymphedema, and wound infection requiring surgical intervention compared to open ILND [14]. A lower rate of complications impacts on quality of life and partially counterbalances the elevated costs of acquisition and maintenance of robotic technology [8]. Creation and management of the working space under the Scarpa’s fascia are two of the more challenging steps of endoscopic approaches to ILND. Some Authors reported the use of different devices to create the surgical space. Hyde et al. used a Spacemaker™ balloon (Medtronic, Minneapolis MN) to aid in blunt dissection [3], while Tamhankar et al. used the PDB 1000 balloon® (Medtronic, Minneapolis MN) [18]. To solve the problem of gas leakage, Patel et al. used the AirSeal assistant trocar (SurgiQuest, Milford, CT, USA) and the Alexis mini Gel-port system (Applied Medical, Santa Margarita, CA, USA) [19], while Fankhauser et al. reduced the first incision with a suture and used a 12-mm Kii balloon port (Applied Medical, Santa Margarita, CA, USA) [20]. During our preliminary experience, we also faced the problems of working space creation and gas leakage during RAIL. At our Institution, AWPR is routinely used during robot-assisted radical prostatectomy and robot-assisted radical cystectomy, and in particular has been proven to be useful during port placement and to allow fast extraction of the specimen for frozen section analysis [21]. This device consists of a wound retractor, a cap and a 12 mm trocar. Thus, we decided to use AWPR during RAIL to seal the first incision, where the camera port was placed. Due to the aforementioned benefits, we hypothesized that AWPR could reduce the gas leakage and help in the working space creation. In the current study, we noticed a decrease of operative time after the introduction of AWPR during RAIL, when compared with our historic simple RAIL cohort. We reported in fact a median operative time for RAIL with AWPR of 225 minutes (IQR 198-243), which appeared lower than that observed in simple RAIL (279; 210-300 min). Despite it is difficult to definitively assess a variable associated with the observed operative time decrease, it is noteworthy that an easier trocar positioning and a sensible reduction of gas leakage was recorded. Unfortunately, no statistical significance could be tested because of the small population size. Moreover, we could not use a specific and reliable method to define an accurate difference for gas leakage if not the surgeon perception, the absence of gas fluctuation and the observation of an adequate stable working space with avoiding continuous adjustment of camera port. In consequence, despite our promising results, future analyses that directly compare the two surgical techniques are required to test the superiority of our novel camera port placement. The AWPR use did not affect the safety of our approach. Nor open conversions neither major intraoperative complications were reported, and blood loss was clinically not significant. Hospital stay was similar with that previously reported for our historic simple RAIL cohort and with those published in literature [8]. Despite the absence of intraoperative complications, two patients reported minor postoperative complications. In this series one skin necrosis, which did not necessitate of any surgical treatment, and one wound infection, which needed antibiotic treatment, were reported. None of these complications was specifically related to AWPR use. A low number of major complications, short hospital stay and fast recovery, due to minimally invasive techniques, have been described to allow a precocious start of adjuvant therapies when needed [22]. In the current study, all patients with nodal invasion received adjuvant treatment within three months after RAIL. In particular, patients with Merkel cell carcinoma underwent adjuvant radiation therapy on the field of surgery, while patients harboring melanoma underwent systemic adjuvant treatments. All patients presented a good performance status at the start of the adjuvant treatment delivery and could complete the whole cycle of treatment proposed. Last, nodal yield after ILND is routinely used as surrogate for oncological adequacy. Even in the absence of an accepted cut-off, according to the current literature at least 6-8 nodes on each side should be removed [15,22,23]. In the current series, median lymph nodes yield for each groin was 9 (7-17), confirming oncologic adequacy. Despite the novelty introduced, we have to acknowledge some limitations in the current study. First, the study is limited by the number of patients enrolled. In consequence, our results should be considered exploratory and should be confirmed by larger series. Second, direct comparisons with other surgical series cannot be derived. Third, this study aims to report surgical feasibility and non-inferiority for pathological results after the novel port placement application. Therefore, oncological outcomes should be tested in future lager cohorts. Last, this series is based on the application of a specific wound retractor. In consequence, it is possible that other wound retractors could be used for the purpose and be compared.
Conclusions
RAIL with the use of AWPR confirmed adequate oncologic outcomes in terms of lymph node yield, no major intra- and postoperative complications, and short hospitalization. AWPR also reduced gas leakage and allowed fast intraoperative removal of the specimen for frozen pathology if needed. Finally, a trend to a shorter operative time than our historical RAIL was observed. However, a larger cohort is necessary to support our preliminary findings.
Acknowledgments
In memory of a beloved friend and colleague Dr. Gabriele Cozzi.
This work was partially supported by the Italian Ministry of Health with Ricerca Corrente and 5x1000 funds.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
Authors declare that they have no conflict of interest.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Cozzi G, Musi G, Ferro M, et al. Robot-assisted inguinal lymphadenectomy: preliminary experience and perioperative outcomes from an Italian referral center. Ther Adv Urol 12 (2020):1756287220913386.
- Francone E, Reina S, Spagnolo F, et al. Combined robotic inguinal and iliac-obturator lymphadenectomy for stage III skin cancers: Surgical technique and preliminary results. Int J Med Robot 18 (2022): e2391.
- Hyde GA, Jung NL, Valle AA, et al. Robotic inguinal lymph node dissection for melanoma: a novel approach to a complicated problem. J Robot Surg 12 (2018): 745-748.
- Gupta MK, Patel AP, Master VA. Technical considerations to minimize complications of inguinal lymph node dissection. Transl Androl Urol 6 (2017): 820-825.
- Trias M, Targarona EM, Piulachs J, et al. Extraperitoneal laparoscopically assisted ilioinguinal lymphadenectomy for treatment of malignant melanoma. Arch Surg 133 (1998): 272-274.
- Ji A, Lyu J, Bai Y, et al. Single-position robot-assisted versus laparoscopic antegrade bilateral inguinal lymphadenectomy for penile cancer: A retrospective controlled study. Asian J Surg 45 (2022): 1530-1534.
- Singh A, Jaipuria J, Goel A, et al. Comparing Outcomes of Robotic and Open Inguinal Lymph Node Dissection in Patients with Carcinoma of the Penis. J Urol 199 (2018): 1518-1525.
- Gkegkes ID, Minis EE, Iavazzo C. Robotic-assisted inguinal lymphadenectomy: A systematic review. J Robot Surg 13 (2019): 1-8.
- Sotelo R, Cabrera M, Carmona O, et al. Robotic bilateral inguinal lymphadenectomy in penile cancer, development of a technique without robot repositioning: a case report. Ecancermedicalscience 7 (2013): 356.
- Zhang SH, Sood AK, Sorosky JI, et al. Preservation of the saphenous vein during inguinal lymphadenectomy decreases morbidity in patients with carcinoma of the vulva. Cancer 89 (2000): 1520-1525.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40 (1987): 373-383.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240 (2004): 205-213.
- Kandasamy SG, Chandran KR, Pooleri GK. Minimal invasive approaches in lymph node management of carcinoma of penis: A review. Indian J Urol 38 (2022): 15-21.
- Rodrigues GJ, Guglielmetti GB, Orvieto M, et al. Robot-assisted endoscopic inguinal lymphadenectomy: A review of current outcomes. Asian J Urol 8 (2021): 20-26.
- Elsamra SE, Poch MA. Robotic inguinal lymphadenectomy for penile cancer: the why, how, and what. Transl Androl Urol 6 (2017): 826-832.
- Abdullatif VA, Davis J, Cavayero C, et al. Single-port robotic inguinal lymph node dissection for penile cancer. Urology 161 (2022): 153-156.
- Josephson DY, Jacobsohn KM, Link BA, et al. Robotic-assisted endoscopic inguinal lymphadenectomy. Urology 73 (2009): 167-170.
- Tamhankar AS, Ojha SP, Ahluwalia P, et al. Technical caveats in robot assisted video endoscopic inguinal lymph node dissection - evolution of a modified technique. Int Braz J Urol 47 (2021): 216-217.
- Patel AS, Isharwal S. Single-port robotic inguinal lymph node dissection: A safe and feasible option for penile cancer. Surg Oncol 38 (2021): 101633.
- Fankhauser CD, Lee EWC, Issa A, et al. Saphenous-sparing ascending video endoscopic inguinal lymph node dissection using a leg approach: surgical technique and perioperative and pathological outcomes. Eur Urol Open Sci 35 (2022): 9-13.
- Almeida GL, Musi G, Mazzoleni F, et al. Intraoperative frozen pathology during robot-assisted laparoscopic radical prostatectomy: Can ALEXIS trocar make it easy and fast? J Endourol 27 (2013): 1213-1217.
- Nabavizadeh R, Petrinec B, Necchi A, et al. Utility of minimally invasive technology for inguinal lymph node dissection in penile cancer. J Clin Med 9 (2020): 2501
- Rossi CR, Mozzillo N, Maurichi A, et al. Number of excised lymph nodes as a quality assurance measure for lymphadenectomy in melanoma. JAMA Surg 149 (2014): 700-706.


 Impact Factor: * 4.2
Impact Factor: * 4.2 Acceptance Rate: 72.62%
Acceptance Rate: 72.62%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 