Oncoplastic Breast Conservation made Possible with Precision Marker for Expansive Disease - A Case Study
Sonia Y Khan1,2, Karla Daniele1,2, Casey Cook2,3, Rakhshanda Layeequr Rahman1,2*
1Department of Surgery, Texas Tech University Health Sciences Center, Lubbock, TX, 79430 USA.
2Breast Center of Excellence School of Medicine, Texas Tech University Health Sciences Center, Lubbock, TX, 79430 USA.
3Department of Radiology, Texas Tech University Health Sciences Center, Lubbock, TX, 79430 USA.
*Corresponding Author: Rakhshanda Layeequr Rahman, Department of Surgery, Texas Tech University Health Sciences Center, Lubbock, TX, 79430 USA.
Received: 15 February 2023; Accepted: 27 February 2023; Published: 13 March 2023
Article Information
Citation: Sonia Y Khan, Karla Daniele, Casey Cook, Rakhshanda Layeequr Rahman. Oncoplastic Breast Conservation Made Possible with Precision Marker for Expansive Disease - A Case Study. Journal of Surgery and Research 6 (2023): 90-93.
View / Download Pdf Share at FacebookKeywords
<p>Breast conservation, Expansive disease, Cancers</p>
Article Details
Introduction
Expansive disease associated with widespread microcalcifications often renders breast conservation difficult if at all possible. This is particularly problematic when lesions are not sonographically visible. Bracketing technique with wires has been utilized to optimize resection volume [1], albeit obtaining negative margins continues to pose a challenge [2,3]. Non-wire markers including radioactive, radar, magnetic, and radiofrequency localization seeds have offered the logistic advantage of avoiding same-day localization for surgery [4,5]. Use of multiple radioactive seeds to bracket the expansive lesions has been recently reported [6]. However, the use of radioactive seeds mandates robust protocol for seed tracking from the time of insertion up to retrieval and appropriate disposal during specimen handling and pathological evaluation [7]. Damage to the seed risks radiation exposure for the staff; though the radiation dose is reportedly acceptably low from a single seed, multiple seeds could potentially pose a problem [8]. We report a case where magnetic occult lesion localization instrument (MOLLI) precision markers were used to guide the extent of disease for oncoplastic breast conservation in a patient with extensive ductal carcinoma in situ with small breast volume.
Materials and Methods
Case
A 62-year-old gravida 3 para 3 post-menopausal female was evaluated in 2022 after an abnormal screening mammogram. Initial mammography showed right-sided extensive microcalcifications with a focal asymmetry extending from the 7 -10 o’ clock position located 7 cm from the nipple. The span of suspicious findings measured 7 x 7 cm of lateral breast parenchyma from the lateral edge of the breast to almost at the areolar border. Stereotactic biopsy revealed ductal carcinoma in situ (DCIS) with solid, cribriform, and micropapillary patterns. Given the extent of the disease, mastectomy was deemed the most reasonable option by her surgeon. The patient refused mastectomy and declined any treatment without the ability to save her breast. She was seen for a second opinion; at this point, an extensive review of her imaging on a high-resolution system was conducted to delineate the exact extent for potential breast conservation. The patient had no pertinent medical or surgical history, no prior history of cancers, no history of substance abuse, no history of radiation, and no history of hormone replacement therapy. She did have family history significant for breast cancer diagnosed in her sister in her early 40s. Her genetic workup was negative for any known mutation.
Multidisciplinary Evaluation and Localization
Patient imaging studies were jointly reviewed in detail by the radiologist and surgeon on a high-resolution imaging system to exactly identify the extent of suspicious calcifications and plan the extent of resection. The decision was made to mark the superomedial extent and inferolateral extent of the disease in order to assist with the limits of resection of the mid-lateral quadrant while utilizing the reduction mammoplasty approach. Given this extent, a modified approach was planned to save the parenchyma just above the inframammary fold (which is typically resected in reduction mammoplasty) and utilize it as a local flap to fill in the defect created by the superior extent of the disease up to 10 ‘clock position in the breast, which needed to be resected (which is not the case in routine reduction). Hence the two precision markers were placed at the superomedial and inferolateral extent couple of days before surgery (Figure 1A and B). In addition, given the risk of invasive disease with extensive calcification,9 and the potential for disruption of lymphatic drainage with a reduction approach,10 a sentinel node biopsy was planned at the same time.
Surgical Resection
On the morning of surgery, a Wise pattern incision was marked on the patient bilaterally. The contralateral symmetry procedure was done with the usual resection of inferolateral and inferomedial parenchymal flaps along with skin with the relocation of the nipple-areolar complex on the inferior pedicle to correct ptosis. Similar skin incisions were planned on the cancer side, however, once the lateral flap was raised similar to the mastectomy approach, an intraoperative MOLLI system wand was used to identify the distance from the markers to ensure adequate margins. At 2 cm from the inferolateral marker, a cautery artifact was created in the parenchyma to mark the inferior and lateral extent of the resection; a similar artifact was created at the superior and medial extent. The en bloc resection was then achieved and a specimen radiograph was obtained to confirm the adequacy of excision (Figure 2A and B). The tumor bed was marked with clips before any further dissection. The nipple-areolar complex was raised on the inferior pedicle albeit care was taken for the pedicle to be medial to the resection mark. The parenchyma saved just superior to the inframammary fold up to the inferior extent planned and guided by the marker was utilized when the superior skin flaps were wrapped around the pedicle. Given the extent of the disease and suspicion for an upgrade to invasive cancer, a sentinel biopsy was also done. Closure was achieved in layers. Postoperative cosmetic outcome is depicted in figure 3.
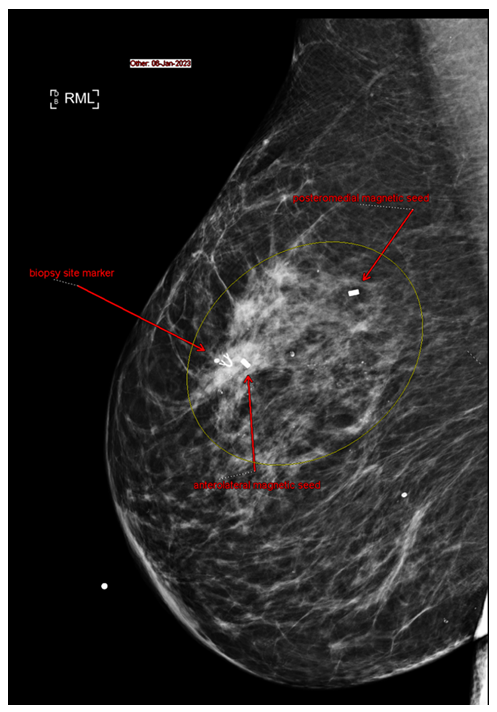
Figure 1A: Mediolateral view of right breast.
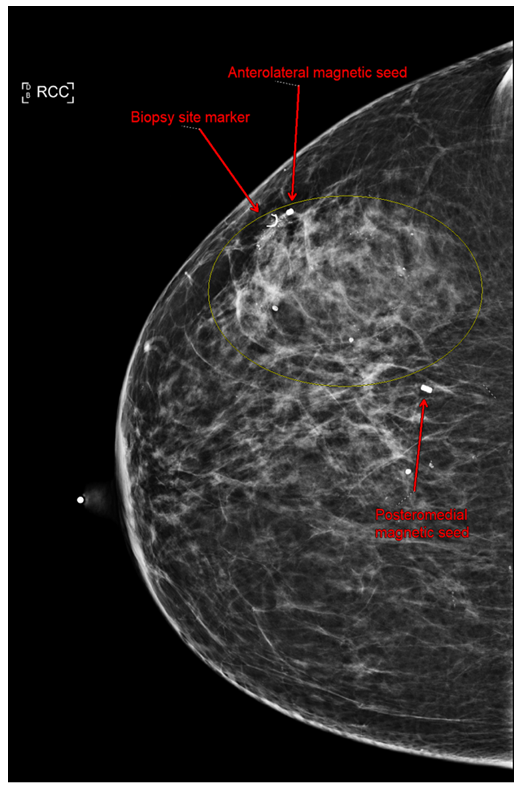
Figure 1B: Craniocaudal view of right breast. Anterolateral magnetic and mediolateral magnetic seeds were placed at tumor borders to guide excision.
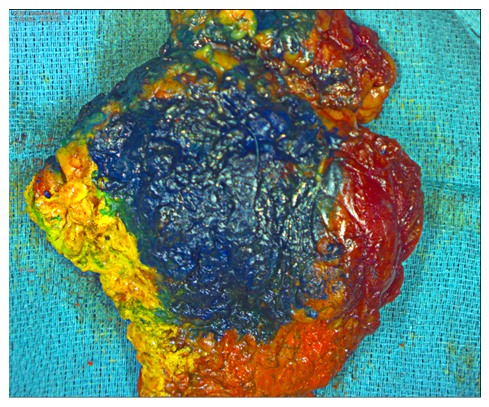
Figure 2A: Painted gross specimen. Red = medial, yellow = lateral, blue = anterior, black = posterior, green = superior, orange = inferior.
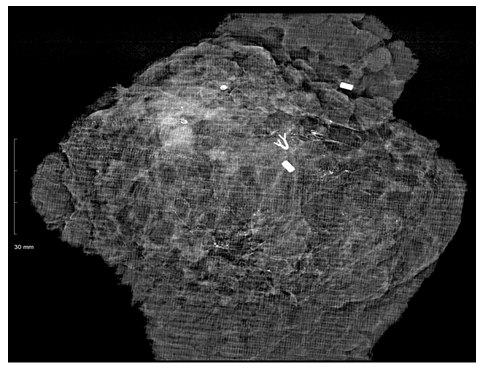
Figure 2B: Resected specimen radiograph. Both the anterolateral and posteromedial clips are present. Tumor within the clip borders with >2cm margins. Biopsy clip present.
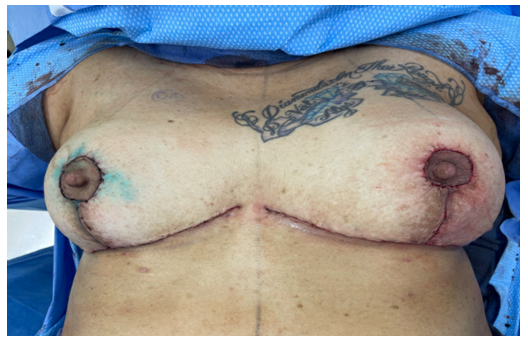
Figure 3:Postop image of bilateral breasts. Blue dye on right breast from sentinel lymph node biopsy.
Pathology
Routine H&E was performed on the resected specimen. The final pathology revealed extensive DCIS spanning 7 cm with four foci of microinvasion. of DCIS and in one of two positive nodes (1.2 cm focus). All margins were > 2 mm from the closest tumor.
Discussion
Optimal wide local excision involves resecting as little healthy tissue as possible while still achieving tumor-free margins. Smaller excisional volumes also lead to improved cosmetic outcomes [11-13]. This necessitates precise knowledge of tumor borders, which can be particularly difficult in tumors with extensive microcalcifications. While the use of sonography in the OR can be advantageous in guiding the excision of solid tumors, it is not a reliable tool to determine the extent of microcalcifications, which mandates the use of localization devices for an accurate demarcation of tumor borders. In addition to providing an accurate assessment of tumor location, localization devices shorten the surgical time for resection [14]. However, not all localizing techniques are created equal. While wire localization can be cost effective and efficient, it needs to be placed on the day of the surgery and is still prone to migration, fractures, and retention [15]. This can lead to excessive excision to ensure tumor-free margins. Failed localization and marker dislodging are less likely with the use of magnetic seeds than wire [16]. Wire markers also impact the choice of incision, potentially compromising the surgical approach and liberty of closure [17]. Since ferromagnetic markers are implanted within the patient, this allows for greater freedom in designing an oncoplastic surgical procedure. In this case, bracketing the superomedial and inferolateral borders of the tumor with MOLLI allowed for successful resection of the tumor and associated suspicious microcalcifications without removal of excess healthy tissue. The most important aspect was the ability to save as much normal tissue as possible to allow for local rotation flaps to achieve an excellent cosmetic outcome. A reduction of the contralateral breast allowed for improved cosmetic outcome and patient satisfaction.
This is a single patient report of someone who was refusing treatment if breast conservation was not an option. However, this is a case in point for determining the accurate extent of disease so minimal resection of healthy tissue and maximal utilization of oncoplastic techniques can offer an ontologically feasible treatment option with excellent patient satisfaction. Successful breast conservation in future patients with multifocal breast cancer may increase the validity of using precision marker-guided bracketing technique. The use of ferromagnetic markers for bracketing also offers an option where radioactivity dose concerns are not a factor. Future cost analysis of wire vs magnetic seed localization may help patients and their surgeons determine which modality they prefer.
Acknowledgments
We would like to thank the patient for allowing us to share this important data. We would also like to thank the healthcare staff for helping us with patient care.
References
- Civil YA, Duvivier KM, Perin P, et al. Optimization of Wire-guided Technique With Bracketing Reduces Resection Volumes in Breast-conserving Surgery for Early Breast Cancer. Clin Breast Cancer 20 (2020): e749-e756.
- Layeequr Rahman R, Puckett Y, Habrawi Z, et al. A decade of intraoperative ultrasound guided breast conservation for margin negative resection - Radioactive, and magnetic, and Infrared Oh My. Am J Surg 220 (2020): 1410-1416.
- Lyons W. Bracketed Localization in Breast-Conserving Surgery: Indications and Success Rates From a Single, High Volume, Academic Breast Cancer Center. Am Surg (2022).
- Cheang E, Ha R, Thornton CM, et al. Innovations in image-guided preoperative breast lesion localization. Br J Radiol 91 (2018): 20170740.
- Jeffries DO, Dossett LA, Jorns JM. Localization for Breast Surgery: The Next Generation. Arch Pathol Lab Med 141 (2017): 1324-1329.
- Guirguis MS. Bracketing with Multiple Radioactive Seeds to Achieve Negative Margins in Breast Conservation Surgery: Multiple Seeds in Breast Surgery. Clin Breast Cancer 22 (2022): e158-e166.
- Graham RP, Jakub JW, Brunette JJ, et al. Handling of radioactive seed localization breast specimens in the pathology laboratory. Am J Surg Pathol 36 (2012): 1718-1723.
- Dauer LT. Radioactive seed localization with 125I for nonpalpable lesions prior to breast lumpectomy and/or excisional biopsy: methodology, safety, and experience of initial year. Health Phys 105 (2013): 356-365.
- Guillot E, Vaysse C, Goetgeluck J, et al. Extensive pure ductal carcinoma in situ of the breast: identification of predictors of associated infiltrating carcinoma and lymph node metastasis before immediate reconstructive surgery. Breast 23 (2014): 97-103.
- Martin TA, Choudhry S, Holton LH, et al. Is Sentinel Lymph Node Biopsy Reliable After Recent Oncoplastic Breast Reduction? Am Surg (2021).
- Vos EL. Preoperative prediction of cosmetic results in breast conserving surgery. J Surg Oncol 111 (2015): 178-184.
- Haloua MH. Intraoperative Ultrasound Guidance in Breast-Conserving Surgery Improves Cosmetic Outcomes and Patient Satisfaction: Results of a Multicenter Randomized Controlled Trial (COBALT). Ann Surg Oncol 23 (2016): 30-37.
- Cochrane RA, Valasiadou P, Wilson AR, et al. Cosmesis and satisfaction after breast-conserving surgery correlates with the percentage of breast volume excised. Br J Surg 90 (2003): 1505-1509.
- Srour MK, Kim S, Amersi F, et al. Comparison of wire localization, radioactive seed, and Savi scout((R)) radar for management of surgical breast disease. Breast J 26 (2020): 406-413.
- Tardioli S. Wire-guided Localization in Non-palpable Breast Cancer: Results from Monocentric Experience. Anticancer Res 36 (2016): 2423-2427.
- Dave RV. Wire- and magnetic-seed-guided localization of impalpable breast lesions: iBRA-NET localisation study. Br J Surg 109 (2022): 274-282.
- Lovrics PJ. A multicentered, randomized, controlled trial comparing radioguided seed localization to standard wire localization for nonpalpable, invasive and in situ breast carcinomas. Ann Surg Oncol 18 (2011): 3407-3414.


 Impact Factor: * 4.2
Impact Factor: * 4.2 Acceptance Rate: 72.62%
Acceptance Rate: 72.62%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 