Oesophageal Atresia: Clinical Outcome After Surgical Treatment
Melanie Kapapaa*, Daniela Webera, Alexandre Serraa
Division of Paediatric Surgery, Department of Surgery, University Medical Centre Ulm, Eythstrasse 24, 89075 Ulm, Germany
*Corresponding Author: Melanie Kapapa, Division of Paediatric Surgery, Department of Surgery, University Medical Centre Ulm, Eythstrasse 24, 89075 Ulm, Germany
Received: 02 October 2023; Accepted: 12 October 2023; Published: 06 December 2023
Article Information
Citation: Melanie Kapapa, Daniela Weber, Alexandre Serra. Oesophageal atresia: Clinical outcome after surgical treatment. Journal of Surgery and Research. 6 (2023): 401-410.
View / Download Pdf Share at FacebookAbstract
Background: To evaluate the clinical outcome of oesophageal atresia (OA) after surgical treatment we performed a retrospective study with a controlled observational design.
Materials and Methods: Data were collected from hospital records, questionnaires and additional interviews with families for all OA children treated at our hospital between 2004 and 2015.
Results: Due to a higher incidence of preterm births, OA children showed lower birth weight (p<0.001), worse post-natal adaption (p<0.001) and frequently higher incidence of concomitant diseases (p<0.001) and malformations (p=0.005) like VACTERL (p=0.006) in comparison with normal controls. The surgical correction was performed primarily in 77.4% of the patients, while 9.7% were submitted to a secondary anastomosis. A contrast oesophagogram was performed routinely in most patients, showing an anastomotic leakage in 26.3% of those (more often in preterm, p=0.044) unrelated to moderate or severe documented anastomotic tension (p=0.071). An association with the use of chest drains and trans- anastomotic gastric tubes on postsurgical oesophageal stenosis could not be excluded. As for the clinical outcome, there was a gastroesophageal reflux in 78.9% and stenosis in 73.3% of the patients, leading to feeding problems and the necessity of multiple dilations and gastric acid suppression, respectively.
Conclusion: These clinical outcomes are difficult to compare due to the lack of national- and international consensus in the management of OA children. The restructuring of medical training and the practical implementation of the distribution of various congenital malformations in the sense of priority care to selected centres in the state is decisive for future-oriented paediatric surgical care.
Keywords
<p>Oesophageal atresia, Anastomoses, Surgery, Stenosis, Outcome</p>
Article Details
Keymessage
Type of Vogt classification, concomitant diseases and malformations have influence on timing of surgical treatment and technique. Clinical course and complications depend on these factors and significantly influence the clinical outcome. Pooling of OA cases in expert centres could promote expertise, outcome comparability and optimise perioperative handling.
Graphical abstract
The first figure describes the internal postoperative treatment after OA correction, based on the classification of our collective according to the modified Vogt classification in figure 2. This is followed by the 3rd figure with the subdivision into the different surgical techniques used.
Introduction
Oesophageal atresia (OA) is characterized by an interrupted oesophageal continuity during the development of the embryo, which presents in 5 major forms. According to the Vogt classification, type IIIb is most common [1]. Other congenital anomalies like a VACTERL association are present in up to 60% of OA cases [2], and surgery is needed to restore oesophageal continuity including a trachea-oesophageal fistula closure (if present) so that oral feedings can be established. Pre-operative complications such as aspiration and infections must be minimized to improve the survival rate and clinical outcome [3]. An optimal preoperative preparation includes oral food restriction, "slurping probe" in the upper blind sac to remove saliva, elevating the upper body and antibiotic prophylaxis [4,5]. Additionally, for an optimal surgical planning the oesophageal gap length must be verified preoperatively. Only with a proper measurement of the gap length (i.e. whether a short (<3cm) and long gap (>3cm) atresia is present) can the decision for a primary or secondary anastomosis be made. This decision also depends on other factors like concomitant malformations. In terms of the technique employed for the procedure, the choice between "open vs. laparoscopic" depends on the training of the surgical team and the hospital’s availability of technical equipment [6]. Complications specific to this malformation include stenoses in 22-78% of the patients, an anastomotic leakage or insufficiency in 3% - 8% and in recurrent fistulas [7]. The morbidity and the preferred surgical methods also vary in accordance to the OA types [8]. In regards to long-term complications, these include oesophageal motility disorder [9], gastro-oesophageal reflux [10], tracheomalacia [11] and the onset of thoracic deformities [12,13]. Therefore, a mmultidisciplinary follow-up care with the participation of all relevant departments is recommended in terms of quality of care [14,15]. For this study, we aimed to analyse our own experience with the surgical management of OA patients focusing on 1) Postnatal aspects, type of Vogt classification and concomitant diseases and/or malformations 2) Timing of surgical treatment and technique, 3) Clinical course and complications, 4) Clinical outcome, using a retrospective, observational controlled-design study.
Methods
Design
This study was designed as a retrospective, observational examination enrolling children with OA who were managed surgically and/or postoperatively at the University of Ulm from January 1st, 2004 to June 30th, 2015. The local ethics committee of the University Medical Centre approved this study (No. 431/15). Information was obtained through internal hospital files and records. Incomplete or additional data were obtained using questionnaires and interviews of the families (Figure 1).
Inclusion criteria
A total of 39 families with children after OA correction (19 in-house and 12 externally-operated children) were cared for during the study period in the outpatient clinic for congenital malformations of the University of Ulm. For comparison purposes a control group (CG) was recruited from age-matched children to OA group (age 0-11 years) without OA or any other congenital malformations, who were treated for other diseases in the paediatric surgery ward (n=30).
Exclusion criteria
OA children who were born outside the study period or had non-atresia-related oesophageal surgeries or battery ingestion (n=12) and those patients with incomplete data (n=1) were excluded from the study.
Mortality
Additional children were excluded due to the mortality of OA infants, which was in total 20.0% (n=8) and therefrom 50% died prior surgery due to severe concomitant malformations and/ or chromosomal abnormalities (n=4). Four children were initially surgically treated (n=4; 10.3%). Cause of death were: trisomy 18 with respiratory insufficiency (14th day of life), Fanconi anemia (with 10 years) and in the case of two children, no documentation on the reasons for death could be obtained (5th and 20th month of life)
Patients and surveyed subjects
The International Classification of Diseases (ICD-9 and ICD- 10) was used to identify all children with the diagnostic codes Q39.0-Q39.9 for congenital malformations of the oesophagus (n=52).
Data collection
Data on postnatal evolution, Vogt classification, concomitant diseases and malformations, timing of surgical treatment and technique, clinical course, complications and clinical outcome were collected by reviewing hospital files, surgery reports, radiological findings as well as via questionnaires and interviews with the families. (Figure 1)
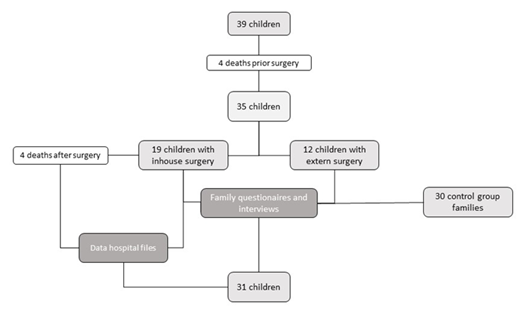
Figure 1: In-house follow-up treatment after OA correction
Statistics
The obtained data were primarily analyzed using descriptive methods, in which Mean, standard deviation, median and range were reported for the quantitative parameters, while absolute and relative frequencies were generated for qualitative parameters. These parameters formed the basis for exploratory tests between subsets. Due to the specific size of the subgroups, t-tests and Fisher's Exact Test were used. The Mann-Whitney-U test was used to calculate differences in ordinally scaled data. Metrically scaled data were tested for correlations using Spearman's correlation. The calculations were based on a confidence interval of 95% and the significance was set at p ≤ 0.05. All statistical tests were performed using IBM SPSS software, version 26 (IBM, Illinois, USA).
Results
Postnatal aspects, Vogt classification and concomitant diseases/ malformations
In 75% of the cases the OA diagnosis was unexpected and occurred postnatally, even though a typical polyhydramnios was significantly more often diagnosed (p<0.001) in OA mothers than in the CG group. Birth occurred significantly earlier in OA children, with a mean gestational age at birth of 35.5 weeks (range 25-40) compared to CG children with 38.8 weeks (range 30-40; p<0.001); consequently, there were significantly more preterm OA than CG children (54.8% (<37th pregnancy week; p<0.001)) which require lung maturation prophylaxis in 35.5% of the cases (p=0.003). (Table 1)
|
Risk factors |
OA (n; %) |
CG (n=30; %) |
p-value |
|
Abnormalities during pregnancy |
29 (31; 93.5%) |
11 (36.7%) |
<0.001 |
|
Polyhydramnios |
20 (31; 64.5%) |
0 (0%) |
<0.001 |
|
OA diagnosed (n; %; pregnancy week) |
5 (20; 25%) (range 20-35) |
0 (0%) |
<0.001 |
|
Gestational age (pregnancy week) |
35.52 (range 25-40) |
38.80 (range 30-40) |
<0.001 |
|
<37th pregnancy week |
17 (54.8%) |
3 (10%) |
<0.001 |
|
Lung alteration medication |
11 (35.5%) |
1 (3.3%) |
0.003 |
Table 1: Specialized family questionnaires for OA and CG parents
Birth weight and body length of OA children were with a mean of 2.247g (range 795-3.410g) and 46.3cm (range 34-53cm) significantly lower than 3.311g (range 1.660-4.500; p<0.001) and 50.8cm (range 39.5-58cm; p<0.001) in CG children; hence 25.8% of OA children were hypotrophic (<10th percentile; n=8) compared to 16.7% in CG (n=5; p=0.534). The APGAR scores of OA children between 1st and 10th minute of life were significantly lower compared to CG children (p<0.001). Concomitant diseases were significantly more often in OA (n=25; 80.6%) compared to CG children (n=2; 6.6%, p<0.001) as well as concomitant malformations (n=24; 77.4% vs. n=4, 13.3%, p=0.005). A VACTERL association was diagnosed in 22.6% of OA (n=7) in contrast to 0% in CG children (p=0.006). (Table 2)
|
Factor |
OA group (n=31; range) |
CG Group (n=30; range) |
p-value |
|
Birth weight (g) |
2.247 (795-3.410) |
3.311 (1.660-4.500) |
<0.001 |
|
Birth length (cm) |
46.3 (34-53) |
50.8 (39.5-58) |
<0.001 |
|
Hypotrophy (<10. percentile) (n) |
8 children (25.8%) |
5 children (16.7%) |
0.534 |
|
APGAR |
average (range) |
average (range) |
|
|
· 1st minute of life |
7.0 (1-10) |
>8 (8-10) |
<0.001 |
|
· 5th minute of life |
8.5 (5-10) |
>8 (8-10) |
<0.001 |
|
· 10th minute of life |
9.5 (7-10) |
>8 (8-10) |
<0.001 |
|
Concomitant diseases (n) |
25 (80.6%) |
2 (6.6%) |
<0.001 |
|
Concomitant malformations (n) |
24 (77.4%) |
4 (13.3%) |
0.005 |
|
· VACTERL |
7 (22.6%) |
0 (0%) |
0.006 |
Table 2: Physical children´s data, APGAR, concomitant diseases and malformations
Within our cohort, the proportion according to Vogt classification was 0% (type I); 3.2% (type II); 6.5 % (type IIIa); 67.7% (type IIIb); 9.7% (type IIIc) and 12.9% (type IV), respectively (Figure 2).
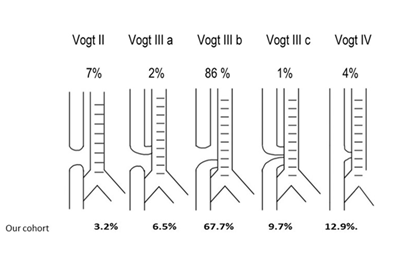
Figure 2: Vogt classification modified [43].
Timing of the surgical treatment and technique
An isolated fistula closure was performed in all type IV OA (n=4; 12.9%), a primary anastomosis was performed in 77.4% (n=24), and a secondary anastomosis was necessary in 3 children (9.7%), diagnosed with “long gap” OA (distance >3cm) and/ or additional malformations. Isolated fistula closures and primary anastomoses were performed on the 2nd day of life (mean 4.47; range 1st - 32nd day of life). In 3 children submitted to a two-stage procedure, the secondary anastomosis took place on the 23rd, 77th day of life and with 5 months of age (Figure 3).
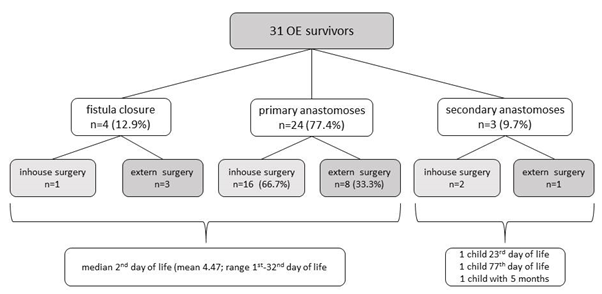
Figure 3: Surgical techniques
Clinical course and complications
Isolated fistula closures: No chest tube was required intraoperatively, the children were extubated directly after the surgical intervention and spent one day on paediatric ICU for monitoring. Antibiotic-therapy was carried out for 6 days, oral feedings began on the 6th postoperative day on average and the intraoperatively placed transanastomotic tube was removed after 14 days as planned without any radiological signs of complications (Table 3).
|
Factor |
Fistula closure (n=1) |
Primary anastomoses (n=16) |
Secondary anastomoses (n=2) |
|
Chest tube (days; range) |
no (0) |
yes (18; range 1-47) |
|
|
Intubation duration (days; range) |
0 |
12.1 (range 1-55) |
4 & tracheotomy |
|
Additional or revision surgeries |
0 (0%) |
4 (25%) |
2 (100%) |
|
Neonatal ICU stay (duration) |
1 day |
31.9 days (range 2-102) |
26 & 43 days |
|
Gastric tube (duration) |
yes, 14 days |
yes, 18.9 (range 6-67) |
yes, 14 days |
|
Routine Oesophagogram before gastric tube removal (multiple answers possible) |
|||
|
Routine Oesophagogram performed |
yes |
yes |
yes |
|
1 Anomalies |
none |
7 (43.8%) |
1 (50%) |
|
2 Leakage |
none |
1 (6.3%) |
0 (0%) |
|
3 Insufficiency |
none |
1 (6.3%) |
0(0%) |
|
4 Stenosis |
none |
5 (26.3%) |
1 (50%) |
|
5 Diverticulum |
none |
1 (6.3%) |
1 (50%) |
|
Antibiotic therapy (day, range) |
6 |
5.1 (1-12 days) |
14. &36. day |
|
Begin of enteral feeding (day, range) |
2 |
9.8 (1-51 days) |
8. & 14. day |
|
Begin oral nutrition (day, range) |
6 |
24.7 (2-68 days) |
24. & 71. day |
|
Discharge home (day, range) |
20 |
57.6 (13-134) day |
26. & 97. Day |
Table 3: Postsurgical outcome in OA children after surgical inhouse treatment (n=19)
Primary anastomosis: chest drains were required in 73.7% (n=14) of the patients and were removed after an average of 9.2 days (range 1-47 days). Mechanical ventilation was necessary on average 12.1 days (range 1-55 days). The average length of stay on the paediatric ICU was 31.9 days (range 1-102 days). Intraoperative trans- anastomotic tube insertion was performed as a splint and to start enteral nourishing at an early stage, it remained on average for 18.9 days (range 6-67 days). Before the final removal of the transanastomotic tube, a contrast oesophagogram was performed and in 7 children abnormalities like an anastomotic stenosis (n=5), a fistula (n=1) or a diverticulum (n=1) were documented. The average duration of antibiotic therapy was 5.1 days (range 1-12 days). The enteral nutrition via gastric tube or gastrostomy started on average after 9.8 days (range 1-51 days), while oral feedings were initiated on average after 24.7 days (range 2-68 days). We observed that, as expected, early enteral feedings led to a significantly shorter hospital stay (p=0.014). OA Children were discharged on average after 57.6 days (range 13-134 days), of which 58.3% were had exclusive oral feedings, while 41.6% needed additional nutritional support such as gastric tube feeding (n=5). A transfer to a hospital closer to the family’s home was possible in 36.8% of the cases (n=7) (Table 4).
(Table 3)
Secondary Anastomosis: One child with a Goldenhar-syndrome was subjected to an initial tracheostomy combined to gastrostomy shortly after birth. The surgical OA reconstruction was performed via gastric elevation on 77th day of life. This child remained in the ICU for further 26 days, oral feedings began after 24 days and the anastomotic tube remained until the 14th PO day. The second child received an end-to-end anastomosis after initial FOKER traction method on the 23rd day of life, the postoperative monitoring on paediatric ICU took 43 days and recurrent infections delayed the ending of the antibiotic therapy (36 days). Oral feedings had to be delayed until the 71st day and needed to be switched to a permanent gastric drip before discharge on the 97th day (Table 3).
Clinical Outcome
Mild to moderate tissue tension during suturing oesophageal anastomosis was documented in 31.6% (n=6), while in 26.3% (n=5) no tension was noted and in 42.1% no specific documentation of the tension was found (p=0.071). An anastomotic leakage was detected via routine contrast oesophagogram in 26.3% (n=5) of OA children, which was successfully treated with nutritional restriction leading to a spontaneous closure of the leakage in 4 children. Only one OA child underwent a surgical revision of the anastomosis, this child had a very low birth weight and was preterm of low gestational age (p=0.044). After discharge one child showed recurrent fistulas which needed to be surgical revised (Table 4).
Clinically suspected gastroesophageal reflux was diagnosed in 78.9% of OA children (n=15). Consequently, Proton-pump inhibitors (PPIs) were initiated in 73.7% (n=14). Respiratory complaints like barking cough (n=9; 56.3%), stridor (n=8; 50%) cyanosis and apnoea attacks (respectively n=3; 18.8%) were reported. The most frequently cited triggers of coughing were infections (n=14; 87.5%), exertion (n=8; 50%) and excitement (n=6; 37.5%).
Feeding problems were reported in 73.7% of OA children, of which a stenosis was confirmed in 14 and treated by bougienage or balloon dilatation (range 1-13 times), with an average age of 3.2 months (range 27 days - 9 months) and 2.4 times/year during 1st year of life in cooperation with the pediatric gastroenterologists. In severe cases an off-label local Mitomycin© application was carried out in 10.5% (n=2), after obtaining informed consent from the parents. In four OA children a suspected “bolus” event occurred and required an endoscopic removal of food scraps in 2 of them (10.5%). A tracheomalacia was documented in 31.6% (n=6), an anastomosis diverticulum in 26.3% (n=5) and a hiatal hernia in 15.8% (n=3), but surgically treatment like a fundoplication (n=1) and an aortopexy (n=1) were seldom necessary (Table 4).
|
Factor |
Measure/ Event |
OA inhouse follow-up (n=19) |
|
Anatomical equivalent |
Mild to moderate anastomotic tissue tension |
6 (31.6%) |
|
Anastomotic Leakage |
5 (26.3%) |
|
|
· conservative treatment |
4 (21.0%) |
|
|
· surgically treated |
1 (5.26%) |
|
|
Hiatal hernia |
3 (15.8%) |
|
|
· conservative treatment |
2 (10.5%) |
|
|
· surgically treated |
1 (5.26%) |
|
|
Diverticulum/ surgical treatment |
5 (26.3%)/ 0 (0%) |
|
|
Tracheomalacia/ surgical treatment (aortopexy) |
6 (31.6%)/ 1 (3.2%) |
|
|
Clinical relationship |
Nutrition at discharge/ transfer |
|
|
· orally fed |
8 (42.1%) |
|
|
· gastric tube/ button fed |
8 (42.1%) |
|
|
· additionally, parental nourishment |
3 (15.8%) |
|
|
Bolus event / endoscopic removal of food scraps |
4 (21.0%)/ 2 (10.5%) |
|
|
Oesophageal reflux |
15 (78.95%) |
|
|
· Respiratory complaints |
||
|
· barking cough |
9 (56.3%) |
|
|
· cyanosis |
3 (18.8%) |
|
|
· apnoea |
3 (18.8%) |
|
|
· stridor |
8 (50%) |
|
|
Triggers of cough |
||
|
· infections |
14 (87.5%) |
|
|
· exertion |
8 (50%) |
|
|
· excitement |
6 (37.5%) |
|
|
Clinical consequences |
Drug therapy for oesophageal reflux |
13 (68.42%) |
|
Oesophageal stenosis |
14 (73.7%) |
|
|
· bougienage (n; %; times) |
13 (68.42%; 1-13 times) |
|
|
· balloon dilatation (n; %; times) |
2; 10.5%; 4 & 5 times |
|
|
· off–label-Use Mitomycin© (n; %; times) |
2; 10.5%; 2 times |
Table 4: Clinical outcome in participants of inhouse follow-up
Additionally, a regression analysis showed no definitive risk factors for oesophageal stenoses and reflux medication. (Table 5).
|
Anastomotic stenosis |
Reflux therapy |
|||
|
Factor |
OR (95% CI) |
P value |
OR (95% CI) |
P value |
|
Preterm (≤37th pregnancy week) |
0.189 (-0.302-0.681) |
0.429 |
-0.64 (-0.643-0.516) |
0.821 |
|
VACTERL |
-0.042 (-0.396-0.313) |
0.813 |
-0.227 (-0.844-0.390) |
0.452 |
|
Long gap (>3cm) |
-0.225 (-0.528-0.078) |
0.136 |
0.404 (-0.210-1.017) |
0.179 |
|
Anastomotic leak |
0.250 (-0.362-0.862) |
0.378 |
0.150 (-0.687-0.987) |
0.685 |
|
Complication oral feeding |
0.187 (-0.321-0.695) |
0.45 |
0.114 (-0.001-0.230) |
0.052 |
Table 5: Linear regression OR (95% CI)
Discussion
Postnatal aspects, type of Vogt classification and concomitant diseases/ malformations
Our results demonstrate an association between prematurity, poor postnatal adaptation and frequent malformations/concomitant diseases which further emphasize the necessity of postnatal stabilisation and interdisciplinary consensus on the optimal time for surgery, as confirmed by Lejeune et al. and Thompson et al. [16,17]. The mortality among our patients was increased among premature babies with underweight in combination with a congenital OA malformation [18], and in patients with associated syndromes the survival rates are lower and did not progress over the last decades [19]. Additionally, we have also observed an increased incidence of VACTERL association in our cohort, which is known to be frequently concomitant to OA [20].
Timing of surgical treatment and technique
A correct preoperative classification of the malformation is fundamental for achieving a primary OA reconstruction (in our case a rate of 77.4%) and establishing an optimal timing of surgery. Similar data have been also discussed by Fuladi et al. [21] and the decision for secondary reconstruction must be made on an individual basis, in which the best interests of the child are central in order to achieve an optimal long-term outcome [22]. The possibility of an H-fistula/type IV Vogt should not be underestimated; hence the experience of interdisciplinary teams is crucial not to overlook mild symptoms [23].
Clinical course and complications
The routine insertion of a chest drain (CD) is widespread established and has been practised also in our collective, although its benefit has been controversial for years [24]. Therefore, an actual discussion among paediatric surgeons and paediatricians worldwide to dispense with the insertion of CD intraoperatively has gained momentum. Some studies vehemently argued against CDs, since there was no proven benefit for them [25] or CDs were associated with a higher number of oesophageal strictures [26]. Another controversial point in the perioperative management of OA is the routine insertion of a gastric tube (GT) to splint the oesophagus and allow early enteral feedings. A large survey of paediatric surgical centres/departments in Belgium and Luxembourg showed that all of them leave a transanastomotic tube in place and then use it to start the enteral feeding; additionally, in 86% of the cases GT were also routinely used for a postoperative contrast study, which is exactly what we did in our cohort [27]. In contrast, LaRusso et al. were able to show that a transanastomotic GT significantly increases the rate of oesophageal strictures and at the same time does not reduce the duration of parenteral nutrition. Therefore, they concluded that the GTs do more harm than good and are therefore unnecessary [28]. These different points of view result from years of experience and the outcomes of recent clinical studies and will not be easily reconciled. Similarly, routine esophagrams are widely used based on the assumption that leakages and insufficiencies can be clearly visualised and result in a prompt surgical intervention. However, studies by Golden et al. show that a routine esophagram is not necessary in asymptomatic patients, limiting this examination to symptomatic patients with clinical abnormalities or radiologically visible insufficiency in plain chest X-rays, as only in these cases a surgical consequence follows and in all other cases a minor leakage heals conservatively under oral food restriction [29]. We support these findings and have started to change our procedures accordingly.
In terms of postoperative antibiotic therapy, a worldwide survey of paediatric surgeons conducted by Lal et al. showed that there is no consensus on intra- or postoperatively antibiotic therapy and administration of acid-suppression medication [30]. The question of the management of nutrition after OA correction is also controversial. In a European study, Zani et al. showed that there is no consensus on the right time to start both enteral and oral nutrition [31]. All these reasons make it difficult to compare the individual studies with each other, as many individual parameters can vary. Furthermore, these results lead to difficulties in establishing a consensus in what concerns the therapy, which hopefully will be regulated by further international associations such as the European Reference Network ERNICA [32].
Clinical Outcome
Typical symptoms after surgical correction of OA are respiratory symptoms such as cough, cyanosis and apnoea attacks [33]. A barking cough may be typical of tracheomalacia [34], which we have also seen in our collective. However, the number of children affected by barking cough was higher than that of children with known tracheomalacia, suggesting surgical trauma to the airways as a possible mechanism for the symptoms.
The postoperative development of stenosis requiring bougienage or balloon dilatation is one of the most common problems after OA, seen in our study in 68.4% of the patients. This incidence remains variable in several studies [35] but different factors have been associated to the development of stenosis. In terms of anastomotic fistulas, Huang et al. showed that low preoperative albumin, low birth weight and long gap defects are risk factors fistulas, which by their turn may favour stenosis [36]. Vergouwe et al. showed that a proportion of children often develop recurrent stenosis after anastomotic leakage and the need for oesophageal dilation less than 1 month after OA repair, these data were seen also in our study [37]. In case all dilatation attempts fail, there is still the controversial option of intra-lesional steroid application and topical “off-label” use of mitomycin. The success of these procedures varies from "no effect" [38,39] up to a 67% success rate [40]. Unfortunately, there is also a wide range of individual decisions in the case of stricture management, which again reveals a lack of consensus [35]. Acid suppression therapy in the controversial discussion of OA management with reflux is no exception [41], since there are institutions that treat all OA children with acid suppression prophylactically while others (like us) treat only symptomatic patients. In cases of further progression of reflux disease, the decision to perform a fundoplication during childhood cannot be taken lightly, since fundoplication in OA children does not lead to success in terms of postoperative complaints such as dysphagia, gagging and bloating in contrast to those without OA [42].
Since there is so much controversy in the postoperative treatment of OA, it is important to coordinate interdisciplinary efforts, especially in the case of long gap atresia. To this effect, Oliver et al. postulate an elective delayed oesophageal dilation in these cases because it allows preservation of the native oesophagus combined with low periprocedural morbidity, leading to favourable functional outcomes in long-gap OA children [22].
As expected, the type of Vogt classification, concomitant diseases and/or malformations have an influence on both the timing of surgical treatment and the technique used. We were able to show that the clinical course and complications depend on these factors and significantly influence the clinical outcome. In our cohort we did not observe a striking difference in treatment outcome compared to other centres. Due to the rare occurrence of OA, most paediatric surgery centres treat an average of 2-5 children per year, with only a few exceeding this number [27]. Consequently, only a pooling of OA cases in expert centres could promote expertise and make outcomes comparable, as well as to optimise perioperative handling. However, this would require that trainees would need to rotate within multiple centres to safely learn comprehensive surgical skills during paediatric surgical training. Furthermore, the wide establishment of thoracoscopic surgical techniques may lead to the fact that many paediatric surgeons will no longer be able to operate on OA at all, as the few cases would then be treated predominantly by a special team per centre.
Conflicts of interest and source of funding
All authors declare that there are no conflicts of interest nor funding for this work.
Author contributions
MK organized the study, performed the clinical examinations and was a major contributor in writing the manuscript. DW contacted the parents, organized the database and analysed the data. AS supervised the project, performed clinical examinations and interpreted the data. All authors read and approved the final manuscript.
Acknowledgements
None
References
- Muensterer OJ, Berdon WE. From Vogt to Haight and Holt to now: the history of esophageal atresia over the last century. Pediatric radiology 45 (2015): 1230-1235.
- Solomon BD. VACTERL/VATER Association. Orphanet journal of rare diseases 6 (2011): 56.
- Sfeir R, Bonnard A, Khen-Dunlop N, et al. Esophageal atresia: data from a national cohort. Journal of pediatric surgery 48 (2013): 1664-1669.
- Holland AJ, Fitzgerald DA. Oesophageal atresia and tracheo-oesophageal fistula: current management strategies and complications. Paediatr Respir Rev 11 (2010): 100-106.
- Parolini F, Bulotta AL, Battaglia S, et al. Preoperative management of children with esophageal atresia: current perspectives. Pediatric Health Med Ther 8 (2017): 1-7.
- Morini F, Conforti A, Zani A, et al. Diagnostic workup of neonates with esophageal atresia: Results from the EUPSA esophageal atresia registry. Frontiers in pediatrics 8 (2020): 489.
- Teague WJ, Karpelowsky J. Surgical management of oesophageal atresia. Paediatr Respir Rev 19 (2016): 10-15.
- Dingemann C, Dietrich J, Zeidler J, et al. Early complications after esophageal atresia repair: analysis of a German health insurance database covering a population of 8 million. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus 29 (2016): 780-786.
- Faure C, Righini GF. Dysmotility in Esophageal Atresia: Pathophysiology, Characterization, and Treatment. Frontiers in pediatrics 5 (2017): 130.
- Allin B, Knight M, Johnson P, et al. Outcomes at one-year post anastomosis from a national cohort of infants with oesophageal atresia. Plos one 9 (2014): e106149.
- Schmedding A, Wittekindt B, Schloesser R, et al. Outcome of esophageal atresia in Germany. Diseases of the esophagus: Official journal of the International Society for Diseases of the Esophagus 34 (2021): 562.
- Wei S, Saran N, Emil S. Musculoskeletal deformities following neonatal thoracotomy: long-term follow-up of an esophageal atresia cohort. Journal of pediatric surgery 52 (2017): 1898-1903.
- Bastard F, Bonnard A, Rousseau V, et al. Thoracic skeletal anomalies following surgical treatment of esophageal atresia. Lessons from a national cohort. Journal of pediatric surgery 53 (2018): 605-609.
- DeBoer EM, Prager JD, Ruiz AG, et al. Multidisciplinary care of children with repaired esophageal atresia and tracheoesophageal fistula. Pediatr Pulmonol 51 (2016): 576-581.
- Fleck P, Kenner C, Board R, Mott S. Mother's lived experience during repair of long-gap esophageal atresia: A phenomenological inquiry. Adv Neonatal Care 17 (4): 313-323.
- Lejeune S, Sfeir R, Rousseau V, et al. Esophageal Atresia and Respiratory Morbidity. Pediatrics 148 (2021): 3961.
- Thompson A, Thakkar H, Khan H, et al. Not all neonates with oesophageal atresia and tracheoesophageal fistula are a surgical emergency. Journal of pediatric surgery 54 (2019): 244-246.
- Rittler M, Campaña H, Heisecke S, et al. Lethality of Birth Defects in Live Born Infants Categorized by Gestational Age and Birth Weight. Am J Perinatol 11 (2021): 42-58
- Tan Tanny SP, Beck C, King SK, et al. Survival Trends and Syndromic Esophageal Atresia. Pediatrics 147 (2021): 89-97.
- Galarreta CI, Vaida F, Bird LM. Patterns of malformation associated with esophageal atresia/tracheoesophageal fistula: A retrospective single center study. Am J Med Genet A 182 (2020): 1351-1363.
- Fuladi A, Suresh S, Thomas R, et al. Multidisciplinary approach to paediatric aerodigestive disorders: A single-centre longitudinal observational study. Journal of paediatrics and child health 56 (2020): 1929-1932.
- Oliver DH, Martin S, Belkis DI, et al. Favorable outcome of electively delayed elongation procedure in long-gap esophageal atresia. Front Surg 8 (2021): 701609.
- Spataru RI, Iozsa DA, Lupusoru MOD, et al. Practical safety in the diagnosis and treatment of congenital isolated tracheoesophageal fistula. Exp Ther Med 21 (2021): 537.
- Anand S, Singh A, Krishnan N, et al. Whether prophylactic intraoperative chest drain insertion in esophageal atresia-tracheoesophageal fistula is an evidence-based practice or just a prejudice: A systematic review and meta-analysis. Journal of pediatric surgery 8 (2021): 14-29.
- Gawad N, Wayne C, Bass J, et al. A chest tube may not be needed after surgical repair of esophageal atresia and tracheoesophageal fistula. Pediatr Surg Int 34 (2018): 967-970.
- Nguyen MVL, Delaplain PT, Lim JC, et al. The value of prophylactic chest tubes in tracheoesophageal fistula repair. Pediatr Surg Int 36 (2020): 687-696.
- Reusens H, Matthyssens L, Vercauteren C, et al. Multicentre survey on the current surgical management of oesophageal atresia in Belgium and Luxembourg. Journal of pediatric surgery 52 (2017): 239-246.
- LaRusso K, Joharifard S, Lakabi R, et al. Effect of transanastomotic feeding tubes on anastomotic strictures in patients with esophageal atresia and tracheoesophageal fistula: The Quebec experience. Journal of pediatric surgery 57 (2022): 41-44.
- Golden J, Demeter NE, J CL, Ford HR, Upperman JS, Gayer CP. Routine post-operative esophagram Is not necessary after repair of esophageal atresia. Am J Surg 213 (2017): 640-644.
- Lal D, Miyano G, Juang D, et al. Current patterns of practice and technique in the repair of esophageal atresia and tracheoesophageal fistua: an IPEG survey. J Laparoendosc Adv Surg Tech A 23 (2013): 635-638.
- Zani A, Eaton S, Hoellwarth ME, et al. International survey on the management of esophageal atresia. Eur J Pediatr Surg 24 (2014): 3-8.
- Dingemann C, Eaton S, Aksnes G, et al. ERNICA Consensus Conference on the Management of Patients with Long-Gap Esophageal Atresia: Perioperative, Surgical, and Long-Term Management. Eur J Pediatr Surg 31 (2021): 214-225.
- Gatzinsky V, Jönsson L, Ekerljung L, Friberg LG, Wennergren G. Long-term respiratory symptoms following oesophageal atresia. Acta Paediatr 100 (2011): 1222-1225.
- Fraga JC, Jennings RW, Kim PC. Pediatric tracheomalacia. Semin Pediatr Surg 25 (2016): 156-164.
- Ten Kate CA, Tambucci R, Vlot J, et al. An international survey on anastomotic stricture management after esophageal atresia repair: considerations and advisory statements. Surg Endosc 35 (2021): 3653-3661.
- Huang JX, Hong SM, Chen Q, et al. Risk factors for anastomotic complications after one-stage anastomosis for oesophageal atresia. J Cardiothorac Surg 16 (2021): 176.
- Vergouwe FWT, Vlot J, H IJ, et al. Risk factors for refractory anastomotic strictures after oesophageal atresia repair: a multicentre study. Arch Dis Child 104 (2019): 152-157.
- Chapuy L, Pomerleau M, Faure C. Topical mitomycin-C application in recurrent esophageal strictures after surgical repair of esophageal atresia. J Pediatr Gastroenterol Nutr 59 (2014): 608-611.
- Madadi-Sanjani O, Zimmer J, Gosemann JH, et al. Topical Mitomycin C Application in Pediatric Patients with Recurrent Esophageal Strictures-Report on Unfavorable Results. Eur J Pediatr Surg 28 (2018): 539-546.
- Ley D, Bridenne M, Gottrand F, et al. Efficacy and safety of the local application of Mitomycin C to recurrent esophageal strictures in children. J Pediatr Gastroenterol Nutr 69 (2019): 528-532.
- Aksionchyk M, Marakhouski K, Svirsky A. Gastroesophageal reflux disease in pediatric esophageal atresia: Assessment of clinical symptoms and pH-impedance data. World J Clin Pediatr 9 (2020): 29-43.
- Van Lennep M, Chung E, Jiwane A, et al. Fundoplication in children with esophageal atresia: preoperative workup and outcome. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus 24 (2022): 1953.
- Krishnan U. Eosinophilic Esophagitis in Esophageal Atresia. Review. Frontiers in pediatrics 24 (2019): 7-12


 Impact Factor: * 4.2
Impact Factor: * 4.2 Acceptance Rate: 72.62%
Acceptance Rate: 72.62%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 