Physiological Function of Blood Vessels from Uncontrolled Donation after Circulatory Death in Pigs
Galina Travnikova, Goditha Premaratne, Deepti Antony, Michael Olausson*
Department of Transplantation Surgery, Institute of Clinical Sciences, Sahlgrenska Academy at University of Gothenburg and Sahlgrenska University Hospital, Gothenburg, Sweden
*Corresponding Author: Michael Olausson, Department of Transplantation Surgery, Institute of Clinical Sciences, Sahlgrenska Academy at University of Gothenburg and Sahlgrenska University Hospital, Bruna Stråket 5, 41345 Göteborg, Sweden.
Received: 09 February 2021; Accepted: 16 February 2021; Published: 16 April 2021
Article Information
Citation:
Galina Travnikova, Goditha Premaratne, Deepti Antony, Michael Olausson. Physiological Function of Blood Vessels from Uncontrolled Donation after Circulatory Death in Pigs. Journal of Surgery and Research 4 (2021): 202-217.
View / Download Pdf Share at FacebookAbstract
Donation after circulatory death (DCD) has become increasingly important in kidney transplantation. Challenges, including early graft loss and delayed graft function, remain. Little is known regarding vascular properties. The aim was to examine effects of DCD on renal artery function in a novel pig extended DCD model, extending the time from circulatory arrest to retrieval of the organ to more than 4 hours, making it more feasible to recruit more organs from DCD. Renal and iliac arteries were obtained from animals immediately after euthanasia (fresh) and 4.5hr after DCD. Vessels were also analyzed after 10hr at 4°C in physiological salt solution (stored). Preparations were mounted for isometric force recording in vitro and examined at optimal stretch for active tension. Iliac arteries were more sensitive to noradrenaline compared to renal artery, possibly reflecting differences in sympathetic control of perfusion in renal and hind limb vascular beds. Active tension (depolarization, noradrenaline activation) was significantly lower in DCD renal arteries compared to fresh vessels. Stored renal arteries were also weaker, but to a lower extent, whereas iliac artery was only marginally weakened in uDCD and unaffected by storage. For both vessel types, DCD caused a significant attenuation of endothelial-mediated relaxation. Kidneys from uDCD donors performed poorly after transplantation, as expected, with a somewhat better result if the donor was given the standard protocol, including cooling after two hours. In conclusion, DCD introduces changes both in contractile function and endothelium-mediated relaxation of renal arteries. Effects were more pronounced than in iliac artery, suggesting that DCD could be one factor affecting the functional circulation of the donor kidney after transplantation. Further studies of uDCD subject to reconditioning before transplantation is underway to study the reversibility of changes to the endothelial fu
Keywords
<p>Kidney transplantation, Renal arteries, Invitro vessel analysis, Warm ischemia, Pig model, Vessel preservation</p>
Article Details
1. Introduction
Blood vessels are procured for organ transplantation when the recipient vessels are suboptimal or too short to perform anastomosis to the donor organ. Vessel procurement may also be carried out if specific actions are required to enable the transplanted organ to function. Other important reasons for vessel procurement are emergencies such as arterial thrombosis that may occur after transplantation and requires an interposition graft, or general hepato-pancreatico-biliary surgery that may require a vascular graft. For example, a portal vein graft may be required to successfully complete pancreas resection [1-3]. In many institutions, like our own, these grafts can be stored in a refrigerator at 4-8 °C for up to 2 weeks before being discarded. Despite using these vascular grafts for decades in the clinical setting, the physiological function of these grafts is not well understood. Alternative approaches such as cryopreserved vessels or artificial vessels [4,5] have also been used but these options are hampered by the risk of strictures [6-9] and thromboses [7-10]. Therefore, we felt it was important to learn more about the effects of circulatory arrest in donors and cold storage on the functional aspects of blood vessels to be able to use these grafts more optimally.
The present study is part of an ongoing project actively exploring novel techniques to improve the success of renal transplantation after uncontrolled circulatory death (uDCD), applying a large animal (pig) model. The model aims at extending the present limits on when a viable organ can be acquired after circulatory death, potentially increasing the available donor pool. We have in this study selected the renal and iliac arteries from the domestic pig to focus on changes in contractile function of these tissues. In ventilated anaesthetized pigs, uncontrolled circulatory death was simulated by switching off the respirator. Comparisons of vessel contractile function were then performed for fresh vessels removed immediately prior to uDCD, stored vessels removed prior to uDCD and then subjected to 10 h cold storage and uDCD vessels removed 4.5 h after circulatory arrest. If blood vessels can be stored for 2 weeks without contact to blood, like in the present clinical practice, it seems logical that a few hours of ischemia after circulatory death would not be detrimental. We report that prolonged warm ischemia time during the uDCD procedure results in significant loss of vascular contractile function and endothelial relaxation.
2. Materials and Methods
2.1 Animals and operative procedures
The study was performed using male and female Swedish domestic pigs weighing 40-50 kg (mean 45 kg, n=56 for physiological studies, n=16 for transplant experiments. Tension curves were established from 14 different animals. The animals were anesthetized with an intramuscular injection of atropine 0.5 mg, xylazine 100 mg, and ketamine 20 mg/kg body weight; and an intravenous injection of fentanyl 4 µg/kg and midazolam 0.4 mg/kg. Thereafter, a catheter was placed in an ear vein and anesthesia was maintained with an intravenous infusion of ketamine (10 mg/kg x h) and rocuronium bromide (1.5 mg/kg x h). Animals were tracheostomized and connected to a respirator with volume-controlled and pressure-regulated ventilation. In the DCD group, DCD was simulated by turning off the respirator mimicking uncontrolled DCD. Subsequent cessation of cardiac beating occurred within 15 to 20 min. After 2 hours, animals were cooled by placing crushed ice in the abdomen. Organs were harvested 4,5 hours (260-285 minutes) after circulatory arrest. The pharmaceutical compounds used for operative procedures were obtained from Fresenius Kabi (rocuronium) Austria GmbH, Graz, Austria; Hameln Pharma Plus GmbH (midazolam), Hameln, Germany; Intervet AB (ketamine), Stockholm, Sweden; Unimedic AB (atropine), Matfors, Sweden; Braun (fentanyl), Melsungen, Germany; and Bayer (xylazine), Solna, Sweden.
Vascular samples were compared from animals following 3 sets of physiological conditions 1) Fresh: samples obtained at a time point immediately prior to induction of uDCD (n=18 animals); 2) Stored: samples obtained fresh and then stored in normal Krebs solution (composition below) at 4 °C for 10 h (n=16 animals); 3) DCD: samples obtained 4.5 h after cessation of cardiac beating in DCD animals (n=16 animals). Kidneys from uDCD donors were transplanted into allogeneic pigs (n=8), after being flushed on back table with 3-500ml of StoreProtect®. Four pigs received no cooling during the uDCD procedure after two hours, while four pigs received standard cooling with ice-slush in the abdomen after two hours. Vascular arterial flow was measured perioperatively with Cardiomed CM-4000, (Medistim a/s, Oslo, Norway), with Medistim® flow probes 3-5mm, at reperfusion and after 90 minutes in all eight transplanted animals. Pigs considered having viable kidneys were allowed to wake up, given analgesic treatment and were carefully observed following recovery from anesthesia until the end of the study.
2.2 Ethical considerations
The Principles of Laboratory Animal Care [9] were followed. In addition, recommendations in the European Guidelines for Animal Research and the national and local regulations [10-13] were employed. The study protocol was approved by the Local Animal Ethical Committee for Animal Experiments in Lund (Permit Numbers: M174-15 and 5.8.18-13977/2018).
2.3 Vessel preparations
The samples were obtained from one of the renal arteries (the main branch approximately 2.5 cm from the aorta) and the iliac artery (a few mm distal to the bifurcation). From each animal and for each vessel type, 4 ring segment preparations were obtained and mounted for isometric force recording in open organ baths (DMT model 750TOBS, Danish Myo Technology, Aarhus, Denmark) at 37°C in normal Krebs solution of the following composition (mmol/L): NaCl 119, KCl 4.6, MgCl2 1.2, NaH2PO4 1.2, CaCl2 1.5, NaHCO3 22, glucose 11, with pH 7.4 when gassed with 95%O2/5%CO2. The segment lengths were approximately 2.6 mm for both vessel types. The segments were gently stretched for 40 min at a passive force of 80 mN. To confirm the stability and integrity of the vessel preparations, they were activated 2 or 3 times for periods of 3 to 8 min (to allow a force plateau to be established) by membrane depolarization using High-K+ solution. The High-K+ solution had the same composition as normal Krebs except that NaCl was omitted and KCl was increased to 127 mmol/L. The vessels were relaxed in normal Krebs using 3 solution exchanges for 10 min between contractions. Preparations were included in subsequent experiments when the last two force responses differed by less than 10%. These experiments examined the circumference-tension relationship, the noradrenaline sensitivity, and the endothelial responses of the vessels.
2.4 Circumference-tension relationship
In initial experiments on fresh preparations, we determined the general characteristics and functionality of the vessels as a basis for subsequent analysis of the different experimental groups. To determine the relation between stretch (i.e. circumference) and passive/active tension, the vessels were repeatedly activated using High-K+ solution (3 to 8 min enabling maximal response at each circumference) and relaxed for 10 min in normal Krebs. Starting at slack length (where passive tension just became noticeable) the vessels were stretched for 1 min prior to each contraction. The change in circumference at each stretch was set at 1 mm and was reduced to 0.5 mm for the last 3 steps when passive tension was less compliant. Each vessel was activated 7 to 8 times at increasing circumferences until the active tension reduced indicating that circumference was above optimal for active tension. Passive tension was measured immediately before the contraction. At the end of the experiment, the vessel dimensions of segment length and circumference were determined using a slide caliper. The wet weight was determined after gently blotting the sample between two sheets of filter paper. From these experiments, we were able to determine the relationships between vessel circumference and tension (passive and active), the maximal active tension for High-K+ activation (mN/mm), passive tension (mN/mm) at optimal stretch, as well as the optimal circumference. Wall thickness was calculated from vessel dimensions and wet weight using a density of 1.06 mg/mm3.
In separate experiments, we determined the smooth muscle content in the vascular wall (i.e. media thickness). Vessels were stretched in normal Krebs to optimal circumference and fixed for histology using 4% paraformaldehyde in phosphate buffered saline (Q-path buffer, VWR), followed by spin tissue processing (Microm STP 120, Thermo Scientific). The samples were embedded in paraffin, and 5 micrometer thick microtome sections were stained with hematoxylin and eosin. The thickness of the smooth muscle layer in the vascular wall was measured on calibrated microscopic photographs.
2.5 Noradrenaline sensitivity and endothelial responses
Renal and iliac arteries from the fresh, the stored, and the DCD groups were stretched to near optimal circumference as determined from the length-tension relationships on fresh vessels. Noradrenaline sensitivity was examined after the initial High-K+ induced contractions using cumulative increases of the agonist concentration in half-logarithmic steps from 10-7 to 3 x 10-5 mol/L. The maximal stable force at each concentration was determined and expressed in relation to the initial High-K+ response.
After determination of the noradrenaline sensitivity, the arteries were relaxed and activated with 3 x 10-6 mol/L noradrenaline. Once a stable force plateau was established (after about 5 min), substance P (1.0 x 10-5 mol/L) was added to activate endothelial dependent relaxation. Finally, papaverine (3 x 10-4 mol/L) was given to induce a full relaxation independent of endothelium, to record the zero-tension level. We confirmed that the noradrenaline induced contraction was stable if substance P or papaverine were not added. The maximal relaxant response to 10-5 mol/L substance P was evaluated and expressed in relation to the noradrenalin-induced force prior to addition of substance P. The pharmaceutical compounds were obtained from Sigma-Aldrich, Stockholm, Sweden.
2.6 Statistics
All data are given as mean ± SEM (Standard Error of the Mean) where the number of observations refers to the number of animals. From each vessel of each animal, 3-4 segments were analyzed and the average result was used representing the data from that animal. Statistical comparisons were made using Student’s t-test or analysis of variance (ANOVA). Post-hoc testing after ANOVA was performed using the Holm-Sidak method. It was confirmed that the parameters passed normality test (Shapiro-Wilk). If not normally distributed, then the non-parametric Rank Sum test and Dunn’s method was used. Analyses were made applying routines implemented in SigmaPlot for Windows 14, Systat Software Inc.
3. Results
3.1 Characteristics of iliac and renal arteries
In pre-study experiments on 8 renal and 7 iliac arteries (from 14 different animals), we determined the optimal circumference (stretch) for active tension development. Between 1 and 8 ring preparations were analyzed from each vessel (i.e. each animal) and the average was used as being representative of that vessel in the subsequent analysis. Figure 1 shows the circumference-tension relationships for the two vessel types. Passive tension increased exponentially with stretch from the slack length. Both vessel types had a bell-shaped dependence of active tension on length, with optimal circumferences at about 13 and 18 mm, for renal and iliac arteries, respectively and the optimal stretch was about 1.75 times the slack length in both vessel types. The data from these experiments are summarized in Table 1.
|
Renal artery |
Iliac Artery |
||
|
Optimal circumference, mm |
13.0±0.7, n=7 |
18.0±0.7, n=9 |
P = 0.0004 |
|
Maximal active wall tension, mN/mm |
32.2±4.9, n=7 |
26.1±1.9, n=7 |
P = 0.266 |
|
Passive wall tension, mN/mm |
24.4±3.7, n=7 |
27.2±3.2, n=7 |
P = 0.578 |
|
Total wall thickness, mm |
0.72±0.05, n=7 |
0.42±0.02, n=7 |
P = 0.000161 |
|
Media thickness, mm |
0.20±0.01, n=5 |
0.20±0.01, n=7 |
P = 0.784 |
Table 1: Characteristics of fresh renal and iliac arteries. The optimal circumference for active tension was determined for each vessel in circumference–tension experiments (cf. Figure 1). Maximal active tension and passive tension were determined at optimal circumference. Total wall thickness was determined from tissue dimensions and wet weight in each experiment. Media thickness was obtained from histological sections of samples fixed at optimal circumference.
The maximal active wall tension was similar in the two vessels. The wall thickness, determined from the preparation wet weight was significantly larger in the renal artery. However, histological examination of samples fixed at optimal stretch showed that the media thickness was similar, suggesting that the preparations of renal artery contained more adjacent adventitial tissue. If the maximal active smooth muscle stress (i.e. active force per media area) is calculated using active tension and media thickness the iliac vessels had slightly higher active stress (renal: 161 mN/mm2; iliac: 130 mN/mm2) suggesting differences in the amount of smooth muscle cells in the media or in the expression/function of contractile components in the cells. In subsequent experiments, examining the reactivity and endothelial relaxation of the two vessels types in the three models we stretched the preparations to a passive tension of 15 mN/mm giving approximately 90% of optimal length and near maximal active tension (cf. Table 1 and Figure 1).
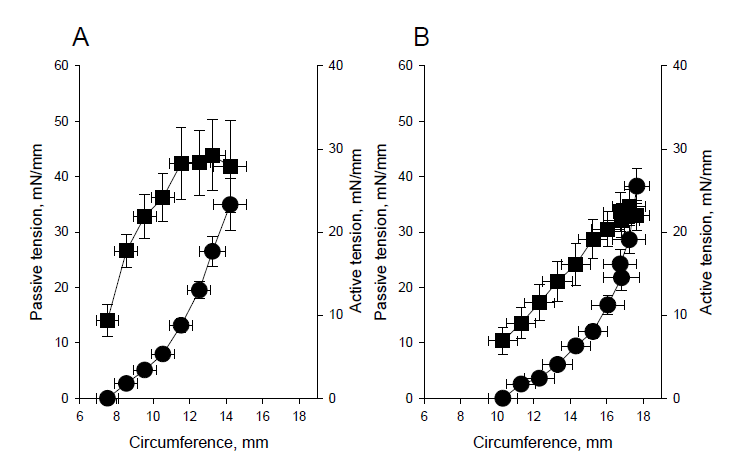
Figure 1: Circumference-tension relationships. Panel A shows renal and Panel B iliac vessels. The active (squares) and passive (circles) tensions are plotted against vessel circumference. N=7.
3.2 Active tension and noradrenaline sensitivity
We determined the active force development after activation with high-K+ and noradrenaline of the two vessel types (renal and iliac) in the three different model conditions. As seen in panel A of Figure 2 the renal arteries were significantly weaker after DCD treatment; the responses to both high-K+ and noradrenaline were significantly reduced. Also, the stored vessels were weakened, but to a lesser extent. The iliac arteries (Panel B, Figure 2) were less affected compared to the renal arteries; the DCD groups appeared somewhat weaker but no significant differences, compared to fresh arteries were observed.
The sensitivity to the physiological contractile agonist, noradrenaline, of the two vessel types in the three different model conditions is shown in Figure 3. In each preparation, we recorded an initial force response to direct activation of Ca++ influx using depolarization with High-K+ and normalized the agonist induced contractions to this value. Figure 3 shows the summarized data. The mean values for tension (y) and concentration (c) are fitted by a hyperbolic equation (y=Max*ch/(ch+EC50h), to determine the maximal response (Max), the concentration giving half-maximal tension (EC50) and the steepness coefficient (h). As seen in the figure, fresh samples of both vessel types gave maximal noradrenaline induced force of about 120% of the high-K+ responses and had h-values around 1. However, the renal vessels were markedly less sensitive to the agonist with a half-maximal tension of about 2 µmol/L (cf Figure 2, EC50 = 5.7 log M), compared to the iliac arteries which exhibited half-maximal response at about 0.2 µmol/L (EC50 =-6.7 log M). The data and fitted curves almost superimpose in each group clearly showing that the two interventions, storage and DCD, did not markedly affect the sensitivity or maximal response to noradrenaline in either vessel.
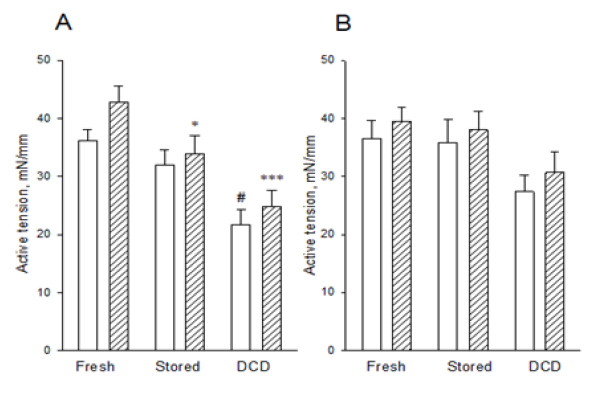
Figure 2: Active tension in fresh, stored and DCD treated, renal (Panel A) and iliac (Panel B) vessels, activated with high-K+ (open bars) and noradrenaline (hatched bars). ANOVA analysis in the high-K+ and noradrenaline groups: # p<0.001 and p<0.05 compared to the fresh group and stored groups, respectively, * p<0.05 compared to the fresh group, *** p<0.001 compared to the fresh group. N=9-14.
3.3 Endothelial dependent relaxation
After recording the noradrenaline sensitivity (cf. Figure 3), the vessels were again activated with 3x 10-6 mol/L noradrenaline to induce a stable, submaximal, contraction. When a plateau force was established, substance P (10-5 mol/L activating the endothelial nitric oxide release and vascular relaxation) was added. After recording the relaxant responses, 3 x 10-4 mol/L papaverine was introduced to fully relax the vessels and enable determination of the baseline tension. Figure 4 shows the relaxant responses to substance P in the different vessels and conditions. In both vessel types, the DCD groups had significantly impaired relaxation. Storage did not affect the iliac arteries, but partly reduced the relaxant responses of renal arteries.
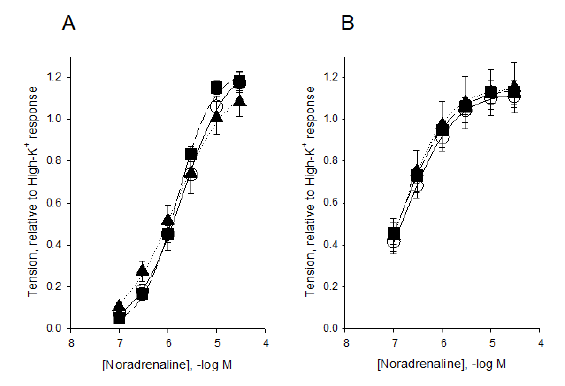
Figure 3: Sensitivity to noradrenaline. Panel A shows renal and Panel B iliac vessels. Tension values are normalized to the initial high-K+ responses. Data are fitted by a hyperbolic equation (see Results). Open circles, full line show data from fresh samples, filled triangles, dotted line data from stored, and filled squares, dashed line data from DCD animals. N= 8-11.
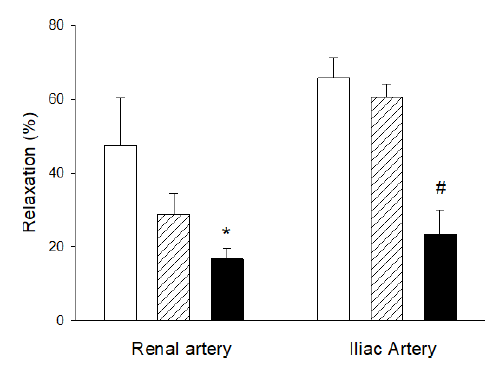
Figure 4: Substance P induced relaxation in renal and iliac arteries. Open bars show fresh samples, hatched bars stored vessels, and filled bars vessels from DCD animals. Data are shown as per cent relaxation relative to the initial noradrenaline (3 µM) contraction. *shows significant difference (p<0.01) compared to fresh samples; #shows significant difference (p<0.001) compared to fresh and stored samples in the same vessel type (ANOVA). N= 5-14.
3.4 Flows and graft survival of pigs receiving an uDCD kidney
Renal arterial flows from eight transplanted pigs can be seen in Figure 5. Two of four animals in the group receiving kidneys from standard cooled uDCD had adequate flows (above 100 ml/min) after 90 minutes and were allowed to recover from anesthesia for further observation. In the group without cooling, none of the four animals showed sufficient arterial flow (below100 ml/min) at reperfusion, and were not recovered from anesthesia. Pigs from the group that had two pigs that recovered from anesthesia and were observed after transplantation failed to eat and thrive. Accordingly, and in compliance with the ethical permit, these animals were sacrificed on day 5. Vascular flow was low and creatinine values of 1370 µmol/L and 1220 µmol/L, respectively, reflected poor kidney function. The explanted kidneys (day 5), from the group receiving kidneys from standard cooled uDCDs, had signs of necrotic areas (dark areas in Figure 6, Panel A), while the remaining kidneys (n=6), sacrificed ninety minutes after reperfusion, all showed signs of poor perfusion, with large parts of the kidney being blue/black (Figure 6, Panel B). Kidneys exhibiting such poor perfusion in the clinical setting would not be accepted for transplantation.
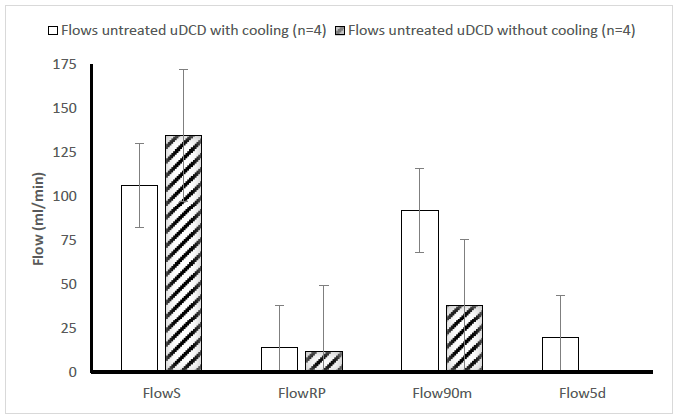
Figure 5: Mean values (+ SEM) of arterial vascular flow in the renal artery, before nephrectomy (FlowS) of the recipient, at reperfusion (FlowRP) and 90 minutes after reperfusion (Flow90m). No filled columns: untreated uDCD pigs with cooling after 2 hours (n=4); Striped columns: untreated uDCD pigs without cooling after two hours (n=4). Two animals in the former group were sacrificed at day 5 in group (Flow5d).
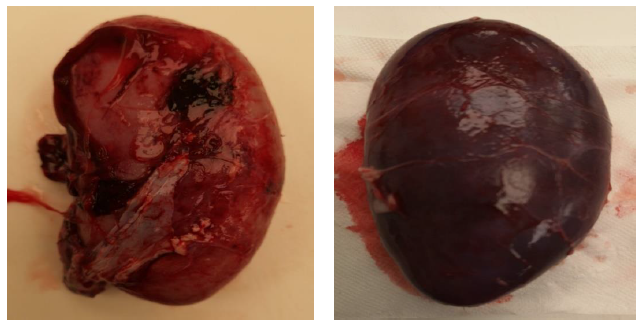
Figure 6: Panel A: Explanted kidney surviving 5 days, having necrotic areas on the surface of the kidney (uDCD with cooling). Panel B: A typical look of severely congested kidney considered not viable, at the day of transplantation, 90 minutes after reperfusion (without cooling).
4. Discussion
Using a porcine model of transplantation to study the effects of circulatory death and storage on donor vascular physiological function, we showed that a long warm ischemia time significantly reduced the contractile function of renal arteries and impaired the endothelial mediated relaxation. These in vitro results were supported by in vivo data using the extended uDCD protocol. In recipients of kidneys from donor animals not subjected to standard cooling and undergoing warm ischemia, kidney vascular flow was inferior compared to recipients who received a donor kidney after standard cooling. Furthermore, the substantial ischemic insult inflicted on the uDCD model showed, as expected, that without proper reconditioning poor outcomes are achieved. Consequently, transplantation based on function of a donor organ subjected to warm ischemia without preconditioning appears futile. Based on these findings, we conclude that changes in vascular function during graft procurement in the clinic could adversely affect kidney perfusion after prolonged warm ischemia in DCD., This is one important factor that needs attention in reconditioning protocols.
Our experimental results from porcine transplanted kidneys, using the extended uDCD protocol (Figures 5,6), confirm that warm ischemia is deleterious to the transplanted organ in the in vivo setting. However, clinical reality suggests that uDCD kidneys are able to overcome the early problems of physiological insult manifesting as delayed graft function (DGF) due to acute tubular necrosis (ATN (16). It has been shown that recipients transplanted with uDCD kidneys recover from the effects of ATN, and regain similar long-term function to that which is typical in kidneys from braindead donors (DBD) (17-19). It is evident that uDCD procured organs require reconditioning if they are to withstand ischemia-reperfusion injury and remain viable for transplantation in the clinic. We now know that pure cold storage is not detrimental to function for the first 24 hours. However, like the duration of warm ischemia time, the development of ATN during cold storage is a function of time. Whether changes to the endothelium can explain the effects of ATN and whether this is the central mechanism for organ failure in uDCD, we are unable to say. To reach a better position to answer these questions, we will study the reversibility of the changes in our uDCD model and compare these effects to the cold ischemia setting. We already know that donor vascular vessels stored in a refrigerator for up to 2 weeks can still be used successfully in a clinical setting, but to-date long term storage of organs for this period has not been successful.
The main focus of our research is to develop techniques which improve renal preservation/reconditioning during DCD transplantation. To this end, we have designed a model of uncontrolled DCD that challenges the limits of the current clinical practice of retrieving and using organs from uDCD. In our model, we allow a 2-hour initial no-touch period, followed by an additional 2 hours of no touch after ice-slush has been placed in the abdominal cavity through a small incision. The donor organs are then retrieved and are placed on the back-table on an ice-bed up to 4.5 hours after cardiac death. The study of improving organ quality after longer preservation times is clinically highly relevant since an extended time allows for a longer period to obtain patient consent. This could ease the logistic problems that many transplant centers face today and allow for a larger number of suitable donor organs. Typically, transplant centers seek donor consent and try to achieve donor organ perfusion within 30-60 minutes after circulatory death (20-26).
The specific aim of this study was to examine possible vascular alterations influencing renal perfusion after kidney transplantation. Therefore, we intentionally chose to study the renal artery and to compared it with a similarly sized muscular artery, the iliac artery. We are aware that these arteries most certainly have different physiological properties, but the iliac vessels serve as a control for systemic and/or general vascular alterations after circulatory death. Accordingly, we characterized these two vessels in vitro to compare their functional properties under defined conditions applying optimal stretch for active tension. Our data show that the mechanical properties can support transmural pressures in the physiological range. If we apply the law of Laplace for thin walled cylindrical vessels [27] then we can make an estimate of the pressure that the vessels can withstand at optimal stretch, when wall tension is supported by both the passive and active components. Using the data in Table 1, we estimate that the renal artery would support a pressure of about 200 mmHg at optimal stretch, whereas for the iliac artery the corresponding pressure would be 140 mmHg. Both values are within the physiological range. It should be recognized that the Laplace’ law is not directly applicable, and that more complex structural/mechanical models might give slightly different results. However, these data suggest that the renal artery has capacity to sustain higher pressure loads which may be a functional consequence of the role that this artery serves in renal blood flow regulation. Interestingly, the renal artery was significantly less sensitive to the contractile agonist noradrenaline (Figure 3) compared to the iliac vessels, which could reflect a difference in efferent sympathetic control between renal and systemic arteries.
Important features of the DCD model are that vessels can be kept within the body and are exposed to blood and changes in temperature during a 4.5-hour ischemic period. This procedure mimics the events that occur during warm ischemia under clinical conditions of terminal circulatory arrest, although the extended time of more than 4 hours is way beyond what is normally considered possible to restore kidney function after reperfusion [28]. In laparoscopic donor nephrectomy, ischemia times beyond 45 minutes are considered detrimental [29]. In partial nephrectomy, beyond 25 minutes of warm ischemia is not recommended [30]. The few clinical programs that use uncontrolled DCD, avoid long warm ischemia times beyond 60 minutes [31]. With regard to the storage model, a situation was created where vessels are maintained in a cold environment but are not exposed to blood. In a clinical setting, cold storage does not affect the function of the kidney, since most kidney transplantations have been performed after static storage for at least ten hours at 4-6 oC, without being in contact with blood.
Under all conditions, regardless of whether the tissue samples came from a fresh, stored or DCD source, both renal and iliac vessels remained viable. These experiments show that the vascular contractile function in larger arteries remain for at least several hours after circulatory death, but exhibit a loss in contractility with the renal arteries most affected. We did not examine nerve induced responses, but we did show that the adrenoceptor sensitivity was unaffected by DCD and storage. The data suggest that the systemic changes in catecholamine levels or alterations in adrenergic tone during circulatory death do not lead to changes in vascular reactivity to sympathetic agonists. The contractile response to direct membrane activation with depolarization and to noradrenaline was significantly impaired in the renal artery after DCD and storage. This suggests that the longer ischemic period (warm or cold) adversely affected the cellular integrity, the contractile system itself or the cellular activation pathways. Interestingly, iliac arteries were less affected compared with renal arteries, although they are of similar size and exhibit similar contractile properties. Physiologically, these observed changes in renal artery contractile function could affect the vascular tone in the kidney and potentially the auto-regulatory mechanisms after DCD. Whether these changes also are responsible for the poor outcome after transplantation in uDCD kidneys remain to be further studied. We also need to find out whether the changes observed are reversible in the established uDCD model.
In contrast to the smooth muscle adrenergic sensitivity, the endothelial mediated relaxation was markedly attenuated by DCD in both vessel types. The change was also observed after storage in the renal artery. The results show that endothelial dysfunction is an important consequence of the DCD procedure. Maximal papaverine-induced relaxation was not affected, which localizes the alteration to the endothelial signaling. It has been shown that endothelium is sensitive to several vascular challenges [32-34]. Several factors including temperature [35-36], low blood flow [37], intravascular coagulation [38], pH changes, and metabolites [39] can be causative. Although cold storage for an extended time has been shown to affect endothelial function [40], it was less detrimental than the effects observed with DCD. This shows that lack of blood flow is not the sole factor for endothelial dysfunction. Rather, the warm ischemia time and accompanying changes in the stagnating blood compartment all contribute to the net adverse effect on the endothelium. It is most relevant that major changes in endothelial responses of the iliac vessel following storage did not occur. This result highlights the difference between the endothelial susceptibility of the two vessels. In most clinical centers the iliac vessels are stored for potential use as vascular grafts. Our data show that the endothelial function is preserved in these vessels, but also indicate that other vessels, such as renal arteries, can be affected by cold preservation. This emphasizes the necessity to further examine alterations and potential reversibility of changes in the vasculature after preservation and extended uDCD.
In summary, a porcine model of uncontrolled DCD reveals poor kidney perfusion after transplantation following warm ischemia with or without cooling during the DCD period. Both cold preservation and uDCD affected contractile function and endothelial relaxation of renal arteries. These findings suggest potential negative effects on blood flow regulation in kidneys and on early graft function following clinical transplantation. Our next step to try to improve donor organ vascular function is to test whether a reconditioning protocol using ex-vivo perfusion of the kidneys is beneficial in the DCD setting.
Conflict of interest
None of the authors reported any potential conflicts of interest
Funding
Supported by grants from IngaBritt and Arne Lundbergs Research Fundation (MO); LUA ALF Grant (MO); The Gelin Foundation (MO) and Hans-Gabriel and Alice Trolle Wachtmeister Foundation for Medical Research (MO).
Ethical approval
This case report did not require ethical approval, as it was exempt at our institution.
References
- Oba A, Ito H, Ono Y, Sato T. Regional pancreatoduodenectomy versus standard pancreatoduodenectomy with portal vein resection for pancreatic ductal adenocarcinoma with portal vein invasion. BJS Open 4 (2020): 438-448.
- Ravikumar R, Sabin C, Abu Hilal M, Bramhall S. Portal vein resection in borderline resectable pancreatic cancer: a United Kingdom multicenter study. J Am Coll Surg. 218 (2014): 401-411.
- Menon VG, Puri VC, Annamalai AA, Tuli R, Nissen NN. Outcomes of vascular resection in pancreaticoduodenectomy: single-surgeon experience. Am Surg. 79 (2013): 1064-7.
- Olausson M, Patil PB, Kuna VK, Chougule P, Hernandez N, Methe K, Kullberg-Lindh C, Borg H, Ejnell H, Sumitran-Holgersson S. Transplantation of an allogeneic vein bioengineered with autologous stem cells: A proof-of-concept study. The Lancet 380 (2012): 230-237.
- Olausson M, Kuna VK, Travnikova G, Bäckdahl H, Patil PB, Saalman R, Borg H, Jeppsson A, Sumitran-Holgersson S. In Vivo Application of Tissue-Engineered Veins Using Autologous Peripheral Whole Blood: A Proof of Concept Study. EBiomedicine 1 (2014): 72-79.
- Kuang AA, Renz JF, Ferrell LD, Ring EJ, et al. Failure patterns of cryopreserved vein grafts in liver transplantation. Transplantation 62 (1996): 742-747.
- Mohkam K, Darnis B, Rode A, Hetsch N. Rescue arterial revascularization using cryopreserved iliac artery allograft in liver transplant patients. Exp Clin Transplant. 15 (2017): 420-424.
- Brown KE, Heyer K, Rodriguez H, Eskandari MK, Pearce WH, et al. Arterial reconstruction with cryopreserved human allografts in the setting of infection: A single-center experience with midterm follow-up. J Vasc Surg. 49 (2009): 660-666.
- Sugawara Y, Makuuchi M, Tamura S, Matsui Y. Portal vein reconstruction in adult living donor liver transplantation using cryopreserved vein grafts. Liver Transpl 12 (2006): 1233-1236.
- Mabrut JY, Abdullah SS, Rode A, Bourgeot JP, et al. Cryopreserved iliac artery allograft for primary arterial revascularization in adult liver transplantation. Clin Transplant 26 (2012): E12-16.
- http://www.jordbruksverket.se/amnesomraden/djur/sveriges3rcenter/
detharar3r.4.48fc962e15ea0a5b2c2a144e.html. - Jordbruksverkets föreskrifter och allmänna råd om försöksdjur (2019).
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes.
- Djurskyddslag 2018:1192 https://www.riksdagen.se/sv/dokument-lagar/dokument/svensk-forfattningssamling/djurskyddslag-20181192_sfs-2018-1192.
- Djurskyddsförordning 2019:66 https://www.riksdagen.se/sv/dokument-lagar/dokument/svensk-forfattningssamling/djurskyddsforordning-201966_sfs-2019-66.
- Peters-Sengers H, Homan van der Heide JJ, Heemskerk MBA, ten Berge IJM, Ultee FCW, et al. Similar 5-Year Estimated Glomerular Filtration Rate Between Kidney Transplants From Uncontrolled and Controlled Donors After Circulatory Death- A Dutch Cohort Study. Transplantation 101 (2017): 1144-1151.
- Zwaini Z, Patel M, Stover K, Dormer J. Comparative analysis of risk factors in declined kidneys from donation after brain death and circulatory death. Medicina 56 (2020): 317.
- Chen G, Wang C, Ko DS, Qiu J, et al. Comparison of outcomes of kidney transplantation from donation after brain death, donation after circulatory death, and donation after brain death followed by circulatory death donors. Clin Transplant. 31 (2017): 11-16.
- Bell R, Farid S, Pandanaboyana S, Upasani V, Baker R, Ahmad N. The evolution of donation after circulatory death renal transplantation: a decade of experience. Nephrol Dial Transplant 34 (2019): 1788-1798.
- Morrissey P, Monaco A. Donation After Circulatory Death: Current Practices, Ongoing Challenges, and Potential Improvements. Transplantation 97 (2014): 258-264.
- Hoogland ER, Snoeijs MG, van Heurn LW. DCD kidney transplantation: results and measures to improve outcome. Curr Opin Organ Transplant. 15 (2010): 177-82.
- Brennan C, Sandoval PR, Husain SA, Tsapepas D. Impact of cold and warm ischemia time on outcomes for kidneys donated after cardiac death. ATC Meeting abstracts. Am J Transplant. 19 (2019).
- Heurn LWE, Talbot D, Nicholson ML, Akhtar MZ, et al. Recommendations for donation after circulatory death kidney transplantation in Europe. Transpl Int. 29 (2016): 780-789.
- Chen J, Mikhail D, Sharma H, Hijazi A. Donor warm ischemic time more than 80 min is an important predictor of kidney graft survival from donors after cardiac death. Meeting abstracts. Am J Transplant. 17 (2017).
- Neyrinck A, Raemdonck DW, Monbaliu D. Donation after circulatory death: current status. Curr Opin Anaesthesiol. 26 (2013): 382-390.
- Osband AJ, James NT, Segev DL. Extraction time of kidneys from deceased donors and impact on outcomes. Am J Transplant. 16 (2016): 700-703.
- Browning DJ. Retinal vein occlusions: evidence-based management. Springer, New York 40 (2010).
- Secin FP. Importance and Limits of Ischemia in Renal Partial Surgery: Experimental and Clinical Research. Adv Urol. (2008) 1-10.
- Pouliot F, Pantuck AJ, Belldegrun AS, Dujardin T. Effect of warm ischemia time on renal differential renal function after renal artery clamping for laparoscopic partial nephrectomy. The J Urol. 181 (2009): 277.
- Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A et al. Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol. 58 (2010): 340-345.
- Hoogland ERP, Smaalen TC, Christiaans MHL, Heurn LWE. Kidneys from uncontrolled donors after cardiac death: which kidneys do worse? Transpl Int. 26 (2013): 477-484.
- Dunzendorfer S, Lee HK,Tobias PS. Flow-dependent regulation of endothelial toll-like receptor 2 expression through inhibition of SP1 activity. Circ Res. 95 (2004): 684-691.
- Chiu JJ, Chien S. Effects of disturbed flow in vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 91 (2011).
- Touyz RM, Alves-Lopes R, Rios FJ, Camargo LL, Anagnostopoulou A, et al. Vascular smooth muscle contraction in hypertension. Cardiovasc Res. 114 (2018): 529-539.
- Fajardo LF, Schreiber AB, Kelly NI, Hahn GM. Thermal sensitivity of endothelial cells. Radiat Res. 103 (1985): 276-285.
- Kasli KK, Chiesa ST, Trangmar SJ, Ali L, Lotlikar MD, et al. Mechanisms for the control of local tissue blood flow during thermal interventions: influence of temperature-dependent ATP release from human blood and endothelial cells. Exp Physiol. 102 (2017): 228-244.
- Barak OF, Mladinov S, Hoiland RL, Tremblay JC, Thom SR, et al. Disturbed blood flow worsens endothelial dysfunction in moderate-severe chronic obstructive pulmonary disease. Nature. Scientific reports. 7 (2017): 169-189.
- Lee DY, Chiu JJ. Atherosclerosis and flow: roles of epigenetic modulation in vascular endothelium. J Biosoc Sci. 9 (2019): 26-56.
- Hoffmann J, Haendeler J, Aicher A, Rössig L, Vasa M, Zeiher AM et al. Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli. Important role of nitric oxide. Circ Res. 89 (2001): 709-715.
- Sandoo A, Zanten JJCSV, Metsios GS, Carroll D, Kitas GD. The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J. 4 (2010): 302-312.


 Impact Factor: * 4.2
Impact Factor: * 4.2 Acceptance Rate: 72.62%
Acceptance Rate: 72.62%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 