Clinicopathological Evaluation of Marjolin’s Ulcer: A Single Center Study
Ekram Hossain Fahim1*, A.K.M Shahidur Rahman2, Bedisha Ahmed3, Shah Md. Rezaul Karim4, Md. Sazzad Khondoker5, Md. Abul Kalam6
1Department of Burn and Plastic Surgery, Dhaka Medical College Hospital (DMCH), Dhaka, Bangladesh
2Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
3Department of Radiology and Imaging, Mugda Medical College Hospital, Dhaka, Bangladesh
4Department of Surgery, Sheikh Hasina Medical College Hospital, Habiganj, Bangladesh
5Department of Burn and Plastic Surgery, Dhaka Medical College Hospital (DMCH), Dhaka, Bangladesh
6Sheikh Hasina National Institute of Burn and Plastic Surgery (SHNIBPS), Dhaka, Bangladesh
*Corresponding Author: Dr. Ekram Hossain Fahim, Assistant Professor, Department of Burn and Plastic Surgery, Ministry of Health and Family Welfare, Dhaka, Bangladesh
Received: 16 August 2022; Accepted: 20 August 2022; Published: 28 September 2022
Article Information
Citation: Fahim EH, Rahman AKMS, Ahmed B, Karim SMR, Khondoker MS, Kalam MA. Clinicopathological Evaluation of Marjolin’s Ulcer: A Single Center Study. Journal of Surgery and Research.5 (2022): 549-558.
View / Download Pdf Share at FacebookAbstract
Marjolin's ulcer is an aggressive lesion that usually develops on chronic ulcers, scars, and osteomyelitis sinuses. This prospective observational study was conducted at the Department of Burn and Plastic Surgery, Dhaka Medical College Hospital (DMCH), Dhaka, Bangladesh on 30 clinically suspected Marjolin’s ulcer patients who underwent excision biopsy for histopathology of the tissue from May 2015 to October 2016. Socio-demographic characteristics of the patients, aetiology of the lesions, sites involved, clinical presentation, latency period, dimensions of the ulcers, histopathological diagnosis, margin clearance, surgical procedures, and the outcome of management was recorded accordingly. In this study, the mean age of the suspected Marjolin’s ulcer cases was 40.47±14.42 years (ranging from 15 to 75 years), and male to female ratio was 2.3:1. The most predominant cause of primary insult was flame burn (82.67%) and only 13.33% was road traffic accident (RTA) cases. The ulcers were found on the extremities in almost all cases (29 out of 30), and the lower limbs were the most predominant sites (19 out of 30). The most frequently observed clinical findings were cutaneous ulcers within the scars (70%), followed by foul-smelling discharge (53.33%) and bleeding from the ulcer sites (26.67%). The mean latency period was 19.5±4.26 years (ranging from 3 to 50 years and 11 months). The mean length of the ulcers was 5.67±3.12 cm and the mean width was 3.47±1.87 cm. According to the histopathology report, 77% of the resected tissues were diagnosed as malignant cases; mostly (63.34%), squamous cell carcinoma (SCC) of different grades, and 17% were benign cases (Chronic non-healing ulcer). Most of the cases (80%) underwent split-thickness skin grafting (STSG), five patients (16.67%) underwent different types of flaps coverage, and amputation followed by direct closure of the wound was done on one patient (
Keywords
<p>Burn; Excision Biopsy, Histopathology, Malignancy, Marjolin's Ulcer, Non-Healing Ulcer, Squamous Cell Carcinoma (SCC)</p>
Article Details
1. Introduction
Breakdown of unstable scar and formation of non-healing ulcer is a frequent finding in large burn centers. Break down of scars is predominant in the deep dermal wounds; more frequently in the full thickness burn wounds that heal by secondary intention [1]. This kind of healing leads to the formation of a weak scar that easily tends to give away and an ulcer is formed [1]. Apart from functional, cosmetic, and social problems, these ulcers have the potential for malignant transformation, which are then known as Marjolin's ulcers (MU) [1,2]. Therefore, Marjolin’s ulcer is a malignant lesion that develops in a burn scar or chronic fistula [2,3]. This is also described as a malignant lesion that arises from the area of chronic irritation or injury and undergoes malignant transformations after many years [4]. Similarly, some studies suggest that Marjolin’s ulcer is mostly a squamous cell carcinoma (SCC) arising from scars of chronic wounds and osteomyelitis discharging sinuses [3-5]. Marjolin’s ulcer may occur at any age, but it is uncommon in children [6]. The age of presentation varies widely, and in most cases, it is between the age of 20 and 90 years [4-6]. Marjolin's ulcer is more common in males, though the ratio varies a lot in different geographic locations [6,7]. Only in the case of malignant melanoma (MM), the incidence in females is greater than that in males [7]. Reports from Western countries reveal that the malignant transformation in men is three times more frequent than in women [8]. The precise mechanism of malignant transformation of chronic ulcers (wounds) is not yet clearly known; many theories have been postulated [9]. Few early theories suggested that cellular mutation from the effects of inflammatory substances which are released by damaged, ischemic, and nutritionally deficient tissues, are responsible for malignant transformation [10]. Some studies proposed that traumatic displacement of living epithelial tissues into the dermis may cause a foreign body response and lead to a deranged regenerative process which results in neoplastic change [11]. A theory of immunologic isolation has also been suggested [12]. Some studies postulate that chronic irritation and repeated damage to the ulcer take place constantly, and continuous mitotic activities of the epidermal cells occur as an attempt to resurface the defect, and this cycle ultimately leads to carcinomatous change [12,13]. In addition, patients with an inherent immune deficiency are at higher risk of developing malignant ulcers [14]. There are two variants of Marjolin’s ulcers; acute and chronic. In the acute form, malignant transformation is observed within 12 months from the date of the initial injury [3-5]. The chronic form is more frequent and malignancy develops slowly after a long latency period [4-6]. The interval period from injury to malignant transformation (latency period) was found different in different previous studies; typically, from 25 to 40 years [15-17]. This type of wide variation is mainly due to variation in genetic predisposition, environmental factors, and exposure to some carcinogens [15-17]. Diagnosis of Marjolin's ulcer is confirmed by histopathology examination of biopsies taken off from different suspected sites of the ulcer; either incisional or excision [16]. Squamous cell carcinoma (SCC) is the most predominant histological type, followed by basal cell carcinoma (BCC), malignant melanoma (MM), sarcomas (fibrosarcoma, liposarcoma, etc.), and others [3,5,8,16,17]. Again, SCC may be of three types; well-differentiated, moderately differentiated, and poorly differentiated [15-17]. The prognosis of squamous cell carcinoma (SCC) of chronic ulcer is the worst in comparison to other types; moreover, it develops de novo and requires aggressive treatment [15-17]. Early recurrence and death within 6 months of surgery have been reported in some studies [15-17]. Re-excision, radiotherapy, and adjuvant chemotherapy are recommended for managing the recurrence [15-17]. The prognosis of Marjolin’s ulcer depends on various factors; most importantly, age, size, type, grade of the disease, stage of disease, presence of local recurrence, and distant metastases [15-17]. Long-term follow-up is recommended in all cases [15-17]. This current study evaluated the clinically suspected Marjolin’s ulcer cases and the histopathological findings of the biopsies obtained from them, and that was conducted at an University teaching hospital in Bangladesh.
2. Methodology
This prospective observational study was conducted in the Department of Burn and Plastic Surgery, Dhaka Medical College Hospital (DMCH), Dhaka, Bangladesh from 1 May 2015 to 31 October 2016. A total of 30 clinically suspected Marjolin’s ulcer patients were selected according to inclusion and exclusion criteria. They underwent excision biopsies of the ulcers; the ulcers were excised with 2 cm margin clearance as well as deep tissue clearance. Patients with cutaneous scars for the last 12 months or more and non-healing ulcers for 12 weeks or more were selected primarily. Then, they were further evaluated with the presence of one or more of the potential clinical presentations; for example, non-healing ulcers with irregular bases and/or margins along with raised edges in any part of the ulcer, foul-smelling discharge, bleeding from the ulcer, uncontrolled growth of granulation tissue or exophytic growth, and regional lymphadenopathy. Finally, the patients who were willing to undergo excision biopsy of their ulcers with 2 cm margin clearance as well as provided informed written consent for this study were included. Data, regarding socio-demographic characteristics of the patients, aetiology of the lesions, anatomical sites, duration of the ulcer at the time of clinical diagnosis, clinical presentations, latency period, dimensions of the ulcers, histopathological diagnosis, margin clearance, deep surface clearance, treatment modalities, and outcomes (of reconstruction) were recorded accordingly. Obtained data were summarized and analyzed using Statistical Package for Social Sciences (SPSS) version 20.
Surgical procedures
The well-practiced option for the treatment of squamous cell carcinoma (SCC) Marjolin’s ulcer is excision of the ulcer along with a 2 cm wide rim of ulcer-free scar or apparently normal tissue circumferentially for the first diagnosis; most preferably along with a frozen section biopsy facility. If any of the margins are found involved, excision of farther 0.5 cm tissue is recommended for re-examination. But in the majority of the recurrent cases, the 2 cm resected safety margins are still found to be involved and necessitate a further 0.5 cm excision for attaining the evidence of tumors-free margin and depth. Therefore, for the purpose of preventing recurrence and avoiding further complications, resection of apparently visible ulcers with a 2 to 2.5 cm safety margin is practiced widely. The biopsy samples should ideally be sent for a frozen section histopathology report prior to any flap reconstruction in the same sitting; if not possible, the surgically created defect should ideally be covered by split-thickness skin grafting (STSG). Alternatively, a full-thickness skin grafting may also be considered where it is appropriate or ideal; especially, over the joints, tendons, or smaller defects. Flap reconstruction may also be required if any of the vital structures are exposed. Amputation is considered in some advanced and/or recurrent cases; especially, in the cases of limb involvement. In this study, split-thickness skin grafting (STSG) was performed among 24 patients, five patients underwent different types of flaps reconstruction, and amputation followed by direct closure was done on one patient.
Follow-up protocol
Regular daily follow-up was carried out until the discharge of the patients. At the time of discharge, the patients were advised to come and visit the research team for follow-ups that were scheduled on the 5th, 7th, 14th, and 28th postoperative days. The outcome of the surgery was evaluated and recorded accordingly.
3. Results and Observations
This study was carried out to evaluate the clinicopathological status of the Marjolin’s ulcer patients who were hospitalized within the duration of this study period. In this study, 30 patients were selected according to the selection criteria. The age range of the patients was set at a range from 2 to 80 years. The mean age was 40.47 years, with a standard deviation (SD) of ±14.42 years. Most of the patients were between 21 and 60 years. The youngest and the oldest patient in this study were 15 years and 75 years old respectively (Table 1). Among these 30 study subjects, 21 were male (70%) and 9 were female (30%); a male predominance was observed with a male-to-female ratio of 2.3:1 (Table 1). It was observed that, seventy percent (70%) of the study patients hand primary and secondary level education and 13.33% of patients were illiterate (Table 1). This data reflect that the lower level of educational status might have a relationship with less awareness of patients about self-care, timely visit to a qualified surgeon, and the need for proper treatment of long-standing scars and non-healing ulcers. Occupation analysis revealed that, majority of the study patients were businessmen (23.33%), housewives (26.67%), and farmers (20%); while, three (10%) were students, another three (10%) were day laborers, two (6.67%) were service holders, and one (3.33%) patient was under the other category (Table 1). These findings reflect that Marjolin’s ulcer is more predominant in the more physically active group of people.
|
Socio-demographic characteristics |
Frequency (n) |
Percentage (%) |
|
Age of patients (years) |
||
|
2-10 |
- |
- |
|
11-20 |
3 |
10 |
|
21-30 |
4 |
13.33 |
|
31-40 |
9 |
30 |
|
41-50 |
8 |
26.67 |
|
51-60 |
4 |
13.33 |
|
61-70 |
1 |
3.33 |
|
>70 |
1 |
3.33 |
|
Mean±SD/(range) |
40.47±14.42/(15-75 years) |
|
|
Gender |
||
|
Male |
21 |
70 |
|
Female |
9 |
30 |
|
Male-to-female ratio |
2.3:1 |
|
|
Education level |
||
|
Illiterate |
4 |
13.33 |
|
Primary |
11 |
36.67 |
|
Secondary |
10 |
33.33 |
|
Higher Secondary |
5 |
16.67 |
|
Graduate and above |
- |
- |
|
Occupation |
||
|
Small business (Shopkeeper) |
7 |
23.33 |
|
Service |
2 |
6.67 |
|
Day laborer |
3 |
10 |
|
Farmer |
6 |
20 |
|
Student |
3 |
10 |
|
Housewife |
8 |
26.67 |
|
Other |
1 |
3.33 |
Table 1: Socio-demographic characteristics of the study patients (N=30)
By evaluating the causes of primary injury demonstrated that; 26 (86.67%) patients developed Marjolin’s ulcer from burn injuries, 4 (13.33%) patients developed it from trauma due to road traffic accidents (RTA) and one patient (3.33%) presented with chronic osteomyelitis, but the primary cause of the insult was a flame burn (Table 2). Out of 26 burn injury patients; 22 (84.62%) were victims of flame burn, three (11.54%) were chemical burn patients, and one (3.84%) patient survived from the electric burn (Table- 2). Most ulcers developed on the extremities [29 (96.66%)]; of them, nineteen (63.33%) patients developed ulcers on their lower limbs, ten patients (33.33%) on the upper limbs, and one of them (3.33%) developed the ulcer on the head/neck/face area (Table 2).
|
The cause, type and site of burn scars |
Frequency (n) |
Percentage (%) |
|
Causes of burn scars |
||
|
Burn |
26 |
86.67 |
|
RTA* Trauma |
4 |
13.33 |
|
Osteomyelitis (partially) and |
1 |
3.33 |
|
Flame burn (partially) |
||
|
Pressure Sore and others |
- |
- |
|
Type of Burn |
||
|
Flame Burn |
22 |
84.62 |
|
Chemical Burn |
3 |
11.54 |
|
Electric Burn |
1 |
3.84 |
|
Scald |
- |
- |
|
Sites |
||
|
Lower limb |
19 |
63.33 |
|
Upper Limb |
10 |
33.33 |
|
Head, Neck, Face |
1 |
3.33 |
|
Trunk |
- |
- |
* RTA= Road Traffic Accident
Table 2: Distribution of the study patients according to the cause, type and site of burn scar formation (N= 30)
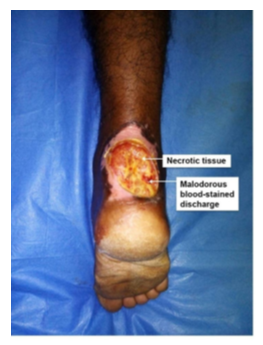
Out of the total 30 study patients, the maximum number [21 (70%)] of them presented the disease as a cutaneous ulcer within the scar, two of them (6.67%) presented with cutaneous ulcers beyond the scars, three (10%) patients presented with fungating ulcer within the scar, another three (10%) patients with fungating ulcer beyond the scar, and one (3.33%) of them presented with the signs of chronic osteomyelitis. In addition, 16 (53.33%) patients presented with foul-smelling discharge, eight (26.67%) patients presented with bleeding, and four (13.33%) patients presented with palpable enlarged regional lymph nodes. No evidence of distant metastasis was observed in this study (Table 3).
|
Clinical presentation* |
Frequency (n) |
Percentage (%) |
|
Cutaneous ulcer within the scar |
21 |
70 |
|
Cutaneous ulcer beyond the scar |
2 |
6.67 |
|
Fungating ulcer within the scar |
3 |
10 |
|
Fungating ulcer beyond the scar |
3 |
10 |
|
Foul-smelling discharge |
16 |
53.33 |
|
Bleeding |
8 |
26.67 |
|
Lymphadenopathy |
4 |
13.33 |
|
Chronic Osteomyelitis |
1 |
3.33 |
*Multiple responses
Table 3: Distribution of the study patients according to clinical presentation
The latency period was observed very long along with a wide range. In six cases (20%), the latency period was found between 21 to 25 years range. In addition, five cases (16.67%) were noted in each of the three ranges of latency periods of 6 to 10 years, 11 to 15 years, and 16 to 20 years; followed by, three cases (10%) in the range of 26 to 30 years, one (3.33%) case in 31 to 35 years, and one case (3.33%) in more than 40 years range group. The minimum latency period was observed as 3 years and the maximum latency period was 50 years and 11 months. The mean latency period was calculated as 19.5 years with a standard deviation (SD) of ±4.26 years (Table 4).
|
Latency period (years) |
Frequency (n) |
Percentage (%) |
Mean±SD (Years) |
|
1-5 |
4 |
13.33 |
19.5±4.26 |
|
6-10 |
5 |
16.67 |
|
|
11-15 |
5 |
16.67 |
|
|
16-20 |
5 |
16.67 |
|
|
21-25 |
6 |
20 |
|
|
26-30 |
3 |
10 |
|
|
31-35 |
1 |
3.33 |
|
|
36-40 |
- |
- |
|
|
>40 |
1 |
3.33 |
|
|
Total |
30 |
100% |
Table 4: Distributions of the study patients according to the latency period (N=30)
In this study; 17 (56.67%) patients were presented with ulcers for 6 to 12 months duration, followed by 6 cases (20%) for 13 to 18 months, 3 (10%) patients for 19 to 24 months, and 4 (13.33%) patinets for more than 24 months (Table 5).
|
Duration of ulcer (Months) |
Frequency (n) |
Percentage (%) |
|
6 - 12 |
17 |
56.67 |
|
13 - 18 |
6 |
20 |
|
19 - 24 |
3 |
10 |
|
>24 |
4 |
13.33 |
|
Total |
30 |
100% |
Table 5: Distribution of the study patients according to the duration of ulcer at the time of clinical diagnosis (N=30)
Regarding the dimension of the ulcers; the mean longitudinal dimension or length was calculated as 5.67 cm with a standard deviation (SD) of ±3.12 cm, and the range was observed from 2 to14 cm. On the other hand, the mean transverse dimension or the width was found 3.47 cm with a standard deviation (SD) of ±1.87 cm, and the range was from 1 to 8 cm (Table 6).
|
Wound Measurement |
Mean (cm) |
SD (cm) |
Range (cm) |
|
Longitudinal dimension/Length (cm) |
5.67 |
±3.12 |
2-14 |
|
Transverse dimension/Width (cm) |
3.47 |
±1.87 |
1-8 |
Table 6: The size of the ulcers at the time of clinical diagnosis
All of the resected tissues (biopsies) were sent for histopathological studies, and most of the cases (63.34%) were diagnosed as squamous cell carcinoma (SCC) which was 19 in number. Out of these 19 cases, 14 were well-differentiated, three were moderately differentiated, and two were poorly differentiated SCC. Next to SCC, four (13.33%) cases were diagnosed simply as Marjolin’s ulcer though the description of the histopathology report mentioned the early stage of malignant changes at the ulcer margins. On the other hand, one resected tissue was diagnosed with hyperkeratosis and another one with pseudo-epitheliomatous hyperplasia at the ulcer margin; both of which are considered an early stage of malignant transformation. Only five (16.67%) cases were reported as chronic non-healing ulcers that were benign in nature (Table 7).
|
Histopathological diagnosis |
Frequency (n) |
Percentage (%) |
|
Squamous cell carcinoma (SCC) |
19 |
63.34 |
|
a) Well-differentiated |
14 (73.68% of 19 cases) |
|
|
b) Moderately differentiated |
3 (15.78% of 19 cases) |
|
|
c) Poorly differentiated |
2 (10.52 of 19 cases) |
|
|
Marjolin’s ulcer |
4 |
13.33 |
|
Basal cell carcinoma (BCC) |
- |
- |
|
Malignant melanoma (MM) |
- |
- |
|
Sarcoma |
- |
- |
|
Hyperkeratosis |
1 |
3.33 |
|
Pseudo-epitheliomatous hyperplasia at the ulcer margin |
1 |
3.33 |
|
Chronic non-healing ulcer |
5 |
16.67 |
|
Total |
30 |
100% |
Table 7: Distribution of the study patients according to the histopathology reports (N= 30)
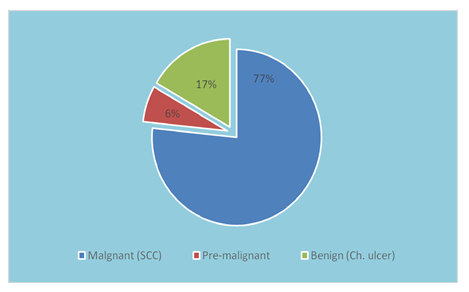
Careful analysis of these data revealed that; 77% of the study patients were confirmed as malignant ulcers with the histopathology report, 6% were pre-malignant cases, and only 17% were chronic non-healing ulcers that were benign ulcers (Figure 3).
In this study, all the patients underwent excision of ulcer with 2 cm rim of surrounding tissue from the apparently healthy margin and then histopathology was performed for the diagnosis to be confirmed as well as for the assessment of the margin clearance. According to the histopathology reports, resected tissues were found free of malignant cells in all four margins as well as on the deep surface in 21 cases which were equivalent to 70 percent of all cases. Tumor-involved medial margin was identified in two (6.66%) resected tissues, and one (3.33%) was involved on the deep surface. All of the corresponding margins were re-excised for a further 0.5 cm, re-examined their histopathology, and then margins were found free of malignant cells (Table 8).
|
Resected margin involvement |
Frequency (n) |
Percentage (%) |
|
Proximal margin involved |
- |
- |
|
Distal margin involved |
- |
- |
|
Medial margin involved |
2 |
6.66 |
|
Lateral margin involved |
- |
- |
|
Deep Surface involved |
1 |
3.33 |
|
All margins are free |
21 |
70 |
|
Margin clearance not done/Not applicable |
6 |
20 |
|
Total |
30 |
100% |
Table 8: Distribution of the study patients according to the margin clearance (histo-pathologically)
In this study, most of our patients [24 (80%)] underwent split-thickness skin grafting (STSG), five of them received different types of flap coverages (16.67%), and amputation followed by direct closure of the wound was performed on one patient (3.33%) (Table 9).
|
Procedure of reconstruction |
Frequency (n) |
Percentage (%) |
|
STSG* |
24 |
80 |
|
Flaps |
5 |
16.67 |
|
Amputation & direct closure |
1 |
3.33 |
|
Total |
30 |
100% |
*STSG= Split-thickness skin grafting
Table 9: Distribution of the study patients according to the reconstruction after the excision (N=30)
Figure 4(A,B,C,D): (A) A 38-year old female with a Marjolin's ulcer on the back of the thigh. The duration of the ulcer was 7 months and the latency period was 8 years. (B) Marked for planned exicision. (C) Excision of the ulcer and release of contracture (D) Split thickness skin grafting- STSG (per-operative).
In most of the STSG [14(46.67%)] cases, the grafted skin was well-taken by the recipient sites, and the outcome was good; In nine (30%) patients, the result was satisfactory with less than five percent loss of grafted skin in each case. The outcomes of all the five (16.67%) cases of flaps were satisfactory with no more than ten percent loss of flap, and the result of only one (3.33%) case of amputation was good (Table 10).
|
Types of surgical procedure done |
Results |
Frequency (n) |
Percentage (%) |
|
STSG* |
1. Good |
14 |
46.67 |
|
2. Satisfactory |
9 |
30 |
|
|
3. Poor |
- |
- |
|
|
Flap |
1. Good |
- |
- |
|
2. Satisfactory |
5 |
16.67 |
|
|
3. Poor |
1 |
3.33 |
|
|
Amputation |
1. Good |
1 |
3.33 |
|
2. Satisfactory |
- |
- |
|
|
3. Poor |
- |
- |
|
|
Total (N) |
30 |
100% |
*STSG= Split-thickness skin grafting
Table 10: Distribution of the study patients according to the postoperative outcome
4. Discussion
Non-healing ulcers on the chronic scars are presented to us with a range of clinical and histo-pathological findings. These ulcers can be very notorious because of having the potential for malignant transformation; hence, they have been termed Marjolin's ulcers [18]. Therefore, the most important thing is to exclude the presence of malignant cells in them. Although some morphological variations are noted, both benign and malignant ulcers have more or less similar clinical courses [15-18]. Therefore, any non-healing ulcer on a chronic scar should be managed aggressively as a malignant one, unless and until proved otherwise [19]. This prospective study was designed to determine the presence of malignancies in the clinically suspected Marjolin’s ulcer cases; the resected tissues were examined for malignant cells by histopathology. The study included 30 patients who presented with non-healing ulcers (at least for twelve weeks) on long-standing scars (at least for twelve months), and this was conducted over a period of 17 months, from May 2015 to October 2016, at one of the largest burn and plastic surgery center in the world. Burn scar carcinoma may occur at any age, and it has no strong race predisposition [15-19]. The ratio of transformation of a long-standing ulcer on the scar into a Marjolin’s ulcer was reported as three times more common in men than women [6,17,20]; and in our study, this ratio was 2.3:1. Among our 30 patients, 21 were males (70%) and nine were females (30%). The reason for this gender discrepancy might be due to the genetic predisposition of the male gender, more strenuous physical activities by the males, due to exposure of males to harsher environmental factors, or occupation-related adverse conditions. Considering the results of different studies, we found some variations in the average age of the malignant transformation; but, many of them were close to the age of 58 years, and the range was from 18 to 84 years [6,20]. The mean age of our study cases was 40.47±14.42 years with an age range from 15 to 75 years. Most of our patients (21 out of 30) were educated up to the high school level (grade 10), four patients never went to school, and only five of them completed the higher secondary (grade 12) level of education. These numbers reflect that more than 80% of our study patients came from a lower level of education which might be related to a lack of awareness about wound care and failure of addressing the potential changes at an early stage. On the other hand, eight of our patients were housewives, seven were businessmen, and six were farmers. The countries, where the number of Marjolin’s ulcers is relatively high, the healthcare system needs to incorporate more precise and effective health education and social awareness campaigns on the prevention, risk factors, and early signs of presentation of malignant transformation as well as the comprehensive treatment protocols without further delay. In this study, the primary cause of scar formation was mostly burn injuries, and this was observed in 86.67% of the study patients. Of them; most of the burn cases (84.62%) were flame burns, followed by 11.54% chemical burns and 3.84% electric burns; while, no patient was a victim of scald. After the burn injuries, road traffic accidents (RTA) and trauma were the other common causes of Marjolin’s ulcer in our study. Moreover, one of our patients was reported with chronic osteomyelitis discharging sinuses for about 3 years, but she was also a victim of a flame burn. This means that the sinuses were developed on this preexisting old flame burn scar. Chalya PL et al. showed in a retrospective study of histologically confirmed cases of Marjolin’s ulcers that burn scars (89.3%) were the most common causative lesions for those ulcers, and this report was very close to our observation [17]. In contrast, Asuquo ME et al. from Nigeria mentioned that trauma was the leading cause of injury for Marjolin’s ulcer formation, while the rest of the patients suffered from diabetic foot ulcers [20]. According to Tavares E et al., osteomyelitis is also a well-known pre-existing condition for developing squamous cell carcinoma (SCC) [16]. The incidence of Marjolin’s ulcer in chronic osteomyelitis is difficult to evaluate; however, studies reported that Marjolin’s ulcers developed in 1.5% of all cases of chronic osteomyelitis [21,22]. Most of our study patients were suffering from ulcers on their limbs (96.67%). Among 30 patients, 29 had ulcers on their limbs; of them, 19(63.33%) were on the lower limbs, and ten (33.33%) were on the upper limbs, only one patient had an ulcer on the scalp which was clinically presented along with chronic osteomyelitis discharging sinuses. Similar results were revealed by different researchers in their studies [6,17]. The most common type of carcinoma reported in almost all previous studies was squamous cell carcinoma (SCC), and it constitutes 75-90% of all cases which was consistent with our study [3,5,8,15-17]. Some pieces of research revealed that malignant transformation occurs after a mean period of 43 years of the initial lesion; however, this period may vary from 10 to 70 years [6,15,17,23]. In our study, we found a mean (±SD) latent period of 19.5(±4.26) years with a range of 3 to 51 years. One of our patients presented with osteomyelitis sinuses that developed on a primary burn scar and developed malignancy after a very long latent period. Many previous studies reported the latent period of Marjolin’s ulcer [3-5, 15-17]. Some studies reported very small latent periods; for example, 7 months, 3 months, or even 6 weeks [3-5]. These are categorized under the acute Marjoiln’s ulcer group [4-6]. Variations in the latency period may occur from some contributing factors, such as environmental factors, immunological status, age of the patient, age at the primary insult, and genetic difference. Most of our clinically suspected Marjolin’s ulcer cases [19 (63.34%)] were suffering from squamous cell carcinoma (SCC); these were confirmed by histopathology examinations. Out of these 19 cases; 14 (73.68%) were well-differentiated, three (15.78%) were moderately differentiated, and two (10.52%) were poorly differentiated SCC. This result was consistent with related previous studies [14,15]. The majority of the scars developed from flame burns, and all of them from the full-thickness (third-degree) burn which was similar to related previous studies [17-19]. Most of our study cases presented the ulcer on their extremities with predominance (63.33%) in the lower extremities; this observation was consistent with many previous studies [6,15-20]. One of the most important aspects of this study was the evaluation of the clinical presentation of Marjolin’s ulcer. Our aim was to find out the most frequent clinical presentations in the suspected Masrjolin’s ulcer cases and to evaluate how many of these suspected cases were confirmed by histopathology. We recorded the cutaneous ulcers within the scars in70% (21 out of 30) cases, cutaneous ulcers beyond the scars in 6.67% (two out of 30) cases, fungating ulcers within the scar in 10% of cases, fungating ulcers beyond the scars in 10% cases, lymphadenopathy was found in four patients (13.33%), and chronic osteomyelitis was the main presentation in one patient (3.33%). We also observed malodorous discharge in 16 (53.33%) out of 30 patients and bleeding in eight (26.67%) patients. The surrounding skin (scar) of the ulcer was hypo-pigmented in all cases of burn injuries. A significant number of patients complained about movement’s restriction due to the presence of ulcers; most of those lesions were in the extremities. This restriction in movements was mostly due to the associated scar contracture. The mean longitudinal dimension of the ulcers of our patients was 5.67±3.12 cm (ranging from 2 to 14 cm) and that of the transverse dimension was 3.47±1.87 cm (ranging from 1 to 8 cm). The majority of them were six centimeters or less in maximum diameter. According to some previous studies, ulcers with a diameter of more than 6 cm are more likely to transform into malignancies [15-18]. The finding of our study correlates that the diameter is not consistent with other reports. This discrepancy may be due to differences in genetic and environmental factors in different populations as well as many other factors. Although variations in diameter exist, clinicians should more aggressively evaluate the large lesions and complaints of increase in sizes for the diagnosing of malignant transformation [5,24].
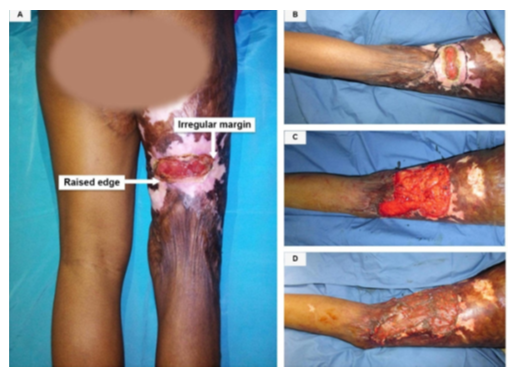
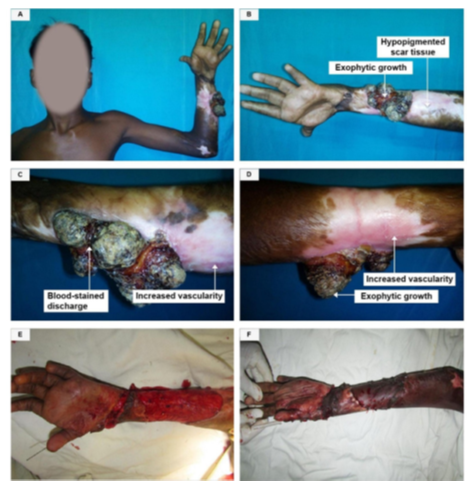
Figure 5 (A,B,C,D,E,F): (A) A 15-year old male with a Marjolin's Ulcer on the left forearm; confirmed as Well differentiated SSC. The duration of the ulcer was 9 months and the latency period was 3 years. (B,C,D) Fungating ulcer beyond the scar crossing one of it’s margins with malodorous blood- stained discharge. (E) After the exicision of the ulcer (per-operation). (F) After split thickness skin grafting- STSG (per-operative).
In this study, histopathology of the resected tissues identified malignancies in 23 (76.67%) patients. Moreover, one of our patients was diagnosed with pseudo-epitheliomatous hyperplasia, one was hyperkeratosis, and the remaining five cases were benign ulcers. All 23 cases were confirmed squamous cell carcinomas of different grades. Most of the related studies on Marjolin's ulcers reported a predominance of squamous cell carcinoma (SCC); but other types were also reported in some related studies [3,5]. The degree of differentiation is also important as the risk of metastasis correlates with the degree of differentiation [6-8]. It was reported that, the incidence of metastasis is 10% for Grade I lesions, 59% for Grade II lesions, and 86% for Grade III lesions [6]; we also documented four cases (13.33%) of lymphadenopathy that indicates the close proximity to the observations in many other previous studies [6-8]. In our study, 19 cases (63.34%) were diagnosed as squamous cell carcinoma (SCC); and out of these 19 cases, 14 were well-differentiated, three were moderately differentiated, and only two were poorly differentiated SCC. Next to SCC, four cases were diagnosed simply as Marjolin’s ulcer (13.33%) which was also regarded as squamous cell carcinomas, and five cases were chronic non-healing ulcers (16.67%). Two other patients were diagnosed as hyperkeratosis and pseudo-epitheliomatous hyperplasia at the ulcer margin respectively which are considered to be the early stages of SCC. These findings are in an agreement with related previous studies [15-18]. As in other related studies, the ulcers of our patients were excised with 2 cm of healthy margin [24,25]. Split thickness skin grafting (STSG) was performed on 24 (80%) patients while five (16.67%) patients required local flaps coverage and one (3.33%) of our patients underwent amputation at below knee level. There is an association between margins and/or deep surface involvement with poor wound healing. Two (6.66%) of our patients had involved medial margin and in one patient (3.33%) the deep surface was involved; while, 21 (70%) were free of all margin. Margin clearance was not relevant for chronic non-healing ulcer cases. The surgical outcome was good in most cases and satisfactory in the rest of the cases except in one case of flap reconstruction. The wound healing was good, and the “Take” of split-thickness skin grafting was satisfactory except in very minimum areas of a few wounds. All patients with Marjolin's ulcers were referred to the oncology department for post-operative evaluation and further management. No mortality was recorded during the study period.
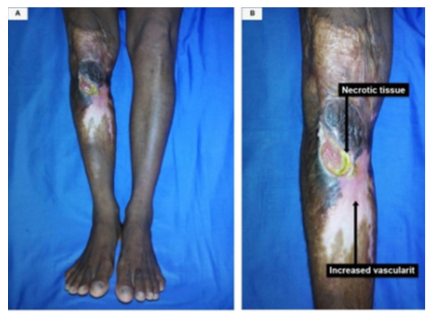
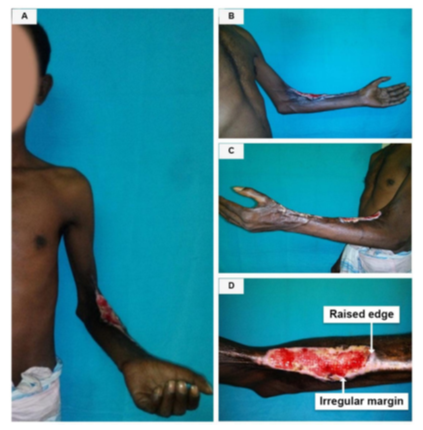
Figure 6 (A,B,C,D): (A) A 45-year-old male with Marjolin's ulcer (Well differentiated SCC) on the left forearm The duration of the ulcer was 18 months with a latent period of 14 years. (B) Medial view: (C) Lateral view. (D) A closure view shows the irregular margins of the ulcer and the edge is raised along the distal margin.
Conclusion
Marjolin’s ulcers are more frequent in the adult age group with male predominance. The majority of Marjolin’s ulcers develop from flame burn injuries followed by trauma, and the limbs are affected most with lower limb predominance. The mean latent period in our study was found 19.5 years; the minimum period was documented as three years and the maximum was 50 years 11 months. In this study, the maximum patients of Marjolin’s ulcers were presented as cutaneous ulcers within the long-standing scars followed by many other forms of clinical presentations; like cutaneous ulcers beyond the scars, fungating ulcers within the scars, fungating ulcers beyond the scars, chronic osteomyelitis, foul-smelling discharge, bleeding, and enlarged regional lymph nodes or other signs of distant metastasis. Most of the Marjolin’s ulcers cases were confirmed as malignant ulcers, and squamous cell carcinoma (SCC) was the most frequent diagnosis by histopathology. Many Majrolin’s ulcer cases are commonly mistaken for long standing non-healing ulcers. Therefore, all chronic ulcers on long-standing scars and wounds are suspicious as malignant ones and should undergo multiple biopsies from clinically suspected sites to avoid missing of malignancies in these ulcers. By developing evidence-based structured clinical diagnostic tools for evaluation of the suspicious malignancies in long-standing scars and by ensuring their proper management at an early stage can save valuable lives of many patients.
Limitation of the study
It was a single-center study.
Recommendation
Long-term multicenter studies with a large number of patients will be more conclusive in identifying the most frequent clinical presentations as well as confirming the presence of malignancies in Marjolin’s ulcer cases.
Conflict of interest
None declared.
References
- Tobin C, Sanger JR. Marjolin's Ulcers: A Case Series and Literature Review. Wounds: A Compendium of Clinical Research and Practice 26 (2014): 248-254.
- Yu N, Long X, Lujan-Hernandez JR, et al. Marjolin’s ulcer: a preventable malignancy arising from scars. World Journal of Surgical Oncology 11 (2013): 1-7.
- Hahn SB, Kim DJ, Jeon CH. Clinical study of Marjolin's ulcer. Yonsei Medical Journal 31 (1990): 234-241.
- Soh LJ, Tan HK. Acute Marjolin's ulcer: a forgotten entity. Annals of the Academy of Medicine, Singapore 42 (2013): 153-154.
- Khan K, Giannone AL, Mehrabi E, et al. Marjolin’s ulcer complicating a pressure sore: the clock is ticking. American Journal of Case Reports 17 (2016): 111.
- Fazeli MS, Lebaschi AH, Hajirostam M, et al. Marjolin's ulcer: clinical and pathologic features of 83 cases and review of literature. Medical Journal of the Islamic Republic of Iran 56 (2013): 215-229.
- Ortiz BD, Riveros R, Gabriela MB, et al. Marjolin ulcer: a case report. Our Dermatol Online 5 (2014): 51-53.
- Wronski K. Marjolin’s ulcer of the thigh after burn injury- case report and review of literature. New Medicine. 18 (2014): 69-75.
- Nthumba PM. Marjolin's ulcers: theories, prognostic factors and their peculiarities in spina bifida patients. World Journal of Surgical Oncology 8 (2010): 1-5.
- Treves N. The development of cancer in burn scars: an analysis and report of thirty-four cases. Surg Gynecol Obstet 51 (1930): 749-82.
- Neuman Z, Ben-Hur N, Shulman J. Trauma and skin cancer: Implantation of epidermal elements and possible cause. Plastic and reconstructive surgery 32 (1963): 649-56.
- Bostwick J, Pendergrast JR WJ, Vasconez LO. Marjolins Ulcer: An Immunologically Privileged Tumor?. Plastic and Reconstructive Surgery 57 (1976): 66-69.
- Copcu E, Aktas A, Sisman N, et al. Thirty-one cases of Marjolin's ulcer. Clinical and Experimental Dermatology: Clinical dermatology 28 (2003): 138-141.
- Trent JT, Kirsner RS. Wounds and malignancy. Advances in skin & wound care 16 (2003): 31-34.
- Shahla A. An overview of heel Marjolin's ulcers in the Orthopedic Department of Urmia University of Medical Sciences 12 (2009): 405-408.
- Tavares E, Martinho G, Dores JA, et al. Marjolin's ulcer associated with ulceration and chronic osteomyelitis. Anais Brasileiros de Dermatologia 86 (2011): 366-369.
- Chalya PL, Mabula JB, Gilyoma JM, et al. Early Marjolin’s ulcer developing in a penile human bite scar of an adult patient presenting at Bugando Medical Centre, Tanzania: A case report. Tanzania Journal of Health Research 14 (2012): 82-95.
- Cruickshank AH, McConnell EM, Miller DG. Malignancy in scars, chronic ulcers, and sinuses. Journal of clinical pathology 16 (1963): 573-580.
- LH K. Carcinoma within a chronic burn scar (Marjolin's ulcer). Report of a case. The Medical Annals of the District of Columbia 33 (1964): 620-622.
- Chalya PL, Mabula JB, Rambau P, et al. Marjolin's ulcers at a university teaching hospital in Northwestern Tanzania: a retrospective review of 56 cases. World journal of surgical oncology 10 (2012): 1-8.
- Asuquo ME, Nwagbara VI, Omotoso A, et al. Marjolin’s ulcer: mismanaged chronic cutaneous ulcers. J Clin Exp Dermatol Res S 6 (2013): 228-239.
- Hobart MH, Miller DS. Unusual complications of osteomyelitis. The American Journal of Surgery 45 (1939): 53-59.
- Bauer T, David T, Rimareix F, et al. Marjolin's ulcer in chronic osteomyelitis: seven cases and a review of the literature. Revue de chirurgieorthopedique et reparatrice de l'appareilmoteur 93 (2007): 63-71.
- Sengul G, Hadi-Kadioglu H. Penetrating Marjolin's ulcer of scalp involving bone, dura mater and brain caused by blunt trauma to the burned area. Neurocirugía 20 (2009): 474-477.
- Johnson P, Brookins S, Beech D, et al. Marjolin's ulcer of a primarily grafted burn. Journal of the National Medical Association 105 (2013): 192.
- Ghalambor A. Marjolin ulcer: how much of safety margin needs resection along Marjolin ulcer squamous cell carcinoma in recurrence cases. Pakistan Journal of Medical Sciences 23 (2007): 394-397.


 Impact Factor: * 4.2
Impact Factor: * 4.2 Acceptance Rate: 72.62%
Acceptance Rate: 72.62%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 