Long-Term Persistence of Diastolic Dysfunction and Dyspnea in Patients who Underwent Aortic Valve Replacement for Aortic Valve Stenosis
Valentin Bridonneau1, Elena Galli1, Elise Paven1, Kyriakos Yiangou2, Jean-Philippe Verhoye3, Christophe Leclercq1, Guillaume Bouzille4, Erwan Donal1*
1Université Rennes 1, Cardiology Department, Rennes University Hospital, Rennes, France
2Cardiology Department, Nicosia General Hospital, Nicosia, Cyprus
3Cardiothoracic and vascular surgery department, Rennes University Hospital, Rennes, France
4Inserm, Rennes University, Rennes University Hospital, Inra, Signal and Image Processing Laboratory, Clinical Data Center, Rennes, France
*Corresponding Author: Erwan Donal, Cardiology Service, Pontchaillou Hospital, Rennes University Hospital, F-35033 Rennes, France
Received: 05 September 2019; Accepted: 20 September 2019; Published: 24 September 2019
Article Information
Citation:
Bridonneau V, Galli E, Paven E, Yiangou K, Verhoye JP, Leclercq C, Bouzille G, Donal E. Long-Term Persistence of Diastolic Dysfunction and Dyspnea in Patients who Underwent Aortic Valve Replacement for Aortic Valve Stenosis. Journal of Surgery and Research 2 (2019): 217-230.
View / Download Pdf Share at FacebookAbstract
Background: Aortic stenosis (AS) requires treatment when is severe and symptomatic. However, after the correction of the AS, some patients still remain symptomatic. Severe AS can lead after aortic valve replacement to heart failure with preserved ejection fraction (HFpEF). We sought to describe the clinical status and to characterized patients based on E/e’ at 1-year follow-up of patients operated for an AS according to current guidelines.
Method and Results: 236 patients were prospectively included before AVR (surgical or transcatheter) and followed-up at one year with a standardized clinical and echocardiographic evaluation. Among 191 patients evaluated at follow-up (FU), 82 (43%) remained symptomatic (NYHA ≥II). Persistence of dyspnea at 1-year FU was associated with more severe echocardiographic diastolic function indices. Diastolic dysfunction (DD) was still demonstrated in 120 (63%) patients at one year. Using E/e’ as a surrogate marker of DD and elevated filling pressures, it was possible to characterize determinants of its persistence (E/e' ≥ 14). Our model demonstrated that patients with severe pre-operative DD (baseline E/e' ratio >16 and mitral E wave velocity >111 cm/s) had persistent elevated LV filling pressures, as opposed to patients who underwent surgical valve replacement with a baseline E/e' ratio ≤ 16 who mostly normalized their LV filling pressure.
Conclusion: 43% of treated AS patients remain symptomatic. DD characterization according to current standards allowed to predict E/e’ at one-year post AVR.
Keywords
<p>Aortic valve stenosis, Diastolic dysfunction, E/e' ratio, Dyspnea</p>
Article Details
Abbreviations:
AF-Atrial fibrillation; AS-Aortic stenosis; AVR-Aortic valve replacement; BSA-Body surface area; CABG-Coronary artery bypass graft; CAD-Coronary artery disease; COPD-Chronic obstructive pulmonary disease; DD-Diastolic dysfunction; HFpEF-Heart failure with preserved ejection fraction; IVSTd-Interventricular septum thickness in diastole; LA-Left atrium/left atrial; LV-Left ventricle/left ventricular; LVEDd-Left ventricular end-diastolic diameter; LVEF-Left ventricular ejection fraction; LVM-Left ventricular mass; SAVR-Surgical aortic valve replacement; TAVR-Transcatheter aortic valve replacement
1. Introduction
Severe aortic stenosis (AS) is responsible for a left ventricular (LV) chronic pressure overload, resulting in LV hypertrophy (adaptative and maladaptive) and subsequent diastolic dysfunction (DD). DD is thus related to LV-remodeling and potential irreversible myocardial damages [1, 2]. Symptoms are common with heart failure and dyspnea induced by stress. Current guidelines strongly recommend (class Ia) aortic valve replacement (surgical (SAVR) or transcatheter (TAVR)) for patients with severe symptomatic high-gradient aortic stenosis or when valve disease is associated with LV systolic dysfunction [3, 4]. All these patients have prior to surgery a DD with different degree of severity. However, a significant proportion of patients experience persistent dyspnea despite that guidelines were followed [5]. For example in the PARTNER II trial [6], 30 to 40% of surviving patients remained in NYHA class II or more 2 years after TAVR or SAVR. After TAVR, a significant proportion of patients might be considered as patients with a heart failure with preserved ejection [7].
Diastolic dysfunction might be valuable to look for before and more applicable that was as been nicely done looking at late gadolinium enhancement [1]. The purposes of our study were (1) to assess the diastolic function before and after AVR and to determine if persistent dyspnea was correlated with persistent diastolic dysfunction (based on E/e’) and (2) to find predictive markers of post-operative diastolic dysfunction (persistence of E/e’).
2. Methods
2.1 Population
We prospectively included 236 patients with preserved left ventricular ejection and severe high-gradient AS (aortic valve area <1 cm² or <0.6 cm²/m², mean transvalvular gradient ≥ 40 mmHg) who underwent a surgical or transcatheter aortic valve replacement. These patients were classical high gradient AS patient with an expected range of age leading to consider both surgical and percutaneous aortic valve replacement. The study was conducted in accordance with the Declaration of Helsinki. The study was reviewed by the independent ethics committees. All of the patients provided written informed consent (ethic committee authorization for the study entitled “AoMyoc”: CPP Ouest V. EudraCT number: 2012-AO1323-40).
Patients were excluded in the presence of a severe concomitant mitral or tricuspid valve disease needing surgery, severe coronary artery disease requiring coronary artery bypass graft (CABG), or reduced LVEF (under 50%). The following clinical characteristics were collected by a standardized questionnaire at baseline and 1 year follow-up: age, gender, anthropometric data (weight, size, body-mass-index [BMI], body surface area [BSA]), cardiac and extra-cardiac comorbidities (coronary artery disease [CAD] including previous myocardial infarction, atrial fibrillation [AF], conduction disorder requiring a permanent cardiac pacemaker, chronic obstructive pulmonary disease [COPD], peripheral artery disease [PAD], end-stage kidney disease), therapeutic regimen, and biology (haemoglobin, creatinine, NT-proBNP). Dyspnea was assessed using the NYHA scale and by a physician non-aware of the protocol and the other results of the evaluation of each patient. The assessment of the clinical status was done by a blinded physician of echocardiographic characteristics.
2.2 Echocardiographic data
Transthoracic Doppler echocardiography was performed at baseline and in 1 year according to the protocol. The exams were performed by board-certified physicians using Vivid ultrasound systems (General Electric Healthcare, Horten, Norway), and were reviewed offline on a dedicated workstation (GE-Healthcare -Echopac, Horten, Norway) at the corelab (CIC-IT, platform INSERM 1414-CHU Rennes). AS severity was assessed according to current guidelines and recommendations [8], by measuring continuous wave doppler peak transvalvular velocity, maximum and mean transvalvular gradients, and calculating aortic valve area using continuity equation. In accordance with current American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI) recommendations [9], four variables were measured to assess diastolic dysfunction: (1) annular e' DTI velocity (considered abnormal if septal e' <7 cm.s-1 or if lateral e' <10 cm.s-1), (2) average E/e' ratio (considered abnormal if average E/e' ≥ 14), (3) left atrial volume index (considered abnormal if ≥ 34 ml/m²), (4) tricuspid regurgitation peak velocity ≥ 2.8 m/s. According to the aforementioned guidelines and recommendations, DD is considered present if more than one-half of the available parameters meet the cut-off values.
For patients with DD in sinus rhythm during the acquisition, we categorized DD severity in four grades based on pulse-wave doppler mitral E and A velocities and E/A ratio. When E/A ratio is <0.8 with E velocity <50 cm/s, DD is defined as grade I. When E/A ratio is >2, DD is defined as grade 3. When E/A ratio is between 0.8 and 2 or E/A ratio is <0.8 with E velocity >50 cm/s, three indicators were used to assess diastolic dysfunction: (1) average E/e' ratio, (2) left atrial volume index, (3) tricuspid regurgitation peak velocity. The same cut-off values as mentioned before were used for atrial fibrillation patients. If more than half of these parameters met the cut-off values, grade II DD was present. If not, grade I DD was present. If only two parameters could be assessed, DD could not be graded if they gave contradictory information. If only one parameter was available, DD could not be graded. All the measurements were made based on at least to average of three consecutives beats and five in case of arrhythmia. In atrial fibrillation, the recommendations were followed [9] and the “automated measurement (available on echoPac) of E and e’ were considered for the multi-beat averaging.
The LV dimensions were measured according to Lang et al. [10]. Measurements included left ventricular end-diastolic diameter (LVEDd), left ventricular posterior wall thickness and interventricular septal thickness (IVSTd). Left ventricular mass (LVM) was calculated from the ASE convention formula: LV-mass= 0.8 x 1.04[(LVDd + LVPWTd + IVSTd)3 – (LVDd)3] + 0.6, and then indexed to the body surface area (BSA). LV ejection fraction (LVEF) was measured using the biplane Simpson method. Stroke volume index, left atrial volume, and right ventricular function were also assessed according to Lang et al. [10]. Left atrial reservoir function was assessed using 2D strain echocardiography on apical 4 cavity view, with a gating on QRS-complexes [11].
2.3 Procedure
All the patients underwent either surgical or transcatheter aortic valve replacement (TAVR). The choice between surgery (SAVR) versus TAVR was made by the local heart team according to contemporary guidelines [3]. Procedures were performed by experienced heart surgeons and interventional cardiologists. The valve and heart characteristics were homogeneous, the choice of the treatment was based on the general status and the co-morbidities.
2.4 Endpoints
Endpoints of the study were (1) post-operative dyspnea, defined by a NYHA functional class ≥ II and (2) presence of a post-operative E/e' ratio ≥ 14 [9].
2.5 Data analysis
Quantitative variables were expressed as mean ± standard deviation for normally distributed variable or median and [inter-quartile range (IQR)]. Qualitative variables were described with count and percentage. We compared groups with the use of either analysis of variance or Student t test for normally distributed quantitative variables, or Kruskal-Wallis or Mann-Withney test. We used Chi squared test or Fisher exact test for comparisons between groups of qualitative variables, as appropriate. Normality of distributions were tested with the Shapiro test. The level of statistical significance was set to 0.05. Primary analysis consisted in the use of machine learning algorithms to assess the probability of the presence of a post-operative E/e’ ratio ≥ 14, given baseline and preoperative variables.
Predictors were prepared in the following fashion:
- Quantitative variables were transformed with log, square root or exponential, power or inverse function, to follow normal distribution, if necessary. The most suited transformation was assessed graphically.
- For qualitative variables, we merged rare modalities and then built dummy variables.
- Missing values were imputed with the use of a bagging imputation procedure independently applied on training and testing blocks during cross validation.
We compared three algorithms known to perform very well in the presence of a high number of predictors, potentially correlated: support vector machine, random forest and gradient boosting machine. These algorithms are highly predictive and can learn nonlinear associations between predictors and target event. Each algorithm has hyper-parameters which need to be optimized in order to provide good predictive capabilities. To determine the best combination of hyperparameters, we used a random search procedure on a set of hyperparameters values available in a knowledge database (http://mlrhyperopt.jakob-r.de/parconfigs).
Tuning step and algorithms performance comparisons were performed in a nested 10 folds cross-validation procedure, to avoid potential overfitting. We assessed performance evaluation of models with the use of area under the receiver operating curve (AUROC). We only retained the best model for further analysis, which was tuned with a simple 10 folds cross-validation to determine optimal hyperparameters. The final model was built on the whole dataset with optimal hyperparameters. Concerning interpretation of the final model, as the algorithm produced a black-box model, we built a surrogate tree model to explain estimated probabilities of the final model to discover simple rules explaining the structure used by the black-box model to predict a post-operative E/e’ <14. We also computed importance of variables based on permutation error, that is to say, the added error rate if a given variable is removed from the predictors. All analyses were performed on R statistical software version 3.4.1. We used the mlr package version 2.11 for machine learning procedures.
3. Results
3.1 Patients' characteristics and intervention
236 patients were included at baseline (Figure 1 Bib-annexes). These patients are, based on the exclusion criteria, homogeneous in term of severity and clinical but also echocardiographic characteristics of the AS. Their clinical baseline characteristics are described in Table 1 BIS (annexes). Mean pre-operative NYHA class was 2.18 ± 0.62. 134 patients (56.8%) underwent surgical aortic valve replacement (7.5% mechanical prosthesis, 92.5% bioprosthesis), and 102 patients (43.2%) underwent transcatheter aortic valve replacement (83.2% transfemoral access, 6.1% transapical access, 6.1% subclavian approach, 2.0% transaortic approach, 2.0% transaortic access). Median time between first evaluation and intervention was 39 [1;84] days
3.2 Clinical status at follow up
Median time between baseline evaluation and follow-up visit was 430 days (mean follow-up duration 469 ± 141 days). 13 patients (6.0%) died, 17 (7.2%) were considered lost at follow-up according to the protocol, because they refused to come back to our center (followed only locally by their physicians so no independent NYHA classification possible and no review of the echocardiographies at the corelaboratory), and NYHA class data collection was incomplete for 15 patients (6.4%). Post-operative NYHA class could be assessed for 191 patients (80.9% of the initial cohort). Figure 1 is the flowchart of the study. 109 patients (57.1% of the remaining cohort) were NYHA class I, 77 patients (42.3%) were NYHA class II, 5 patients (2.6%) were NYHA class III. (Figure 2 bis- annexes). Mean post-operative NYHA class was 1.46 ± 0.55. 5 patients (2.6%) were re-admitted at hospital for heart failure related issues. Mean improvement from baseline to follow-up in NYHA was 0.73 class ± 0.734, with 60.0% of patients reporting improvement of at least 1 NYHA class (NYHA 1: 46.8%, 2: 12.1%, 3: 1.1%). For 38.4% of patients, NYHA class was the same at baseline and at follow-up. For 1.6% of patients, there was a worsening in one grade of the NYHA class.
3.3 Baseline characteristics associated with persistence or worsening of dyspnea
At baseline, mean age was 76 ± 9 years. The subgroup of dyspneic patients at follow-up was older (median age 80 years [76, 95] vs 77 years [69, 82] (p= 0.003), with a higher percentage of women (men 46.3%, women 62.4%), and a higher prevalence of atrial fibrillation on baseline electrocardiogram (19.0% vs 7.5%, p=0.046). Patients with dyspnea at follow-up had higher plasmatic NT pro-BNP levels (median NT pro-BNP 1031 pg/ml [487, 2134] vs 646.5 pg/ml [304,1343], p = 0.02). There was no significant difference between subgroups at baseline according to COPD, or CAD or kidney disease, or anaemia at baseline.
Echocardiographic parameters at baseline are described in Table 1. There was no significant difference between both subgroups according to LV systolic parameters such as LV ejection fraction (63% ± 9% vs 63% ± 7, p= 0.8), or LV global longitudinal strain (-17.5 ± 3.9% vs -16.9% ± 3.3%, p=0.3). We did not either observe any difference according to LV geometry, as there was no observed difference in LV mass index, LV diastolic diameter or interventricular septum diastolic thickness, or according to aortic stenosis severity indices such as aortic valve area and aortic mean gradient.
We could assess if diastolic dysfunction (with the 4 parameters of the recommendations [12]) was present at baseline echocardiography in 155 patients (81.2%). Its prevalence was different between both subgroups: 81.4% for patients with dyspnea at follow up vs 74.1% for asymptomatic patients (p=0.02). When it was present, its grade could be evaluated for only 79 patients (65.8% of patients with DD). Grade 2 DD was the most prevalent in both subgroups (45.9% vs 42.8% of DD patients in both subgroups). There was no significant difference between subgroups according to DD grade at baseline echocardiography. One by one comparison of each diastolic function index at baseline echocardiography according to subgroups did not demonstrate any difference between left atrial volume index, mitral E wave velocity, mitral annulus e' wave velocity, or E/e' ratio. However, tricuspid regurgitation velocity was higher at baseline in the subgroup of patients with dyspnea at follow-up (2.80 ± 0.46 m/s vs 2.61 ± 0.51 m/s, p=0.04), and left atrial reservoir function was lower (17 [11, 24]% vs 21 [15, 26], p=0.03).
3.4 Follow-up characteristics and persistent dyspnea
Follow-up characteristics are presented in Table 2. At follow-up, we did not observe any difference between both subgroups according to LVEF (64 ± 8% vs 64 ± 6%, p= 0.5), LVM index (94 [77, 124] g/m² vs 97 [81, 122] g/m², p=0.8) or prosthesis function parameters such as prosthetic valve area (1.5 [1.3, 1.7] cm² vs 1.6 [1.3, 1.9] cm², p=0.1) or mean transprothetic gradient (12 [9, 17] mmHg vs 12 [9, 17] mmHg, p=0.6). Dyspneic patients did not have significantly greater degree of diastolic dysfunction (68.1% vs 57.5%, p=0.18).
However, comparisons of isolated diastolic function indices between these subgroups revealed that patients with dyspnea had higher mitral E wave velocities (91 [76, 121] cm/s vs 85 [70, 102] cm/s, p=0.09), were more prone to have an elevated E/e' ratio with a cut-off at 14 (50% vs 33%, p=0.02) and had higher tricuspid regurgitation velocities (2.8 ± 0.5 vs 2.6 ± 0.5, p=0.04). Dyspneic patients also had higher plasma NT pro-BNP levels at follow-up (median NT pro-BNP 438.5 pg/ml vs 270.5 pg/ml, p = 0.006). Haemoglobin was similar in both groups.
3.5 Prediction of an elevated E/e' ratio at follow-up (≥ 14)
As detailed in Table 2 BIS (annexes), in univariate analysis, an elevated post-operative E/e' ratio was associated with an older age, male sex, higher blood pressure, TAVR procedure, congestive heart failure at baseline, physical examination and loop diuretics prescription, lower LV global longitudinal strain values and left atrial reservoir function value, a higher mitral regurgitation grade, higher E and A mitral wave velocities, higher tricuspid regurgitation peak velocity, higher pre-operative E/E' ratio, higher left atrial volume index, and higher DD grades on baseline TTE.
A model to predict a post-operative ratio ≥ 14 based on all the baseline data available (clinical, electrocardiogram, laboratory, echocardiography) has been built. The most accurate model was obtained using the random forest algorithm. The 30 most important variables used and their relative importance is displayed in Figure 3 bis (annexes). The prediction of estimated elevated filling pressures (defined by E/e' ≥ 14) has been characterized by the ROC analyses and an area under the curve reaching 0.80 (Figure 2). By this approach, the best cut-offs were defined.
Our simplified model could define 4 groups of patients (Figure 2 bis, Table 3 bis (annexes)):
- The first, defined by patients with pre-operative E/e' ratio ≤16 who underwent SAVR, could predict with a good accuracy a post-operative E/e' ratio <14.
- The second, defined by patients with pre-operative E/e' ratio ≤ 16 who underwent TAVR, had a low predictive value on the post-operative E/e' ratio.
- The third, defined by patients with pre-operative E/e' ratio >16 with pre-operative E mitral wave <110 cm/s, had a low predictive value on the post-operative E/e' ratio.
- The fourth, defined by patients with pre-operative E/e' ratio >16 with pre-operative E mitral wave >110 cm/s, predicted with a good accuracy a post-operative E/e' ratio ≥ Table 3 Bis (annexes) is summarizing these groups and their main differences.
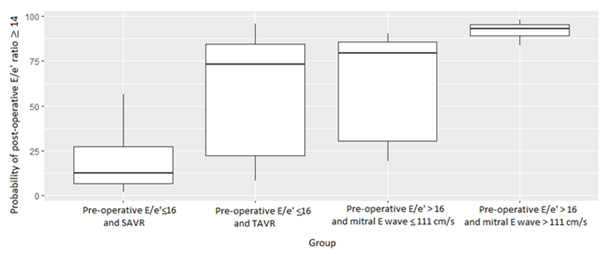
Figure 1: Probability of post-operative E/e' ratio ≥ 14 for each group of patients defined by the simplified model.
Random Forest algorithm was selected for our model as its predictive accuracy was the highest.
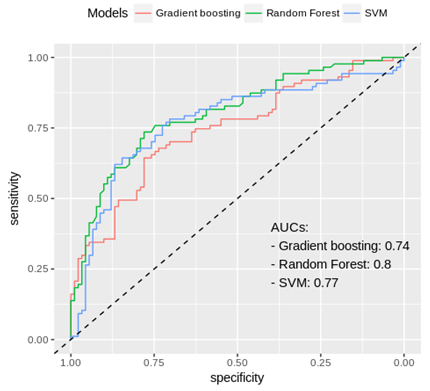
Figure 2: Receiver operating channel curves of different models to predict post-operative E/e' ratio.
|
Variable |
NYHA (n=109) |
NYHA ≥ II at follow-up |
P value |
|
Diastolic IVST (mm) |
14 [12, 15] |
14 [12, 14] |
0.290 |
|
End-diastolic LV diameter (mm) |
47.00 [44, 50] |
46 [42, 50] |
0,237 |
|
LV mass index (g/m²) |
126 [106, 152] |
124 [108, 145] |
0.756 |
|
Aortic Valve Area (cm²) |
0.67 ± 0.17 |
0.67 ± 0.19 |
0.776 |
|
Mean Aortic Gradient (mmHg) |
54 [45, 63] |
53 [43, 61] |
0.241 |
|
LVEF (%) |
64 ± 7 |
63 ± 9 |
0.812 |
|
LV global longitudinal strain (%) |
-16.9 ± 3.3 |
-17.5 ± 3.9 |
0.333 |
|
Left atrial volume index (ml/m²) |
39.10 [33, 51] |
43 [34, 53] |
0.339 |
|
Mitral E wave velocity (cm/s) |
85 [70, 102] |
91 [76, 121] |
0.092 |
|
Mitral E/A ratio |
0.80 [0.60, 1.00] |
0.80 [0.60, 1.10] |
0.422 |
|
Mitral annulus e' wave velocity (cm/s) |
6.0 [5.0, 7.0] |
6.0 [5.0, 7.5] |
0.632 |
|
E/e' ratio |
14 [12, 19] |
15 [11, 20] |
0.643 |
|
E/e' ratio >14 (%) |
49 (48.5%) |
40 (57.1%) |
0.267 |
|
Tricuspid regurgitation velocity (m/s) |
2.6 ± 0.5 |
2.8 ± 0.5 |
0.043 |
|
Diastolic dysfunction n (%) |
63 (74.1%) |
57 (81.4%) |
0.279 |
|
Diastolic dysfunction grade |
- |
- |
0.877 |
|
Grade 0 (no DD) |
22 (25.9%) |
13 (18.7%) |
- |
|
Grade 1 |
1 (1.1%) |
1 (1.4%) |
- |
|
Grade 2 |
39 (45.9%) |
30 (42.8%) |
- |
|
Grade 3 |
4 (4.7%) |
4 (5.7%) |
- |
|
DD – grade unknown |
19 (22.4%) |
22 (31.4%) |
- |
|
Left atrial reservoir function (%) |
21 [15, 26] |
17 [11, 24] |
0.035 |
Values are expressed as mean ± SD, median [IQR] or frequency [n (%)]; LV-left ventricle; IVST-interventricular septal thickness; LVEF-left ventricular ejection fraction; DD-diastolic dysfunction
Table 1: Baseline echocardiographic characteristics according to follow-up dyspneic status.
|
Variable |
NYHA (n=109) |
NYHA ≥ II at follow-up |
P value |
|
LV mass index (g/m²) |
94 [77, 124] |
97 [81, 122] |
0.821 |
|
Prosthetic Valve Area (cm²) |
1.6 [1.3, 1.9] |
1.5 [1.3, 1.7] |
0.120 |
|
Mean transprosthetic Gradient (mmHg) |
12 [9, 17] |
12 [9, 17] |
0.620 |
|
Paravalvular leak |
- |
- |
0.510 |
|
No leak |
48 (44.9%) |
34 (42.0%) |
- |
|
Mild |
43 (40.1%) |
31 (38.3%) |
- |
|
Moderate |
16 (15.0%) |
16 (19.7%) |
- |
|
LVEF (%) |
64 ± 6 |
64 ± 8 |
0.518 |
|
Left atrial volume index (ml/m²) |
39 [31, 48] |
40 [32, 52] |
0.448 |
|
Mitral E wave velocity (cm/s) |
92 [70, 115] |
100 [80, 131] |
0.033 |
|
Mitral E/A ratio |
0.82 [0.70, 1.06] |
0.88 [0.70, 1.40] |
0.122 |
|
Mitral annulus e' wave velocity (cm/s) |
7.5 [6.5, 8.5] |
7.5 [6.0, 9.0] |
0.693 |
|
E/e' ratio |
11.5 [9.0, 15.7] |
14.3 [10.5, 17.9] |
0.063 |
|
E/e' >14 (%) |
32 (32.7%) |
33 (50.0%) |
0.026 |
|
Tricuspid regurgitation velocity (m/s) |
2.6 ± 0.4 |
2.7 ± 0.4 |
0.018 |
|
Diastolic dysfunction n (%) |
46 (57.5%) |
47 (68.1%) |
0.182 |
|
Diastolic dysfunction grade |
- |
- |
NA |
|
Grade 0 (no DD) |
34 (42.3%) |
22 (31.9%) |
- |
|
Grade 1 |
0 (0.0%) |
0 (0.0%) |
- |
|
Grade 2 |
29 (36.3%) |
25 (36.2%) |
- |
|
Grade 3 |
0 (0%) |
0 (0.0%) |
- |
|
DD – grade unknown |
17 (21.3%) |
22 (31.9%) |
- |
|
NT pro-BNP (pg/ml) |
270 [152, 575] |
438 [202, 967] |
0.006 |
|
Haemoglobin (g/dl) |
13.18 ± 1.44 |
13.62 ± 1.54 |
0.079 |
Values are expressed as mean ± SD, median [IQR] or frequency [n (%)]; LV-left ventricle; IVST-interventricular septal thickness; LVEF-left ventricular ejection fraction; DD-diastolic dysfunction; NA-not available
Table 2: Follow-up echocardiographic and laboratory findings according to follow-up dyspneic status.
4. Discussion
According to the current guidelines [3], it still remains challenging to define the best timing for treatment of the AS. The question could be reversed: should some patients be treated before symptoms not only to protect the heart’s systolic function but also diastolic function?.
4.1 Epidemiology of long-term persistence of dyspnea after aortic valve replacement
In our study, despite most of the patients being asymptomatic (NYHA I) at long-term follow-up, 44.9% still experience various degrees of dyspnea, with a vast majority of NYHA class II patients (assess by an independent heart failure specialist). In their cohort of 3875 patients who underwent TAVR, Lange et al. reported an improvement in NYHA class for 68.9% of patients at a 1 year follow-up, most patients being NYHA class I and II post-operatively with a quite similar distribution in NYHA classes as in our study [12]. Kim et al. [13] meta-analyzed 62 observational TAVR studies which confirmed this outcome data. They found an improvement of 0.8 to 2.1 NYHA grade at 12 to 23 months follow-up. This meta-analysis reports a better improvement than our data, as compared to the 0.73 class difference between pre and post-operative NYHA grade, presumably explained by less symptomatic patients at baseline in our study (most being NYHA class II). Auensen et al. [14] reported a higher proportion of post-SAVR NYHA class I patients than in TAVR studies. Our population was significantly older and we have mixed both SAVR and TAVR patients (which fit with the daily clinical routine).
4.2 Diastolic dysfunction as a cause of persistent dyspnea at follow-up
Patients who were in NYHA class II or more after aortic valve replacement had higher E/e' ratio, tricuspid regurgitation velocity and plasma NT pro BNP levels, while their LV systolic function, prosthesis function indices or extra-cardiac comorbidities (COPD, anaemia) did not differ from asymptomatic patients. Diastolic dysfunction measured before the correction of the AS seems to be the main cause of these patients' persistent dyspnea. Few studies [15, 16] demonstrated that dyspnea was associated with indices like E/e' ratio, left atrial volume index or the ratio E/e’ got from strain rate data [17]. Muratori et al. [18] reported that improvement of symptoms after TAVR was correlated to changes in diastolic function grade and reduction of LV filling pressures, and that improvement in diastolic function was correlated with LV and LA reverse remodeling.
However, the pre-operative severity of diastolic dysfunction was not predictive for post-operative dyspnea. DD characterization could be challenging. For some patients the application of guideline lead to a class “indeterminate” [9]. Morris et al. [19] had pointed this out in a cohort of patients with a HFPEF, the same observation. The addition of LA reservoir function in the way diastolic dysfunction was characterized could have changed the absence of clear demonstration of the link between DD and symptoms. In the present cohort, the LA reservoir function at baseline was significantly different in symptomatic and asymptomatic patients.
The rhythm is also an issue when grading DD [9]. In our study, AF at baseline electrocardiogram was associated with worse functional status at follow-up. It has been demonstrated that pre-operative atrial fibrillation confers a worse prognosis after aortic valve replacement and is associated with congestive heart failure history [20]. It also has been demonstrated that DD is an independent risk factor for AF [21]. Our study however was not designed to study the link between DD and AF and to determine whether AF was an independent risk factor for persistent dyspnea.
4.3 Predictors of persistent high filling pressure
E/e' ratio is one of the most commonly used parameters for characterizing LV filling pressures. It has been used in many studies as a surrogate marker of DD and high filling pressure. Despite having limitations, it has been demonstrated for its prognostic value in various populations [22-25]. We demonstrated that more altered pre-operative diastolic function (E/e' ratio >16, mitral E wave velocity >111 cm/s) was, more persistent DD at follow-up was. On the other hand, patients with more preserved pre-operative diastolic function and lower LV filling pressures (E/E' ratio ≤ 16) were at lower risk for DD and estimated high filling pressure at follow-up if a surgical aortic valve replacement was performed. Asami et al. [25] reported the association between DD grade and all-cause mortality, cardiovascular death, and major adverse cardiac and cerebrovascular events in post TAVR patients. Biner et al. [21] found the same association between E/e' ratio (as a surrogate marker of DD) and outcomes in patients with aortic stenosis who did not undergo intervention. Dahl et al. with a smallest population, was focused on a more controversial index of diastolic function based on the strain rate [17].
A comprehensive evaluation of diastolic function is probably something to consider carefully. Patients having a significant diastolic dysfunction, might take advantage of an earlier aortic valve replacement. Obviously, further studies are needed. Patients undergoing SAVR seemed to have better outcomes on follow-up E/e' ratio, but the intervention variable was not independently associated with post-operative E/e' in the model we built, suggesting that SAVR does not itself confer better results but that TAVR patients have more risk factors to have persistent diastolic dysfunction (Age might have an impact on that result) [26].
4.4 Study limitations
This is a single-center prospective study performed in a limited number of patients. The diastolic function assessment was based on the 4 parameters required by the current recommendation [9]. It could have been valuable to include more parameters like advocated in guidelines or in Dahl et al. [17]. The population was chosen to be representative of a classical cohort proposed to an aortic valve replacement nowadays. These are not the sickest patients. LVEF had to be preserved and CAD or other valvular significant abnormalities were exclusion criteria. The follow-up was limited to one-year.
5. Conclusions
43% of treated AS patients remain symptomatic, and persistent dyspnea is related with diastolic dysfunction. DD-characterization according to current standards is suboptimal but it is possible to predict E/e’ at one year based on pre-operative clinical and echocardiographic data. That might be something to explore for preventing post-operative symptoms and for preventing post-operative HFpEF.
Conflicts of Interest
There are no commercial products involved in this study.
Acknowledgement
Acknowledgements to the CORECT, the financial support obtained for the study at the CHU de RENNES
References
- Everett RJ, Tastet L, Clavel MA, et al. Progression of Hypertrophy and Myocardial Fibrosis in Aortic Stenosis: A Multicenter Cardiac Magnetic Resonance Study. Circ Cardiovasc Imaging 11 (2018): e007451.
- Singh A, Chan DCS, Greenwood JP, et al. Symptom Onset in Aortic Stenosis: Relation to Sex Differences in Left Ventricular Remodeling. JACC Cardiovasc Imaging 12 (2019): 96-105.
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 38 (2017): 2739-2791.
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 135 (2017): 1159-1195.
- Gotzmann M, Pljakic A, Bojara W, et al. Transcatheter aortic valve implantation in patients with severe symptomatic aortic valve stenosis-predictors of mortality and poor treatment response. Am Heart J 162 (2011): 238-245.
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 374 (2016): 1609-1620.
- Krane M, Deutsch M-A, Piazza N, et al. One-Year Results of Health-Related Quality of Life Among Patients Undergoing Transcatheter Aortic Valve Implantation. Am J Cardiol 109 (2012): 1774-1781.
- Baumgartner H, Hung J, Bermejo J, et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J-Cardiovasc Imaging 18 (2017): 254-275.
- Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29 (2016): 277-314.
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J-Cardiovasc Imaging 16 (2015): 233-271.
- Gan GCH, Ferkh A, Boyd A, et al. Left atrial function: evaluation by strain analysis. Cardiovasc Diagn Ther 8 (2018): 29-46.
- Lange R, Beckmann A, Neumann T, et al. Quality of Life After Transcatheter Aortic Valve Replacement: Prospective Data From GARY (German Aortic Valve Registry). JACC Cardiovasc Interv 9 (2016): 2541-2554.
- Kim CA, Rasania SP, Afilalo J, et al. Functional Status and Quality of Life After Transcatheter Aortic Valve Replacement: A Systematic Review. Ann Intern Med 160 (2014): 243-254.
- Auensen A, Hussain AI, Bendz B, et al. Morbidity outcomes after surgical aortic valve replacement. Open Heart 4 (2017): 000588.
- Dahl JS, Christensen NL, Videbæk L, et al. Left Ventricular Diastolic Function Is Associated With Symptom Status in Severe Aortic Valve Stenosis. Circ Cardiovasc Imaging 7 (2014): 142-148.
- Park SJ, Enriquez-Sarano M, Chang SA, et al. Hemodynamic Patterns for Symptomatic Presentations of Severe Aortic Stenosis. JACC Cardiovasc Imaging 6 (2013): 137-146.
- Dahl JS, Barros-Gomes S, Videbæk L, et al. Early Diastolic Strain Rate in Relation to Systolic and Diastolic Function and Prognosis in Aortic Stenosis. JACC Cardiovasc Imaging 9 (2016): 519-528.
- Muratori M, Fusini L, Tamborini G, et al. Sustained favourable haemodynamics 1 year after TAVI: improvement in NYHA functional class related to improvement of left ventricular diastolic function. Eur Heart J-Cardiovasc Imaging 17 (2016): 1269-1278.
- Morris DA, Belyavskiy E, Aravind-Kumar R, et al. Potential Usefulness and Clinical Relevance of Adding Left Atrial Strain to Left Atrial Volume Index in the Detection of Left Ventricular Diastolic Dysfunction. JACC Cardiovasc Imaging 11 (2018): 1405-1415.
- Saxena A, Dinh DT, Reid CM, et al. Does Preoperative Atrial Fibrillation Portend a Poorer Prognosis in Patients Undergoing Isolated Aortic Valve Replacement? A Multicentre Australian Study. Can J Cardiol 29 (2013): 697-703.
- Rosenberg MA, Gottdiener JS, Heckbert SR, et al. Echocardiographic diastolic parameters and risk of atrial fibrillation: the Cardiovascular Health Study. Eur Heart J 33 (2012): 904-912.
- Biner S, Rafique AM, Goykhman P, et al. Prognostic Value of E/E′ Ratio in Patients With Unoperated Severe Aortic Stenosis. JACC Cardiovasc Imaging 3 (2010): 899-907.
- Acil T, Wichter T, Stypmann J, et al. Prognostic value of tissue Doppler imaging in patients with chronic congestive heart failure. Int J Cardiol 103 (2005): 175-181.
- Sharp ASP, Tapp RJ, Thom SAM, et al. Tissue Doppler E/E′ ratio is a powerful predictor of primary cardiac events in a hypertensive population: an ASCOT substudy. Eur Heart J 31 (2010): 747-752.
- Kuznetsova T, Thijs L, Knez J, et al. Prognostic Value of Left Ventricular Diastolic Dysfunction in a General Population. J Am Heart Assoc 3 (2014): 000789.
- Asami M, Lanz J, Stortecky S, et al. The Impact of Left Ventricular Diastolic Dysfunction on Clinical Outcomes After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 11 (2018): 593-601.


 Impact Factor: * 4.2
Impact Factor: * 4.2 Acceptance Rate: 72.62%
Acceptance Rate: 72.62%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 