Cut Surface Biliary Complications in Open and Laparoscopic Liver Resections - A Single Center Analysis
Sebastian Recknagel1, Uwe Scheuermann1, Hanna Guice1, Elisabeth Sucher2, Andri Lederer1, Claudia Höhne3, Daniel Seehofer1, Robert Sucher1*, Sebastian Rademacher1
1Department of Visceral-, Transplantation-, Thoracic and Vascular Surgery, University Hospital Leipzig, Germany
2Department of Gastroenterology and Oncolgy, Division of Hepatology, University Hospital Leipzig, Germany
3Department of Anesthesiology, Pain Therapy, Intensive Care and Emergency Medicine, DRK Hospital Berlin-Koepenick, Berlin, Germany
*Corresponding Author: Robert Sucher, Department of Visceral-, Transplantation-, Thoracic and Vascular Surgery, University Hospital Leipzig, Germany
Received: 08 September 2022; Accepted: 14 September 2022; Published: 08 March 2023
Article Information
Citation: Sebastian Recknagel, Uwe Scheuermann, Hanna Guice, Elisabeth Sucher, Andri Lederer, Claudia Höhne, Daniel Seehofer, Robert Sucher, Sebastian Rademacher. Cut Surface Biliary Complications in Open and Laparoscopic Liver Resections - A Single Center Analysis. Journal of Surgery and Research. 6 (2023): 39-48.
View / Download Pdf Share at FacebookAbstract
Background/Aims: Biliary leakage (BL) is a major cause of postoperative morbidity after liver resection. Aim of our study was to analyse surgical parameters and postoperative morbidity with special emphasis on BL, after launching a minimally invasive liver resection program.
Methods: A prospectively maintained medical database of patients who required a liver resection was used for analysis.
Results: A total of n=156 patients were divided into a group of n=47 patients (30.1%) receiving laparoscopic (LLR) and n=109 patients (69.9%) undergoing open liver resections (OLR). Patient age (OLR: 59.4 ± 16.0 vs. LLR: 57.9 ± 14.2 years) and male to female ratio (OLR: 63/46 vs. LLR: 25/22) were comparable. We performed n=75 (68.8%) major OLR and n=31 (66.0%) major LLR. Operation time was OLR 342.8 ± 110.5 min vs. LLR 287.3 ± 132.6 min (p=0.014) and the average blood loss was OLR 523.5 ± 428.6 ml vs. LLR 355.5 ± 459.2 ml. Morbidity and mortality was observed in n= 29 (18.6 %) and n= 7 (4.5 %) patients, respectively. The overall biliary leakage (BL) rate was 5.1% (n= 8). Majority of BL were detected in OLR with biliodigestive anastomosis (BDA) (n=2 (11.0%)) followed by OLR without BDA (n=6 (6.5%)). No BL were detected in patients with LLR. Hospital stay was significantly prolonged after OLR in patients with BL (38.4 ± 20.1 vs. 17.4 ± 11.1 days, p< 0.001).
Conclusion: The introduction of different transection techniques in laparoscopic liver resections did not increase morbidity and BL- rate.
Keywords
<p>Biliary leakage, Liver resection, Laparoscopic liver resection, Complications after liver surgery</p>
Article Details
Introduction
Modern surgical treatment of the liver includes the use of minimally invasive resection techniques for benign and malignant disease [1]. The initial experience with laparoscopic liver surgery demonstrated that even major resections could be performed with acceptable morbidity and mortality, in a selected group of patients and in specialized centers. A multimodal therapeutical approach introducing state-of-the-art intraoperative visualization techniques [2,3] and advanced resection strategies [4] may furthermore increase the feasibility and safety of laparoscopic liver resections. However, because of a steep learning curve, a formal structure of minimally invasive liver surgery training and application might be of particular importance for surgeons working in specialized liver tumor centers.
Biliary leakage (BL) remains the Achilles heel of liver surgery and accounts for a major fraction of postoperative complications [5]. BL is defined as fluid with an elevated bilirubin level in the abdominal drain or intraabdominal fluid and can be graded as A, B or C depending on the required treatment method. This can comprise (A) no or little change in the patient’s clinical management, (B) a change in the clinical management (e.g. additional diagnostics and interventional procedures) without the need for re-laparotomy and (C) a bile leakage requiring re-laparotomy [6]. We were able to demonstrate that in open liver resections the intraoperative placement of a T drainage may reduce the incidence of biliary leakage [7] and that the intraoperative placement of external biliary drains may prevent, and worst case, also treat biliary leakages after extended liver resections [8]. Further data indicate that the incidence and severity of post hepatectomy bile leakages may be affected by surgical indications, preoperative chemotherapy and surgical procedures. Although laparoscopic liver surgery has demonstrated several advantages when compared to open liver surgery a recent meta-analysis revealed a higher bile leakage rate for laparoscopic left hemi-hepatectomies for patients with hepatolithiasis (OR, 1.7, p=0,01) [9]. Although no causal explanation for this phenomenon could be given, one possible reason might be differences in the technique of parenchymal transection and bile duct closure during implementation of laparoscopic liver surgery. Like in other minimally invasive procedures, many surgeons are using either advanced bipolar or ultrasonic shears or linear cutters for parenchymal transection rather than a ultrasonic dissector as mainly used in open surgery [10]. It remains unclear, if the change of the parenchymal transection with e.g. use of ultrasonic shears might be afflicted with a higher rate of bile leaks or if this might only be the case when used in inflamed or cholestatic liver parenchyma [9].
In view of the formation of a specialized high-volume liver tumor center, we recently introduced minimally invasive liver resections into the portfolio of our surgical treatment modalities. The aim of our study was to analyze our patient data with regard to surgical outcome and post-operative morbidity. Special emphasis was given to biliary leak occurrence after open (CUSA based) and laparoscopic (ultrasonic shears based) liver resections.
Methods
Study design and Patient demographics
The medical data of patients who required liver resections for various indications at our department between April 2016 and September 2017 was reviewed retrospectively. All consecutive patients receiving a liver resection were included, there were no exclusion criteria. Medical data analysis comprised patient demographics (age, sex, body mass index, ASA-classification), peri and intraoperative surgical data (operation time, blood loss, transfusion requirement (defined as units of red cell concentrate, albumin or fresh frozen plasma substitution) pringle maneuver, bile duct drain and T-drain placement, intraabdominal drain placement, extent of resection, performed lymphadenectomy), histopathologic findings and postoperative data (hospital stay, postoperative complications during the hospital stay with special emphasis on BL). Biliary leakage was detected via macroscopic and serologic detection of bile in the abdominal drainages. Furthermore, patients suspicious for bile leakage (increase infection parameters CRP > 5 mg/l, leucocytosis > 10.000/µl, body temperature >38.5°, tachycardia >100 bpm) and with radiological signs (determined by US or CT) of intraabdominal fluid collections after surgery underwent US or CT guided needle puncture and drain placement.
Data were collected from anaesthesiologic and operative documentation of the patient’s history, the intraoperative procedure, histological results, the process at the intensive care unit and the medical discharge report. Patients were split according to the surgical technique into laparoscopic liver resections (LLR) and open liver resections (OLR). The preoperative performance status was evaluated according to ASA (American society of Anaesthesiology) class I to III. OLRs were graded according to the Brisbane 2000 terminology of liver anatomy and resections [11] and LLRs were graded according to the difficulty scoring system described by Di Fabio et al [12]. Biliary leakages were graded according to Koch et al [6]. Primary endpoint of the study was the evaluation of biliary leakage after liver resection. Secondary endpoints were short-term complications and short-term survival. The study was approved by the ethics committee of the University of Leipzig, number 142/18-EK and the study protocol was performed in accordance with the relevant guidelines. Informed consent was waived by the ethics committee of the University of Leipzig due to the retrospective nature of the study.
Surgical Technique of Open Liver resection (OLR)
Open liver resections were performed as described earlier by our group [13,14]. Vascular inflow and outflow control before parenchymal transection remain the mainstay of our technique for anatomic resections. For the vascular inflow control a tourniquet was placed around the hepatoduodenal ligament and closed on demand. Parenchymal transection was performed with the Caviton Ultrasonic Surgical Aspirator (CUSA). Whereas smaller vascular and biliary structures were divided between titanium clips, larger structures were ligated or sutur-ligated. Additional hemostasis was performed by bipolar forceps and irrigation. No hemostatic agents or other sealants of the transection surface was used. A White-Test for intraoperative bile leak testing was performed in all open liver resections. It was performed either via the T-tube or otherwise a cannula was inserted into the cystic duct after cholecystectomy, and a fatty emulsion (5% fat content parenteral nutrition supplement) was injected with gentle pressure. In case of a biliary leakage extravasation of fatty emulsion at the resection surface or structures of the biliary tree a PDS 5-0 suture was placed accordingly to close the leakage (15). In case of long exposure of central biliary structures, suture of central biliary ducts, hints for an increased biliary pressure or continued leakage during the ‘white test’ a T-tube was inserted for biliary decompression. Abdominal drains were avoided as far as possible, especially in patients with liver cirrhosis. However, in patients with bilioenteric anastomosis or extended lymphadenectomy in the retro- / peripancreatic area or in patients with persistent oozing form the transection surface abdominal drains were routinely used.
Surgical Technique of Laparoscopic Liver Resection (LLR)
For laparoscopic resections a supine split-leg patient position (‘French position’) was applied and the operation table was moved into the Trendelenburg or reverse Trendelenburg position, depending on the operative steps performed. The surgeon was standing between the legs and the assistant was placed to the left side of the patient.
For the vascular inflow control a tourniquet was placed around the hepatoduodenal ligament before parenchymal resection to facilitate an external Pringle maneuver. For right/ extended right hemihepatectomies a laparoscopic liver hanging maneuver was considered to reduce bleeding during parenchymal transection [4].
During LLR ultrasonic shears (Harmonic ACE, Ethicon®) were used as mainstay of tissue dissection and parenchymal transection. Coagulation of the cut-surface was performed using bipolar forceps with irrigation, if needed. Smaller biliary and vascular structures were divided with the ultrasonic scissors, medium and larger structures using titanium or absorbable clips.
A laparoscopic CUSA was only used during right or extended right hemihepatectomies for exposure of the middle hepatic vein and its tributaries and for intrahepatic exposure of the right bile duct. The other parenchymal division was performed by ultrasonic shears.
Intraoperative ultrasound was used to visualize the tumor and the vascular anatomy in every OLR and LLR procedure. If appropriate, indocyanine green (ICG) fluorescence staining was used for anatomic liver resections to visualize the vascular and biliary anatomy of the liver [2]. For direct or indirect tumor staining of e.g. HCC, CCA and CRLM, ICG was applied at different timepoints before surgery [3]. Due to inapplicability, a white test was not performed in laparoscopic resections. Also, a T-tube was not used during LLR at that time.
All patients with liver resections received at least overnight intensive care and were transferred to the normal ward earliest the day after surgery.
Statistical Method
The software SPSS 25 was used for the statistical retrospective analysis of the data. Welch’s t-test for independent samples was used for metric data, so differences in the variability of means could be ignored. Only metric data with sample sizes (n1, n2) smaller than 25 was interpreted with the Wilcoxon-Mann-Whitney-test (MWMU-test). Categorial characteristics were evaluated with the Chi-quadrat-test (χ²-Test), if the sample was higher than 40 in total and if less than 20% of the expected rates where smaller than five. Otherwise the Fisher‘s exact test was used. A p value of <0,05 was accepted as significant.
Results
Patient demographics
In the indicated timespan, a total of n=156 patients underwent liver surgery in our department. Eight patients received repeat resections within the relevant timespan between both interventions and hence each resection was analysed and counted individually. Patient demographics and perioperative data are provided in table 1.
|
Patients |
OLR |
LLR |
P |
|
Mean age, y |
59.4 +/- 16.0 |
57.9 +/- 14.2 |
0.564* |
|
Sex, male/female (%) |
63/64 (57.8/42.2) |
25/22 (53.2/46.8) |
0.594** |
|
BMI |
26.3 +/- 4.5 |
27.6 +/- 5.0 |
0.122 |
|
ASA-Score, n (%) |
0,002§ |
||
|
I |
2 (1.8) |
5 (10.6) |
|
|
II |
47 (43.1) |
28 (59.6) |
|
|
III |
60 (55.0) |
14 (29.8) |
|
|
Diagnosis, n (%) |
|||
|
Primary malign hepatic zumours |
|||
|
HCC |
17 (15.6) |
14 (29.8) |
|
|
CCA |
21 (19.3) |
2 (4.3) |
|
|
Others |
6 (5.5) |
0 (0.0) |
|
|
Hepatic metastases |
|||
|
Colorectal |
37 (33.9) |
7 (14.9) |
|
|
Others |
12 (11.0) |
2 (4.3) |
|
|
Benigne lesions |
|||
|
Benigne tumours |
11 (10.1) |
17 (36.2) |
|
|
Others |
5 (4.6) |
5 (10.6) |
|
|
Major/minor resection, n (%) |
75/34 (68.8/31.2) |
31/16 (66.0/34.0) |
0.726** |
|
Billidiestive anastomosis, n (%) |
17 (15.6) |
0 (0.0) |
|
|
Operation time, min |
342.8 +/- 110.5 |
287.3 +/- 132.6 |
0.014* |
|
Intraoperative blood loss, ml |
532,5 +/- 428.6 |
355.5 +/- 459.2 |
0.051* |
|
Transfusion, n (%)# |
17 (15.7) |
0 (0.0) |
0.004** |
|
Bile duct drainage used, n (%) |
63 (57.8%) |
0 (0.0) |
<0.001** |
|
T-Drainage used, n (%) |
37 (33.9) |
0 (0.0) |
|
|
Biliary leakage, n (%) |
8 (7.3) |
0 (0.0) |
0.107§ |
|
Lymphadenectomy, n (%) |
56 (51.4) |
2 (4.3) |
<0.001** |
|
Abdominal drainage, n (%) |
68 (62.4) |
24 (51.1) |
0.187** |
|
Prigle manoevre, n (%) |
73 (67.0) |
22 (46.8) |
0.018 |
|
Length of hospital stay, days |
16.4 +/- 13.4 |
8.6 +/- 5.8 |
<0.001* |
T-test/** Chi-quadrat-test/§ Fisher´s exact test/# one anaestheasia case file missing, n=108
Table 1: Patient demographics and surgical data for open (OLR) and laparoscopic liver resections (LLR)
In short, n=47 (30.1%) liver resections were performed by laparoscopic surgery (LLR) when compared to open liver resections (OLR) in 109 cases (69.9%). The gender distribution in OLR and LLR was comparable (OLR male: 57.8% vs LLR male 53.2%) and the average age distribution was also similar (OLR: 59.4 ± 16.0 vs. LLR: 57.9 ± 14.2 years). The preoperative ASA performance status was significantly worse in patients with OLR (ASA I: n=2 (1.8%); ASA II: n=47 (43.1%) and ASA III: n=60 (55.0%)) compared to patients with LLR (ASA I: n=5 (10.6%); ASA II: n=28 (59.6%); ASA III n=14 (29.8%)).
Indications and extent of liver resections
The study population was subsequently graded according to the dignity of the disease into a group of patients with malign disease, comprising patients with hepatocellular carcinoma (HCC, n= 31 (19.9 %)), cholangiocarcinoma (CCA, n= 23 (14.7 %)), and patients with liver metastases (colorectal liver metastases CRLM, n= 44 (28.2 %), and non-colorectal liver metastases nCRLM, n= 14 (9.0 %)). Rare tumor entities (like gallbladder carcinoma n= 4 (2.6 %), angiosarcomas n= 1 (0.6 %) and leiomyosarcomas n= 1 (0.6 %)) were summarized as other malignant tumors. Benign indications for liver resection mainly included patients with adenomas (n=7 (4.5%)), focal nodular hyperplasia (n=7 (4.5%)), haemangioma (n=7 (4.5%)), the Caroli disease (n=5 (3.2%)), cholangitis (n=3 (1.9%)), echinococcus cyst (n=2 (1.3%)), benign bile duct stenosis (n=2 (1.3 %)), haematoma (n= 2 (1.3%)), angiomyolipoma (n=1 (0.6%)), liver abscess (n=1 (0.6 %)) and polycystic liver disease (n=1 (0.6%)).
With regard to the extent of liver resections, both OLR (major OLR: n= 75 (68.8%) vs minor OLR n= 34 (31.2%)) and LLR (major LLR: n= 31 (66.0 %) vs minor LLR n= 16 (34.0 %)) contained a higher percentage of major resections. Seven liver resections which started by minimally invasive surgery were completed as OLR (conversion rate n=7 (13.0%)). Reasons for the conversion was stagnation in the process of the operation due to laparoscopic inaccessibility (n=5 (9.3 %)), intraabdominal adhesions (n=1 (1.9 %)), and bleeding from the portal vein (n=1 (1.9 %)).
Oncologic LLR were predominantly performed in patients with HCC (n = 14 (29,8 %)), followed by patients with CRLM (n = 7 (14.9 %)) and a small percentage of patients with CCA (n = 2 (4.3 %)). In parallel LLR accounted for a good amount of liver resections in patients with benign liver lesions. Radical Lymphadenectomy was predominantly performed in OLR (Lymphadenectomy OLR: n=56 (51.4%) vs Lymphadenectomy LLR: n=2 (4.3%)). A detailed description of the distribution of LLR and OLR with regard to diagnosis is also given in figure 1.
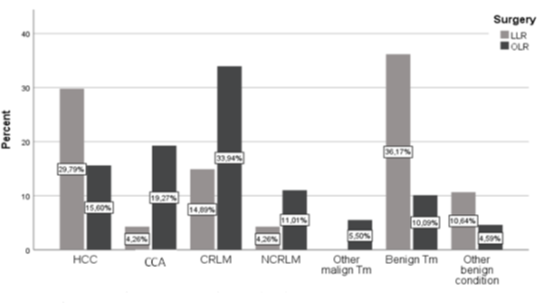
Figure 1: Distribution of LLR and OLR with regard to diagnosis
Surgical parameters
The total operation times of laparoscopic resections were significantly shorter (LLR: 287.3 ± 132.6 min) when compared to OLR (OLR: 342.8 ± 110.5 min, p=0.014). Intraoperative blood loss was slightly higher in OLR (OLR blood loss: 523.5ml ± 428.6ml vs LLR blood loss: 355.5ml ± 459.2ml, p=0.051). Transfusion requirements were generally low (n=17 (10.9%)) and only required in patients with OLR (transfusion requirements OLR n=17 (15.7%) vs. transfusion requirements LLR n=0 (0.0%)).
Intermittent vascular inflow control (Pringle manoeuvre) was used more often in OLR (Pringle manoeuvre OLR: n=73 (67.0%)) than in LLR (Pringle manoeuvre LLR: n=22 (46.8%) p=0.018). Intraabdominal drains were furthermore placed in half of OLR (abdominal drain OLR: n=68 (62.4%)) and LLR (abdominal drain LLR: n=24 (51.1%)). Bile duct drainages were exclusively used in OLR cases (bile duct drainages OLR n=63 (57.8%) vs bile duct drainages LLR: n=0 (0.0%), p<0.001). The length of hospital stay, was almost twice as high for patients with OLR (hospital stay OLR: 16.4 ± 13.4 days vs hospital stay LLR: 8.6 ± 5.8 days, p<0.001).
Morbidity and mortality
Postoperative complications were graded according to the Clavien-Dindo (CD) classification [16]. A detailed CD statement is provided in table 2. Overall morbidity defined as a CD scoring of 3b or higher was n= 29 (18.6%). In LLR morbidity was significantly lower than in OLR (morbidity (CD) ≥3b OLR: n= 26 (23.9 %) vs morbidity (CD) ≥3b LLR: n= 3 (6.4%), p= 0.01). Our overall mortality rate was n= 7 (4.5%). LLR were performed without mortality (mortality LLR: n=0 (0%) vs mortality OLR: n=7 (6.4%), p=0.103).
|
Postoperative complications |
OLR |
LLR |
P |
|
Clavien-Dindo I, n (%) |
18 (16.5) |
0 (0.0) |
<0.001** |
|
Clavien-Dindo II, n (%) |
4 (3.7) |
0 (0.0) |
|
|
Clavien-Dindo IIIa, n (%) |
7 (6.4) |
1 (2.1) |
|
|
Clavien-Dindo IIIb, n (%) |
11 (10.1) |
2 (4.3) |
|
|
Clavien-Dindo IV, n (%) |
8 (7.3) |
1 (2.1) |
|
|
Clavien-Dindo V, n (%) |
7 (6.4) |
0 (0.0) |
|
|
Morbidity, n (%) |
26 (23.9) |
3 (6.4) |
0.010** |
|
Mortality in hospital, n (%) |
7 (6.4) |
0 (0.0) |
0.103§ |
Morbidity = >/= CD IIIb
**Chi-quadrat-test/§ Fisher´s exact test
Table 2: Clavien-Dindo-Classification
Biliary leakage
The overall BL rate was 5.1 % (n= 8). All BL were detected in OLR. The incidence of BL and morbidity with regard to performed surgery is provided in figure 2. BL were predominantly detected in OLR with BDA (n=2 (11.8%)) followed by OLR without BDA (n=6(6.5%)). No BL were detected in LLR. One biliary leak was graded as type A, n=4 were graded as type B and n=3 were graded as type C leakages. Hence, three patients required relaparotomy (relaparotomy due to BL n=3 (37.5%)) and one patient died because of BL complications (BL associated mortality (n=1 (12.5%)). Furthermore, BL occurred after five major resections (biliary leakage in major OLR: n=5, 6.7%) and three minor resections (biliary leakage in minor OLR: n=3, 8.8%).
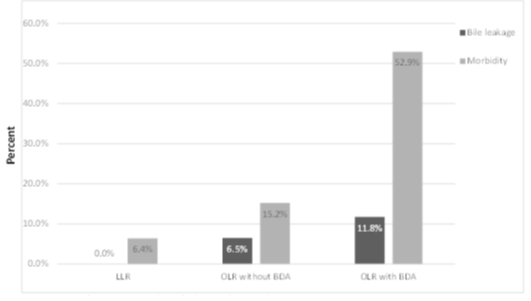
Figure 2: Bile leakage and morbidity with regard to performed
Figure 3 demonstrates the incidence of biliary leakages within the different liver pathologies operated and table 3 describes the influencing variables for the appearance of a biliary leakage after OLR. Patients who underwent liver resection for CCA and gallbladder carcinoma and required an extrahepatic bile duct resection, were more likely to develop a postoperative biliary leakage, although the difference was not statistically significant (incidence of biliary leakage in patients with CCA: n= 3 (13.0%) and incidence of biliary leakage in patient with gallbladder carcinoma: n= 2 (50.0%)). Furthermore, patients with open liver resections for benign lesions had a slightly lower risk for getting a postoperative bile leakage (incidence biliary leakage benign lesions: n=1 (6.2%) vs incidence biliary leakage malign lesions n=7 (7.5%)). Moreover, biliary leakages were more often detected in OLR patients with an intrabdominal drain in place (biliary leakage with drain: n= 7 (10.3%) vs biliary leakage without drain n = 1 (2.4%), p=0.254) and in patients with bile duct drainage (biliary leakage with bile duct drainage: n = 6 (9.5%) vs biliary leakage without bile duct drainage n= 2 (4.3%) table 4). Biliary leakage after OLR was associated with a significantly longer hospital stay (hospital stay with biliary leakage: 38.4 ± 20.1 days vs. hospital stay without biliary leakage: 17.4 ± 11.1 days, p< 0.001).
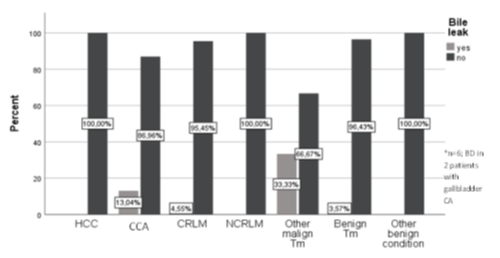
Figure 3: Biliary leak within the liver
|
Patients |
Biliary leakage, n (%) |
P |
|
|
Yes |
No |
||
|
Sex, male/female (%) |
4/4 (6.3/8.7) |
59/42 (93.7/91.3) |
0.719§ |
|
Hospital stay, days, mid. Rang |
38.4 +/- 20.1 (96.1) |
17.4 +/- 11.1 (51.8) |
<0.001# |
|
Major/minor resection, n (%) |
5/3 (6.7/8.8) |
70/31 (93.3/92.5) |
0.703§ |
|
Dignity, benign/malign (%) |
1/7 (6.2/7.5) |
15/86 (93.8/92.5) |
1.000§ |
|
Amdominal surgery in history, n (%) |
1.000§ |
||
|
Yes |
6 (8.2) |
67 (91.8) |
|
|
No |
2 (5.6) |
34 (94.4) |
|
|
Cirrhosis, n (%) |
0.549§ |
||
|
Yes |
1 (10.0) |
9 (90.0) |
|
|
no |
7 (7.1) |
92 (92.9) |
|
|
Bile duct drainage, n (%) |
0.4633§ |
||
|
Yes |
6 (9.5) |
57 (90.5) |
|
|
No |
2 (4.3) |
44 (95.7) |
|
|
Bilidigestin´v anastomsis, n (%) |
0.608§ |
||
|
Yes |
2 (11.8) |
15 (88.2) |
|
|
No |
6 (6.5) |
86 (93.5) |
|
|
t-drain used, y/n (%) |
3/3 (8.1/5.5) |
34/52 (91.9/94.5) |
0.982§ |
|
Abdominal drainage, n (%) |
0.254§ |
||
|
yes |
7 (10.3) |
61 (89.7) |
|
|
No |
1 (2.4) |
40 (97.6) |
|
|
Pringle-manoeuvre, (%) |
0.718§ |
||
|
Yes |
5 (6.8) |
68 (9.2) |
|
|
No |
3 (8.3) |
33 (91.7) |
|
|
Lymphadenectomy, n (%) |
1.000§ |
||
|
Yes |
4 (7.1) |
52 (92.9) |
|
|
No |
4 (7.5) |
49 (92.5) |
|
|
Transfusion at surgery, n (%) |
0.110§ |
||
|
Yes |
3 (17.6) |
14 (82.4) |
|
|
No |
5 (5.5) |
86 (94.5) |
|
|
Reabsfusion at ICZ, n (%) |
0.185§ |
||
|
Yes |
2 (18.3) |
9 (81.8) |
|
|
No |
6 (6.1) |
92 (93.9) |
|
- Fisher´s exact test/# MWMU test
Table 3: Influencing variables fort he apperance of biliary lleakage after OLR
Discussion
Our study demonstrates that the implementation and adaption of laparoscopic liver surgery does not adversely impact morbidity and mortality in a surgical high-volume liver centre. The introduction of laparoscopic liver resections, which accounted for a fraction of more than 30% of all procedures, was accompanied by a first series of feasible and save surgical interventions. This included 66% major and technically major liver resections affecting benign and malign lesions also in the posterolateral segments (VII, VIII and IVa) of the liver [12]. Smaller parenchyma sparing resections were intuitively performed applying the Diamond resection technique as described by Cipriani et al [17]. Our data are in line with the literature that laparoscopic liver resections allows for shorter operation times, reduced intraoperative blood loss, less wound infections, faster recovery and hence shorter hospital stay [18]. Until recently, liver resections In Germany were predominantly performed by open surgery. In contrast, specialized groups in Asia [19], the United States [20], Italy [18] France [21] and the United Kingdom [22] can draw on more than twenty years of experience in this still maturing field of minimally invasive surgery. Since the first international position statement on laparoscopic liver surgery [23] major scientific effort has propelled the application of minimally invasive surgical techniques in Europe. Major concerns regarding morbidity and mortality [24], especially after complex laparoscopic liver surgery with high death rates have been reported [25]. However, oncological inferiority and technical inapplicability have been scientifically disproved by a solid amount of research data [26].
Our findings support the position that laparoscopic surgery is well suited for resectable HCCs in cirrhotic livers [21,27], and resections for HCC also accounted for the major part of our oncologic LLRs in this study. International data also demonstrate that in selected patients with HCC, liver resection does not increase the morbidity or impair long-term survival following liver transplantation [28]. Therefore, laparoscopic liver resection prior to transplantation has been integrated into our treatment strategy for HCC as well.
Biliary leakage remains a serious complication after liver resection with an incidence of up to 12% [29]. Intraperitoneal septic complications resulting from biliary leakages may lead to secondary organ failure and even death [30,31].
Leakages can derive from previously ligated bile ducts at the resection surface of the liver, either based on a separated biliary system or based on an elevated intrabiliary pressure due to downstream stenosis of the biliary system or papillary dysfunction. Moreover, leakage from a bilioenteric anastomosis if performed, is another source of BL [32]. Risk factors include prolonged operation time, age, preoperative chemotherapy and special types of liver resections such as left hemihepatectomy [33]. In our study bile leaks were detected after five major (6.7%) and three minor open liver resections (8.8%). An increased incidence for biliary leakages after left hemihepatectomie has also been recently highlighted in a meta-analysis on laparoscopic liver resections [9]. Fortunately, we did not detect any biliary leakages in our series of LLR which also included ten laparoscopic left hemihepatectomies.
Despite a very low morbidity and especially BL rate in OLR without bilioenteric anastomosis the introduction of a minimally invasive liver resection program did not increase the BL-rate, despite modifications in the technique of parenchymal transection. It could be shown by others, that BL is a persisting problem in modern liver surgery. Whereas, despite an increasing number of complex liver resections the overall morbidity remains stable, but the rate of bile leaks is increasing with an increasing complexity of liver resection [34] leading to an incidence of BL of more than 7% even in high volume centres [35].
Prevention of bile leaks may include several measures as: (1) omitting of abdominal drains, (2) meticulous anatomy orientated liver surgery, (3) pre- or intraoperative recognition of variants of the biliary system, (4) application of bile leak tests and (5) eventually biliary decompression e.g. by means of T-tubes in case of complex liver surgery. However, the latter is not supported by enough evidence, so reduction of postoperative BL (OR 0.3, p=0.002) [15], however it was not used in LLR. Other measures, which did not prove effective in the prevention of BL like routine application of fibrin sealant was not used at all in the present series. However, surveys e.g. from the Netherlands have shown that more than around 25% of surgeons use fibrin sealants on a frequent/routine basis [38], among others to reduce resection surface-related complications like BL. However, the use of fibrin sealants disproved to reduce the incidence of BL in a meta-analysis [39]. However, insertion of a T-tube was afflicted with a (slightly) higher incidence of BL in the present series. Since a T-tube was used mainly in complex cases or in case of long central exposure of the bile duct, this phenomenon is supposed to be based on a consecutive selection of a high risk population for BL based on our previous experience [7] rather than an increased rate of bile leaks caused by T-tube insertion itself. However, this hypothesis cannot be finally proven, but no patient developed BL caused by the T-tube itself, e.g. at the site of insertion into the common bile duct. Influencing factors and the distribution of T-tubes within open liver resected patients (without a BDA) are demonstrated in table 4.
|
Patients |
T-drain used, n (%) |
P |
|
|
Yes |
No |
||
|
Diagnosis, n (%) |
|||
|
Primary malign hepatic tumours |
|||
|
HCC |
7 (18.9) |
10 (18.2) |
|
|
CCA |
6 (16.2) |
5 (9.1) |
|
|
Gallbladder CA |
2 (5.4) |
1 (1.8) |
|
|
Others |
0 (0.0) |
2 (3.6) |
|
|
Hepatic metastases |
|||
|
Colorectal |
13 (35.1) |
23 (41.8) |
|
|
Others |
5 /13.5) |
6 (10.9) |
|
|
Benign lesions |
|||
|
Benign tumours |
3 (8.1) |
8 (14.6) |
|
|
Others |
1 (2.7) |
0 (0.0) |
|
|
Major/minor resection, n (%) |
26/11 (43.3/34.4) |
34/21 (56.7/65.5) |
0.404** |
|
Operation time, min |
352.4 +/- 82.3 |
303.5 +/- 98-7 |
0.012* |
|
Intraoperative blood loss, ml |
572.0 +/- 323.6 |
447.5 +/- 386.9 |
1.124** |
|
Transfusion, n (%)# |
4 (10.8) |
6 (10.9) |
0.988** |
|
Lynphadenectomy, n (%) |
24 (64.9) |
19 (34.5) |
0.004** |
*t-test/** Chi-quadrat-test/# one anaesthesia case file missing, n=108
Table 4: Surgical data and existence of T-drain after ORL without BDA
However, the incidence of BL in our series compared well with other analyses, e.g. of the National Clinical Database (NCD) of Japan including 14,970 patients with a revealed a BL-rate of 8.0% [40]. In this analysis gallbladder cancer and extrahepatic bile duct carcinoma were also unravelled as risk factors for BL, moreover peripheral vascular disease and open wounds were pointed out as further risk factors.
The rate of other complications was low in the present analysis for LLR as well as OLR, including blood loss and blood transfusions, e.g. compared to NSQIP data [41], where transfusions were required in 33% of patients. Also, the overall morbidity was very low after LLR and significantly reduced compared to OLR. Even the morbidity of the OLR group compares well with other reports from other European centres [35] and clinics from North America [42]. However, since patient characteristics differed between both groups, a direct comparison of both groups is debatable. Especially the mortality seems to be rather dependant on patient characteristics than on surgical technique. Likewise, in an analysis using data on 7621 hepatectomies form the US- NSQIP-database [42] it has been shown that mortality after liver resection is predominantly seen in elderly patients in combination with major liver resection.
A recent randomized controlled trial which compared laparoscopic and open liver resection for CRLM indicated superiority of laparoscopic surgery in terms of postoperative complications and cost effectiveness [43]. Almost 15% of our minimally invasive operated patients were treated for CRLM and laparoscopic parenchyma sparing liver surgery was furthermore included into our multimodal treatment strategy for this disease [44]. Recent short-term data also indicate that repeat laparoscopic liver resection after open liver resection might be associated with favourable outcomes in selected groups of patients including those with CRLM [45].
Reports on laparoscopic liver resection for patients with cholangiocarcinoma are still scarce. In our cohort, only two patients with intrahepatic Cholangiocarcinoma were treated by laparoscopic liver resection. All other CCA patients, and especially those with perihilar cholangiocarcinoma received an open liver resection, as described by our group earlier [13]. To date, due to oncological superiority, portal vein embolization for preoperative future liver remnant augmentation and “hilar on block resection” remains our treatment strategy for central bile duct carcinomas [14]. However, this type of resection which also requires vascular reconstruction of the portal vein, to date was not performed laparoscopically by our group. Furthermore, postoperative hepatic insufficiency and bile leakage after demanding biliary reconstruction, often with several small orifices, contribute to the postoperative complication rate of this complex surgical disease pattern [46]. In our study presented here postoperative biliary leakages were predominantly detected in patients with Cholangiocarcinoma or gallbladder carcinoma, which in five cases required extrahepatic bile duct resection. All patients were operated by means of open surgery and received a bilioenteric anastomoses with external biliary drainage, if applicable. A recent study revealed that external biliary drainage following major liver resection did not necessarily decrease the risk of biliary anastomotic leakage and was furthermore associated with an increased incidence of post hepatectomy liver failure [47]. Our incidence in post hepatectomy liver failure however was comparatively low, probably due to extensive perioperative liver function testing including the LiMAx test for this selected group of patients.
Our study has several limitations. A direct comparison of the minimally invasive and open liver resections is invalid, since we did not perform a propensity score matching. Patients in the LLR group hence displayed a better ASA performance status and comprised fewer complex resections including extrahepatic bile duct resections. Furthermore, laparoscopic resections included a significant smaller number of radical lymphadenectomies. The extrahepatic bile duct obtains its blood supply mainly from small branches of the hepatic artery. Hence, extensive lymphadenectomy and long segment bile duct exposure can cause blood deprivation and segmental ischemia induced necrosis which ultimately results in bile leakage. Our overall biliary leakage rate was low when compared to the international literature [48,49]. Increased experience is commonly paralleled by increased confidence to perform more complex resections. Our overall morbidity and mortality rates are satisfying, especially when compared to the overall German average [50].
We were able to safely develop a minimally invasive liver resection program at our institution which so far resulted in an excellent patient outcome. It could be shown, that technical modifications of the surgical approach, like introduction of ultrasonic shears for parenchymal transection are safe and especially not afflicted with an increased rate of biliary leakage or bleeding complications.
Declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of the University of Leipzig, number 142/18-EK and the study protocol was performed in accordance with the relevant guidelines. Informed consent was waived by the ethics committee of the University of Leipzig due to the retrospective nature of the study.
Consent for publication
The current study doesn't involve identity revealing human information, consent for publication is hence not required.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interest
The authors declare that they have no competing interests
Funding
We acknowledge support from the German Research Foundation (DFG) and Leipzig University within the program of Open Access Publishing.
Acknowledgements
Nothing to acknowledge
Authors contributions
Substantial contributions to the conception: RS, SR, HG, DS
Design of the work: RS, SR, HG, ES, AL, TB, CH, DS
Acquisition, analysis of data: SR, HG
Interpretation of data: SR, HG,
Drafted the work or substantively revised it: RS, SR, HG, ES, AL, TB, CH, DS
Have approved the submitted version (and any substantially modified version that involves the author's contribution to the study): RS, SR, HG, ES, AL, TB, CH, DS (all authors)
Have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature: RS, SR, HG, ES, AL, TB, CH, DS (all authors)
References
- Sucher E, Sucher R. Minimally invasive liver surgery: a field is maturing. Laparoscopic Surgery (2019).
- Sucher R, Rademacher S, Lederer A, et al. Laparoscopic Left Hemihepatectoy Applying Intraoperative Indocyanine Green Fluorescence Detection Counter Perfusion Method for Visualization. Zentralbl Chir 145 (2020): 135-137.
- Sucher R, Brunotte M, Seehofer D. Indocyanine green fluorescence staining in liver surgery. Chirurg (2020).
- Sucher R, Hau HM, Rademacher S, et al. Totally minimally invasive extended right hepatectomy using the intracorporal liver hanging maneuver. Videoscopy 29 (2019): 435-442.
- Nagano Y, Togo S, Tanaka K, et al. Risk factors and management of bile leakage after hepatic resection. World J Surg 27 (2003): 695-698.
- Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 149 (2011): 680-688.
- Eurich D, Henze S, Boas-Knoop S, et al. T-drain reduces the incidence of biliary leakage after liver resection. Updates Surg 68 (2016): 369-376.
- Strucker B, Stockmann M, Denecke T, et al. Intraoperative placement of external biliary drains for prevention and treatment of bile leaks after extended liver resection without bilioenteric anastomosis. World J Surg 37 (2013): 2629-2634.
- Yin X, Luo D, Huang Y, Huang M. Advantages of laparoscopic left hemihepatectomy: A meta-analysis. Medicine (Baltimore) 98 (2019): e15929.
- Hibi T, Cherqui D, Geller DA, et al. Expanding indications and regional diversity in laparoscopic liver resection unveiled by the International Survey on Technical Aspects of Laparoscopic Liver Resection (INSTALL) study. Surg Endosc 30 (2016): 2975-2983.
- Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg 12 (2005): 351-355.
- Di Fabio F, Samim M, Di Gioia P, et al. Laparoscopic major hepatectomies: clinical outcomes and classification. World J Surg 38 (2014): 3169-3174.
- Neuhaus P, Jonas S, Bechstein WO, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg 230 (1999): 808-818.
- Neuhaus P, Thelen A, Jonas S, et al. Oncological superiority of hilar en bloc resection for the treatment of hilar cholangiocarcinoma. Ann Surg Oncol 19 (2012): 1602-1608.
- Linke R, Ulrich F, Bechstein WO, et al. The White-test helps to reduce biliary leakage in liver resection: a systematic review and meta-analysis. Ann Hepatol 14 (2015): 161-167.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240 (2004): 205-213.
- Cipriani F, Shelat VG, Rawashdeh M, et al. Laparoscopic Parenchymal-Sparing Resections for Nonperipheral Liver Lesions, the Diamond Technique: Technical Aspects, Clinical Outcomes, and Oncologic Efficiency. J Am Coll Surg 221 (2015): 265-272.
- Aldrighetti L, Belli G, Boni L, et al. Italian experience in minimally invasive liver surgery: a national survey. Updates Surg 67 (2015): 129-140.
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 261 (2015): 619-629.
- Reddy SK, Tsung A, Geller DA. Laparoscopic liver resection. World J Surg 35 (2011): 1478-1486.
- Fuks D, Aldrighetti L, Jiao LR, et al. Laparoscopic Management of Hepatocellular Carcinoma: A Critical Reappraisal. Surg Laparosc Endosc Percutan Tech 27 (2017): 203-215.
- Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg 268 (2018): 11-18.
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 250 (2009): 825-830.
- He J, Amini N, Spolverato G, et al. National trends with a laparoscopic liver resection: results from a population-based analysis. HPB (Oxford) 17 (2015): 919-926.
- Kameda M. Medical bodies launch system to track laparoscopic surgeries. The Japan Times (2015).
- Gavriilidis P, Roberts KJ, Aldrighetti L, et al. A comparison between robotic, laparoscopic and open hepatectomy: A systematic review and network meta-analysis. Eur J Surg Oncol 21 (2020): 5645-5652.
- Seehofer D, Sucher R, Schmelzle M, et al. Evolution of laparoscopic liver surgery as standard procedure for HCC in cirrhosis? Z Gastroenterol 55 (2017): 453-460.
- Belghiti J, Cortes A, Abdalla EK, et al. Resection prior to liver transplantation for hepatocellular carcinoma. Ann Surg 238 (2003): 885-892.
- Reed DN Jr, Vitale GC, Wrightson WR, et al. Decreasing mortality of bile leaks after elective hepatic surgery. Am J Surg 185 (2003): 316-318.
- Norton L, Moore G, Eiseman B. Liver failure in the postoperative patient: the role of sepsis and immunologic deficiency. Surgery 78 (1975): 6-13.
- Yanaga K, Kanematsu T, Takenaka K, et al. Intraperitoneal septic complications after hepatectomy. Ann Surg 203 (1986): 148-152.
- Lam CM, Lo CM, Liu CL, et al. Biliary complications during liver resection. World J Surg 25 (2001): 1273-1276.
- Lo CM, Fan ST, Liu CL, et al. Biliary complications after hepatic resection: risk factors, management, and outcome. Arch Surg 133 (1993): 156-161.
- Zimmitti G, Roses RE, Andreou A, et al. Greater complexity of liver surgery is not associated with an increased incidence of liver-related complications except for bile leak: an experience with 2,628 consecutive resections. J Gastrointest Surg 17 (2017): 57-64 .
- Dokmak S, Fteriche FS, Borscheid R, et al. 2012 Liver resections in the 21st century: we are far from zero mortality. HPB (Oxford) 15 (2013): 908-915.
- Shwaartz C, Fields AC, Aalberg JJ, et al. Role of Drain Placement in Major Hepatectomy: A NSQIP Analysis of Procedure-Targeted Hepatectomy Cases. World J Surg 41 (2017): 1110-1118.
- Squires MH, Weber SM, Brinkman A, et al. Value of primary operative drain placement after major hepatectomy: a multi-institutional analysis of 1,041 patients. J Am Coll Surg 220 (2015): 396-402.
- Boonstra EA, Molenaar IQ, Porte RJ, et al. Topical haemostatic agents in liver surgery: do we need them? HPB (Oxford) 11 (2009): 306-310.
- Sanjay P, Watt DG, Wigmore SJ. Systematic review and meta-analysis of haemostatic and biliostatic efficacy of fibrin sealants in elective liver surgery. J Gastrointest Surg 17 (2013): 829-836.
- Yokoo H, Miyata H, Konno H, et al. Models predicting the risks of six life-threatening morbidities and bile leakage in 14,970 hepatectomy patients registered in the National Clinical Database of Japan. Medicine (Baltimore) 95 (2016): e5466.
- Day RW, Brudvik KW, Vauthey JN, et al. Advances in hepatectomy technique: Toward zero transfusions in the modern era of liver surgery. Surgery 159 (2016): 793-801.
- Tzeng CW, Cooper AB, Vauthey JN, et al. Predictors of morbidity and mortality after hepatectomy in elderly patients: analysis of 7621 NSQIP patients. HPB (Oxford) 16 (2014): 459-468.
- Fretland AA, Dagenborg VJ, Bjornelv GMW, et al. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann Surg 267 (2018): 199-207.
- Seehofer D, Neuhaus P. Current status of multimodal therapy for colorectal liver metastases. Zentralbl Chir 136 (2011): 343-351.
- Wakabayashi T, Felli E, Memeo R, et al. Short-term outcomes of laparoscopic repeat liver resection after open liver resection: a systematic review. Surg Endosc 33 (7): 2083-2092.
- Seehofer D, Kamphues C, Neuhaus P. Resection of Klatskin tumors. Chirurg 83 (2012): 221-228.
- Olthof PB, Coelen RJ, Wiggers JK, et al. External biliary drainage following major liver resection for perihilar cholangiocarcinoma: impact on development of liver failure and biliary leakage. HPB (Oxford) 18 (2016): 348-353.
- Erdogan D, Busch OR, Van Delden OM, et al. Incidence and management of bile leakage after partial liver resection. Dig Surg 25 (2008): 60-66.
- De Castro SM, Kuhlmann KF, Busch OR, et al. Incidence and management of biliary leakage after hepaticojejunostomy. J Gastrointest Surg 9 (2005): 1163-1171.
- Filmann N, Walter D, Schadde E, et al. Mortality after liver surgery in Germany. Br J Surg 106 (2019): 1523-1529.


 Impact Factor: * 4.2
Impact Factor: * 4.2 Acceptance Rate: 72.62%
Acceptance Rate: 72.62%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 