De-Implementation of Urinary Catheters in Neurosurgical Patients during the Operation and on the Ward: A Mixed-Methods Multicentre Study Protocol
Jeanne-Marie Nollen1*, Anja H Brunsveld-Reinders2, Wilco C Peul1, Wouter R Van Furth1
1Department of Neurosurgery, Leiden University Medical Center, The Netherlands
2Department of Quality and Patient Safety, Leiden University Medical Center, The Netherlands
*Corresponding Author: Jeanne-Marie Nollen RN, Department of Neurosurgery, Leiden University Medical Center, The Netherlands
Received: 08 March 2022; Accepted: 16 March 2022; Published: 24 March 2022
Article Information
Citation:
Jeanne-Marie Nollen RN, MSc, Anja H Brunsveld-ReinderJeanne-Marie Nollen RN, MSc, Anja H Brunsveld-Reinders RN, PhD, MSc, Wilco C Peul MD, PhD, MPH, MBA, Wouter R Van Furth MD, PhD. De-Implementation of Urinary Catheters in Neurosurgical Patients during the Operation and on the Ward: A Mixed-Methods Multicentre Study Protocol. Journal of Surgery and Research 5 (2022): 145-158.Jeanne-Marie Nollen RN, MSc, Anja H Brunsveld-Reinders RN, PhD, MSc, Wilco C Peul MD, PhD, MPH, MBA, Wouter R Van Furth MD, PhD. De-Implementation of Urinary Catheters in Neurosurgical Patients during the Operation and on the Ward: A Mixed-Methods Multicentre Study Protocol. Journal of Surgery and Research 5 (2022): 145-158.s RN, PhD, MSc, Wilco C Peul MD, PhD, MPH, MBA, Wouter R Van Furth MD, PhD. De-Implementation of Urinary Catheters in Neurosurgical Patients during the Operation and on the Ward: A Mixed-Methods Multicentre Study Protocol. Journal of Surgery and Research 5 (2022): 145-158.
View / Download Pdf Share at FacebookAbstract
Background
Indwelling urinary catheters (IDUCS) are routinely inserted during transsphenoidal pituitary gland tumour surgery or spinal fusion surgery, despite literature stating that there are no indications for using IDUCS during or following these surgeries. The aim of the study is to reduce the number of inappropriately inserted IDUCS during or post transsphenoidal pituitary gland tumour surgery and spinal fusion surgery with an operation time of less than 4 hours.
Methods
A pragmatic, before-and-after mixed-methods observational study was initiated in a multicentre neurosurgical context. This study includes medical chart analysis, satisfaction surveys with patients and healthcare professionals, and multidisciplinary group interviews to assess the effectiveness of, and experiences with, a multifaceted non-invasive de-implementation strategies The study has a timespan of 2.5 years starting in 2020.
Discussion
This paper presents the study protocol of a multi-centred before and after trial that aims to reduce inappropriate IDUC use after transsphenoidal pituitary gland tumour surgery and spinal fusion surgery, thereby reducing UTIs, shortening length of hospital stay, and increasing patient comfort. The results can be used to de-implement IDUCS after a broad range of surgeries on several wards.
Keywords
<p>De-implementation, Urinary catheters, Mixed-methods, Healthcare professionals, Patients</p>
Article Details
Trial registration
The study has been submitted to the Dutch Trial Register (NTR).
Contributions to the literature
- The implementation of a variety of de-implementation strategies focussed on the healthcare professional as well as patients on reducing indwelling urinary catheter use and its complications;
- A greater understanding of patients’ experiences with urinating after transsphenoidal resection of pituitary gland tumours and spinal fusion operations;
- Facilitates multidisciplinary discussion on the use of IDUCS in the postoperative phase.
1. Background
Indwelling urinary catheter (IDUC) placement in instances of neurosurgical interventions such as anterior skull base operations (e.g. transsphenoidal resection of pituitary gland tumours) and spinal fusion operations (spondylodesis) has become standard practice for various reasons [1,2]. In patients where an IDUC was not placed during surgery, these will frequently be inserted upon their return at the recovery room or the neurosurgical ward.
Current literature highlights a distinction between appropriate and inappropriate IDUC use in daily practice. The following reasons are generally viewed as appropriate:
- Urinary retention and obstruction of the bladder [3];
- Surgery time > 4 hours [4];
- Mobility restriction ≥ 24 hours postoperative [3];
- Administration of large contents of infusion fluid and/or diuretics during operation [3];
- The need to measure the urine production every hour postoperative [5].
Despite abovementioned appropriate reasons for IDUC placement, there are a number of arguments to be made against IDUC use including prolonged recovery time and increased health risks of a different nature. It is commonly known that IDUCS are associated with urinary tract infections (UTIs), non-infection complications (e.g. pain, discomfort, haematuria, mobility restriction and the feeling the need to urinate) and delayed mobilization [6]. UTIs need to be treated with antibiotics which can lead to antibiotic resistance [7,8], and cases of hospital acquired UTIs are associated with longer hospital stay and additional costs [9]. The restriction on a patient’s ability to mobilize due to the IDUC prolongs their recovery time as research shows that early mobilization postoperatively decreases the risk complications and morbidity (e.g. respiratory decompensation/pneumonias, deep venous thrombosis/pulmonary embolism) [10]. If an IDUC is inserted pre- or postoperatively, literature, regardless of the surgical diagnosis, indicates that IDUCS should be removed promptly, preferably within 24-hours postoperatively. This is due to the fact that with every extra day an IDUC remains in place, the patient risk for developing a urinary tract infection increases by 3-7% [3,11]. However, it is unknown to what extent IDUCS are removed within this 24-hour timeframe after transsphenoidal resection of pituitary gland tumours and spinal fusion operations. The decision to insert an IDUC pre- and postoperative transsphenoidal resection of pituitary gland tumours and spinal fusion operations, is based on a number of considerations including but not limited to: the detection of the post-surgical complications diabetes insipidus (DI), post-operative mobility restriction (maximum 24-hours), urinary retention, and convenience for nurses when a patient has an IDUC.
Diabetes insipidus
The key argument in favour of IDUC placement is that it helps ensure close monitoring of fluid balance, which is key to early detection and diagnosis of the most common postoperative complication after pituitary surgery is diabetes insipidus. This condition is characterized by polydipsia and polyuria and can lead to dehydration when left undetected [12]. Although IDUCS are known to increase accuracy with regards to measurement of fluid output, hourly measurement of the fluid balance postoperatively, which is indicated an appropriate indication for IDUC use, is not a requirement [13]. Monitoring the fluid balance closely every 6-12 hours following transsphenoidal pituitary surgery is sufficient for ensuring early detection and diagnosis of diabetes insipidus (DI) [2,14,15]. The absence of additional risk of fluid disturbance following spondylodesis operations reduces the need to monitor fluid balance postoperatively [16].
Mobility restriction
In general, postoperative mobilisation restriction occurs only in rare instances following transsphenoidal resection of pituitary gland tumours and spondylodesis operations, and the duration of the bedrest generally does not exceed the twenty-four hour limit, which is the cut-off-point for an appropriate IDUC indication [3,4,15,17-20].
Urinary retention
Another common reason IDUCS are inserted postoperatively is due to post-operative urinary retention (POUR). Urinary retention is the inability to empty the bladder despite being full [21]. POUR is common following anesthesia and surgery without IDUC placement, with reported incidence of 5%-70% after general surgery and up to 50% after spinal surgery [22,23]. Despite POUR being indicated as an appropriate reason for IDUC insertion, intermittent catheterization has been described in literature as preferred intervention due to a lower risk of UTIs [3].
Convenience
IDUCS are frequently inserted after surgery due to convenience of care for nurses, especially after pituitary surgery where one of the main tasks for nurses is to monitor the fluid balance [24]. IDUCSS reduce nurses’ workload as there is no need to mobilize patient to the restroom and collect the urine in bedpans [25].
Alternatives
Instead of inserting an IDUC without an appropriate reason, the urinary output can be collected and measured with the aid of non-invasive, lower risk tools including an urinal or bedpan [20]. When a patient is unable to urinate postoperatively, bladder scanners can help assess the urinary retention after which intermittent catheterization can be executed [26]. Since IDUCS might be used to a greater extent and possibly for a longer period than is deemed appropriate by literature following these surgeries, this protocol describes a study to evaluate the effectiveness of multiple de-implementation strategies to reduce the inappropriate use of IDUCS during the operation and in the postoperative phase on the ward. Therefore, the goal of this study is: “no IDUC, unless…”
2. Methods
Design
This pragmatic, mixed-methods observational study collects medical chart data, satisfaction survey data and multidisciplinary group interviews data to assess the effectiveness of and experiences with various non-invasive de-implementation strategies aimed at decreasing the number of inappropriate IDUCS inserted during and after transsphenoidal pituitary gland tumour surgery and spinal fusion surgery in a multicentre context. The study has a before-and-after design and a timespan of 2.5 years starting in 2020. The medical chart assessment continues throughout the entire duration of the study whereas the satisfaction surveys and group interviews take place both before and after the de-implementation strategies are implemented. The surveys will be held with both patients and healthcare professionals whereas the group interviews will involve healthcare professionals only. Quantitative methods are used to assess the effect of the de-implementation strategies on IDUC related outcomes including IDUC placement, complications and patients’ and healthcare professionals’ experiences. The group interviews are used to gather insight into the role of each specific professional regarding IDUC use in the patients’ journey from pre-operative consult to discharge.
We have six specific aims:
- To reduce the number of inappropriate inserted IDUCS in the hospital during and after transsphenoidal pituitary gland tumour surgery and spinal fusion surgery with an operation time of less than 4 hours;
- To assess the frequency of intermitted urinary catheterization after transsphenoidal pituitary gland tumour surgery and spinal fusion surgery;
- To reduce the number of UTIs following transsphenoidal pituitary gland tumour surgery and spinal fusion surgery;
- To assess the number of urinary retention bladders in relation to the number of IDUCS placed during and after transsphenoidal pituitary gland tumour surgery and spinal fusion surgery;
- To better understand patients’ experiences and to provide a broad understanding of potential factors contributing to patient satisfaction in relation to urinating in the postoperative phase;
- To investigate healthcare professionals’ experiences with IDUCS and the experienced consequences after IDUC de-implementation.
Setting
This is a multicentrae study and will take place in one university hospital in which both transsphenoidal pituitary gland tumour surgery and spinal fusion surgery are executed, and four general hospitals where only spinal fusion surgery is performed. The multifaceted de-implementation strategies will be implemented in four intervention hospitals: the university hospital and three general hospitals. One general hospital is designated for the control group since, according to the hospitals’ neurosurgeons, IDUCS are not routinely placed in this hospital. All hospitals are located in the Randstad, which is the most densely populated area in the Netherlands and selected based on the following criteria: 1. transsphenoidal pituitary gland tumour surgery and spinal fusion surgery is executed and 2. IDUC use is routinely reported in the medical chart.
Study population
The study population consists of two groups: 1. patients who underwent/will undergo transsphenoidal pituitary gland tumour surgery or spinal fusion surgery and 2. healthcare professionals (e.g. neurosurgeons, neurosurgical residents, operation assistants, recovery nurses, neurosurgical ward nurses). All patients who underwent transsphenoidal pituitary gland tumour surgery or spinal fusion surgery in 2019 and 2020, and are aged 18 and older, are included in the medical chart assessment. Patients who will undergo transsphenoidal pituitary gland tumour surgery or spinal fusion surgery in 2021 or 2022, and are aged 18 and older, are eligible for the study and have to give consent for the medical chart assessment and survey. Patients who meet any of the following criteria will be excluded from participation: an operation time > 4 hours; having a mobility restriction ≥ 24 hours postoperative, having pre-existing bladder complications for which an IDUC is used pre-operatively; peri- or postoperative neurological deficit (e.g. paresis, paralysis); having pre-existing psychological problems; being unable to understand and/or execute instructions from healthcare professionals and not speaking fluent Dutch or English fluently. If patients are underwent surgery in 2021 or 2022 and informed consent is not obtained for the medical chart assessment or the survey, they will be excluded from the study. Healthcare professionals working as neurosurgeons, neurosurgical residents, operation assistants, recovery nurses or neurosurgical ward nurses are eligible for participation in the survey and group interviews. All participants must be aged 18 or older and provide consent to participate. Healthcare professionals who do not give consent for the survey and/or the group interviews are excluded from the study.
Main outcome
The primary study parameter is the number of IDUCS that are placed during and after transsphenoidal pituitary gland tumour surgery and spinal fusion surgery (spondylodesis).
Secondary outcomes
Secondary outcomes linked to the medical chart assessment are:
- incidence of intermittent urinary catheterization,
- incidence and volume of urinary retention bladders,
- incidence of urinary tract infections.
Secondary outcomes from the patients’ surveys are the postoperative experiences with and without IDUC use and the implications for the recovery process. Outcomes related to healthcare surveys are experiences with postoperative IDUC use and the consequences of de-implementing IDUCS. Secondary outcomes related to the group interviews are perceptions on the role of each specific professional regarding IDUC use in the patients’ journey from pre-operative consult to being discharged.
Medical chart assessment
During the pre-operative consult, patients will be asked to participate in the study, thereby participating in the medical chart assessment, by the nurse or resident who attends the consult. The following items will be systematically collected from each medical record:
- The incidence of IDUC placement, including date of insertion, time of placement, location of insertion, reason of insertion and which discipline inserted the IDUC;
- The incidence of intermitted urinary catheterization, including date and time of insertion, location of insertion, reason of insertion and which discipline inserted the catheter;
- The incidence and volume of urinary retention bladders, including: date of urinary retention and where the urinary retention was noticed. We defined a retention bladder as a urine volume of more than 500 milliliter (ml) [27];
The incidence of urinary tract infections. The diagnosis of a symptomatic urinary tract infection, with or without an IDUC, is the detection of bacteria and leukocytes in the presence of clinical symptoms [28]. This definition is chosen since asymptomatic urinary tract infections can be expected when testing urine from an IDUC without the presence of symptoms and do not require antibiotic therapy [29]. Clinical symptoms include painful and frequent urination, fever, flank pain and general malaise [30]. The pathogen and leukocytes are detected and identified by using midstream urine for a urine sediment. The sediment must contain >103 cfu/mL bacteria and >5 leukocytes [28,31];
- The operation time in minutes;
- The duration of stay in recovery room in minutes;
- The date of operation;
- Age;
- Gender;
- Length of hospital stay in days;
- Data is stored in Castor EDC.
Satisfaction surveys
The patient satisfaction survey will be designed to gather insight into patient experiences postoperatively and to provide a broad understanding of potential factors contributing to patient satisfaction in relation to urinating in the postoperative phase. The healthcare professional satisfaction survey will be designed to acquire a greater understanding of healthcare professionals’ experiences with IDUCS and the experienced consequences after IDUC de-implementation. Both surveys will be tested by pilot participants selected from the neurosurgical ward and the operation room. After piloting and revision, the surveys will be sent to all eligible healthcare professionals via their work-email in the before and after measurement phase. During the pre-operative consult, patients will be asked to participate in the survey by the nurse or resident who attends the consult. Patients will receive a hardcopy of the survey if they are admitted to the hospital.
Group interviews
A purposive sampling method will be used to create a diverse and representative sample of at least one professional from each profession. Healthcare professionals will be asked to participate in the focus group via their work e-mail. The participants are all working in one of the five hospitals and there will be no mixing between hospitals as policies and procedures can differ per site. The group interviews will be held at a date and place most suitable for the participants in a meeting room in the specific hospital. The group interview will focus on the following topics: 1. participants’ experiences with the current IDUC policy per- and postoperatively, 2. perceptions and experiences with intercollegiate collaboration and communications regarding IDUC use and 3. perceptions regarding the patients’ role. In addition, demographics including information on age, working experience and gender will be collected at the beginning of the group interviews. The interviews will be led by an independent moderator. At least one of the researchers will also attend the group interviews to answer specific questions related to the topics. The expected duration of the interviews is 60-80 minutes. The interviews will be taped and transcribed verbatim.
De-implementation strategies
In this study, multifaceted de-implementation strategies will be used to decrease the number of inserted IDUCS in pituitary and spinal fusion patients. The de-implementation strategies focus primarily on healthcare professionals. The rationale behind using multiple strategies is that the components positively influence one another and add to acquiring the wanted effect [32]. The de-implementation strategies will take place in the four intervention hospitals. There will be no strategies implemented in the control hospital.
Flowcharts
Three flowcharts (figures 1, 2 and 3) were created based on the indications for appropriate IDUC use in combination with the treatment of POUR. The flowcharts advocate intermittent catheterization over inserting an IDUC, as this intervention has a lower risk of UTIs [3]. A bladder scanner can be used if a patient is unable to urinate to detect the urinary retention [26]. Based on literature and the hospitals’ urinary retention policy, we used 500 ml urine in the bladder as cut-off-point for intermittent catheterization and 100 ml in the bladder as post-void residual [33,34]. Flowchart 1 is designed to use during prior to the operation when deciding on IDUC placement. Flowchart 2 can be used in the recovery room and helps determine actions necessary when a patient is unable to urinate
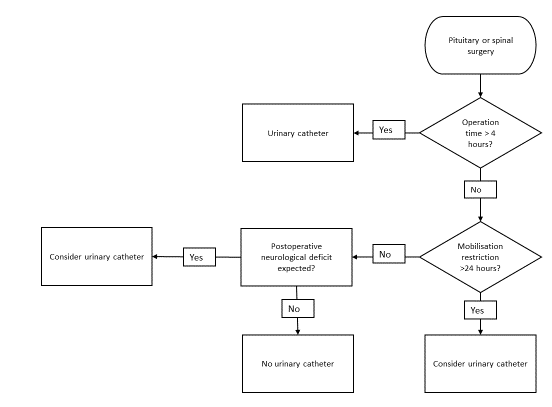
Figure 1: Flowchart IDUC placement during surgery
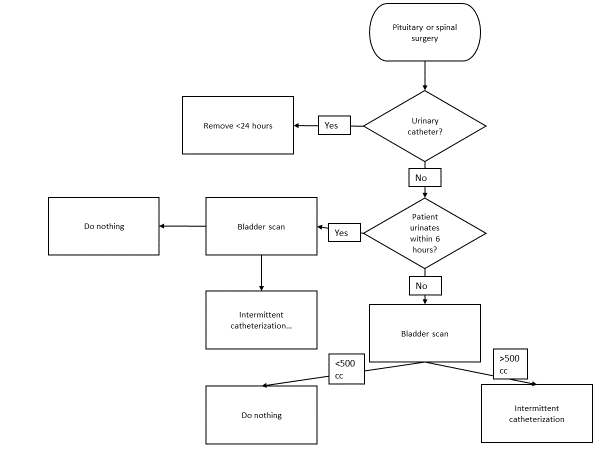
Figure 2: Flowchart IDUC placement in the recovery room
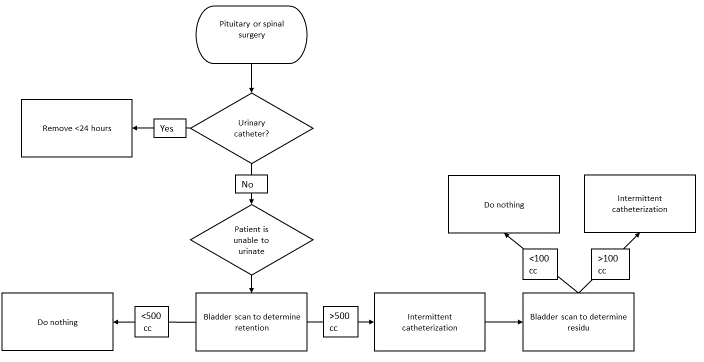
Figure 3: Flowchart IDUC placement in neurosurgical ward
Education
Neurosurgeons, neurosurgical residents, operation assistants, recovery nurses and neurosurgical ward nurses will receive education regarding appropriate and inappropriate IDUC use during and after pituitary and spondylodesis surgery. The information sessions consist of a presentation delivered by the researcher and will take place once at each intervention hospital . Figures 1, 2 and 3 will be used as basis for the educational programme. Additionally, the importance of reducing IDUC use as well as possible complications will be discussed. Healthcare professionals will receive information on how to document IDUC use comprehensively and thoroughly (e.g. date and time of insertion, location of insertion, reason of insertion, which discipline inserted the IDUC, date and time of urinary retention and volume of urinary retention) in the medical chart.
Information
Information regarding the existence of the study will be distributed among the healthcare professionals in the hospitals to create awareness. Intranet, social media and hospital newsletters will be used for dissemination.
Reminders
Informational posters regarding (in)appropriate IDUC use will be placed in the breakrooms of the operation theatre, neurosurgical residents and on the neurosurgical ward.
Organizational strategies
To ensure a structural change in IDUC use, the new policy will be established in the formal and informal rules of each hospital. This means that procedures and protocols regarding inserting an IDUC will be changed.
Feedback
The outcome measures of the collected data regarding IDUC at baseline and after measurements will be communicated to each hospital during the study once per month by sending newsletters to participating healthcare professionals.
Patient information
During the pre-operative consult with the neurosurgeon, all patients will receive information regarding the use of an IDUC during and after the surgery. Patients will receive an infographic explaining the reason for IDUC reduction including alternatives use.
Sample size
In the academic hospital, approximately 150 patients undergo a transsphenoidal pituitary gland tumour surgery per year. In all five hospitals combined, approximately 657 patients undergo a spinal fusion surgery (spondylodesis) per year. The duration of the study is 2.5 years which means that the medical charts of a total of 375 pituitary patients and 1643 spondylodesis patients can be included in the study. Patients will be asked to fill in the patient satisfaction survey for a period of two months during the basement measurement period as well as in the after measurement period. Per month, 12-13 patients will undergo pituitary surgery. Therefore, a total of 48-52 pituitary patients will be asked to participate in the survey. In the hospitals combined, 55 patients will have a spondylodesis operation every month, which means that over a period of four months 219 patients will be asked to fill in the survey. For all five hospitals combined, there are 650 healthcare professionals who are involved in the care for pituitary and/or spondylodesis patients. These participants will be asked to participate in a satisfaction survey at baseline measurement as well as the after measurement. Group interviews will be held in each intervention hospital in the baseline and after measurement phase. The group interviews will consist of six to eight participants as literature indicates that this number is sufficient (35). Per hospital 12-16 healthcare professionals will be asked to participate. In total, 60 to 80 healthcare professionals will be asked to participate in the group interviews.
3. Analysis
We used a combination of qualitative and quantitative data to answer the primary and secondary outcomes. The medical chart research and the satisfaction surveys will be analysed using quantitative techniques while the group interviews will be analysed with the aid of qualitative methods. A deletion method will be used to eliminate missing data.
Primary outcome
The primary study parameter will be the number of IDUCS that are placed during and/or after transsphenoidal pituitary gland tumour surgery and spinal fusion surgery (spondylodesis). The number of inappropriately/appropriately placed IDUCS will be determined with the aid of figures 1, 2 and 3. The software programme SPSS is used during the analysis. The data will be analysed for all hospitals combined with a logistic regression with corrections for several baseline characteristics of the population (e.g. age, sex, type of operation, hospital and COVID-19 period). Data corresponding with the IDUC placement (e.g. date of insertion, time of placement, location of insertion, reason of insertion and which discipline inserted the IDUC) will be analysed with descriptive statistics. The data will be analysed per hospital with the aid of descriptive statistics. Continuous data will be presented as median (interquartile range) or mean (standard deviation) and where appropriate categorical variables as number (percentages). Graphic data displays may also be used to summarize the data. Descriptive statistics will also be presented for the baseline measurement and the after measurement separately. Since the control hospital states that there a no IDUCS inserted during/after spondylodesis operations prior to the study, the extent to which the data from this hospital can be incorporated in the analysis will be determined after the baseline measurement. If there are (almost) no IDUCS inappropriately placed, the data will only be analysed with descriptive statistics. The data will be incorporated in the logistic regression if IDUCS are frequently inappropriately inserted.
Secondary outcome
Medical chart
The incidence of intermittent urinary catheterization, the incidence of urinary retention and the incidence of urinary tract infections will be analyzed equally to the primary study parameter.
Surveys
The surveys from the healthcare professionals will be analyzed with the aid of a paired non-parametric T-test. The surveys from the patients will be analyzed with a non-paired non-parametric test. Demographics will be analyzed with descriptive statists.
Group interviews
Following transcription of the interviews, the software program Atlas.ti will be used to analyze the data. The grounded theory will be used as a framework for the analysis [36,37]. This analysis involves three sequential phases of coding: open, axial and selective coding [38]. An iterative approach was used which implies that data collection and analysis occurred simultaneously [39,40]. Two researchers will independently code the transcripts and afterwards discuss the findings to reach consensus about the interpretation.
Ethics and funding
Approval from the Ethical Committee was obtained for all five hospitals either at site level or, where this did not exist, from a scientific committee at the site. The researchers will adhere to ethical standard for research involving people. Additionally, all researchers will follow their institutional ethical requirements. Funding sources did not partake in the writing of this manuscript or the decision to submit the publication. Patients and healthcare professionals will be given an informed consent form as well as information on the study and the participants rights, prior to the operations, the surveys and the group interviews,. It will be specifically stated that participation is voluntary, that participants can withdraw at any time, and that confidentiality is guaranteed through anonymization. Per request, the results of the study will be communicated to the participants by email.
4. Discussion
This paper presents the study protocol of a multi-centred before and after trial that aims to reduce inappropriate IDUC use after transsphenoidal pituitary gland tumour surgery and spinal fusion surgery, thereby reducing UTIs, shortening hospital stay and increasing patient comfort. Besides developing and executing de-implementation strategies to accomplish a reduction of used IDUCS, the study focusses on patient and healthcare professional experiences with IDUCS in daily practice and the consequences for the care system. Several challenges are anticipated while executing the study. Since this is a study executed in five hospitals, frequent and clear communication between the researchers and the different departments in each hospital is needed. Additionally, in light of busy schedules of our professionals, planning the group interviews ahead is necessary to ensure a sufficient number of participants. The results from this study can be used to de-implement IDUCS after a broad range of surgeries on several wards.
List of abbreviations
DI = Diabetes Insipidus
DURIN-study = De-implementation of urinary catheters in neurosurgical patients
IDUC = Indwelling Urinary Catheter
POUR = Post-Operative Urinary Retention
UTI = Urinary Tract Infection
Declarations
Ethics approval and consent to participate
The Medical Ethics Committee of the university hospital and the four general hospital approved the study (N20. 152).
Consent for publication
Patients operated in 2021 or 2022 will sign informed consent regarding publishing their data. All healthcare professionals will sign informed consent regarding publishing their data.
Availability of data and materials
Data will be available on Zenodo.
Competing interests
The authors have no conflicts of interest to report.
Funding
Funding was received for this study Radboudumc. Specifically from the department IQ Healthcare program "Doen of laten? Het bevorderen van gepaste zorg”.
Authors' contributions
All authors contributed to the study conception and design. Material preparation and data collection will be performed by JMN. Data analysis will be performed by JMN and AHBR. The first draft of this manuscript was written by JMN. AHBR, WvF and WP commented on previous versions of the manuscript. JMN, AHBR, WvF and WP read and approved the final manuscript.
Acknowledgements
Not applicable.
References
- Strickland AR, Usmani MF, Camacho JE, et al. Evaluation of risk factors for postoperative urinary retention in elective thoracolumbar spinal fusion patients. Global Spine Journal 11 (2021): 338-344.
- Edate S, Albanese A. Management of electrolyte and fluid disorders after brain surgery for pituitary/suprasellar tumours. Hormone research in paediatrics 83 (2015): 293-301.
- Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 31 (2009): 319-326.
- Sadeghi M, Leis JA, Laflamme C, et al. Standardisation of perioperative urinary catheter use to reduce postsurgical urinary tract infection: an interrupted time series study. BMJ Quality & Safety 28 (2019): 32.
- Nicolle LE. Urinary Catheter-Associated Infections. Infectious Disease Clinics of North America 26 (2012): 13-27.
- Saint S, Trautner BW, Fowler KE, et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Internal Medicine 178 (2018): 1078-1085.
- Chardavoyne PC, Kasmire KE. Appropriateness of antibiotic prescriptions for urinary tract infections. West J Emerg Med 21 (2020): 633-639.
- Pujades-Rodriguez M, West RM, Wilcox MH, et al. Lower Urinary Tract Infections: Management, Outcomes and Risk Factors for Antibiotic Re-prescription in Primary Care 14 (2019): 23-31.
- Mitchell BG, Ferguson JK, Anderson M, et al. Length of stay and mortality associated with healthcare-associated urinary tract infections: a multi-state model 93 (2016): 92-99.
- Epstein NE. A review article on the benefits of early mobilization following spinal surgery and other medical/surgical procedures. Surg Neurol Int 5 (2014): S66-S73.
- Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control 3 (2014): 23.
- Lamas C, del Pozo C, Villabona C. Clinical guidelines for management of diabetes insipidus and syndrome of inappropriate antidiuretic hormone secretion after pituitary surgery. Endocrinol Nutr 61 (2014): e15-24.
- Garrahy A, Moran C, Thompson CJ. Diagnosis and management of central diabetes insipidus in adults. Clinical endocrinology 90 (2019): 23-30.
- Prete A, Corsello SM, Salvatori R. Current best practice in the management of patients after pituitary surgery. Ther Adv Endocrinol Metab 8 (2017): 33-48.
- Zada G, Woodmansee W, Iuliano S, et al. Perioperative Management of Patients Undergoing Transsphenoidal Pituitary Surgery. Asian journal of neurosurgery 5 (2010): 1-6.
- Lall M. Nursing care of the patient undergoing lumbar spinal fusion. Journal of Nursing Education and Practice 8 (2017): 44.
- Shimanskaya V, Wagenmakers M, Bartels R, et al. Toward shorter hospitalization after endoscopic transsphenoidal pituitary surgery: day-by-day analysis of early postoperative complications and interventions. World Neurosurgery 111 (2018).
- Burgess LC, Wainwright TW, editors. What is the evidence for early mobilisation in elective spine surgery? A narrative review. Healthcare: Multidisciplinary Digital Publishing Institute (2019).
- Gould C, Umscheid C, Agarwal R. Toolkit for Reducing Catheter-Associated Urinary Tract Infections in Hospital Units: Implementation Guide (2019).
- Christian E, Harris B, Wrobel B, et al. Endoscopic endonasal transsphenoidal surgery: Implementation of an operative and perioperative checklist. Neurosurgical focus 37 (2014): E1.
- Palese A, Buchini S, Deroma L, et al. The effectiveness of the ultrasound bladder scanner in reducing urinary tract infections: a meta?analysis. Journal of clinical nursing 19 (2010): 2970-2979.
- Boulis NM, Mian FS, Rodriguez D, et al. Urinary retention following routine neurosurgical spine procedures. Surgical Neurology 55 (2001): 23-27.
- Baldini G, Bagry H, Aprikian A, et al. Postoperative urinary retention: anesthetic and perioperative considerations. Anesthesiology 110 (2009): 1139-1157.
- Hu FW, Yang DC, Huang CC, et al. Inappropriate use of urinary catheters among hospitalized elderly patients: Clinician awareness is key. Geriatr Gerontol Int 15 (2015): 1235-12341.
- Quinn M, Ameling JM, Forman J, et al. Persistent Barriers to Timely Catheter Removal Identified from Clinical Observations and Interviews. The Joint Commission Journal on Quality and Patient Safety 46 (2020): 99-108.
- Brouwer TA, Van den Boogaard C, Van Roon EN, et al. Non-invasive bladder volume measurement for the prevention of postoperative urinary retention: validation of two ultrasound devices in a clinical setting. J Clin Monit Comput 32 (2018): 1117-1126.
- Lee YY, Tsay WL, Lou MF, et al. The effectiveness of implementing a bladder ultrasound programme in neurosurgical units. J Adv Nurs 57 (2007): 192-200.
- Schmiemann G, Kniehl E, Gebhardt K, et al. The diagnosis of urinary tract infection: a systematic review. Dtsch Arztebl Int 107 (2010): 361-367.
- Trautner BW, Cope M, Cevallos ME, et al. Inappropriate treatment of catheter-associated asymptomatic bacteriuria in a tertiary care hospital. Clinical Infectious Diseases 48 (2009): 1182-1188.
- Vasudevan R. Urinary tract infection: an overview of the infection and the associated risk factors. J Microbiol Exp 1 (2014): 00008.
- Van Pinxteren B, Knottnerus B, Geerlings S, et al. NHG-Standaard Urineweginfecties (derde herziening). Huisarts Wet 56 (2013): 270-280.
- Colla CH, Mainor AJ, Hargreaves C, et al. Interventions Aimed at Reducing Use of Low-Value Health Services: A Systematic Review. Medical Care Research and Review 74 (2016): 507-550.
- Choi S, Mahon P, Awad IT. Neuraxial anesthesia and bladder dysfunction in the perioperative period: a systematic review. Canadian Journal of Anesthesia/Journal canadien d'anesthésie 59 (2012): 681-703.
- Thanagumtorn K. Accuracy of post-void residual urine volume measurement using an ultrasound bladder scanner among postoperative radical hysterectomy patients. J Med Assoc Thai 99 (2016): 1061-1066.
- Nyumba T, Wilson, Derrick C, et al. The use of focus group discussion methodology: Insights from two decades of application in conservation. Methods in Ecology and Evolution 9 (2018): 20-32.
- Van Houdt S, Sermeus W, Vanhaecht K, et al. Focus groups to explore healthcare professionals' experiences of care coordination: towards a theoretical framework for the study of care coordination. BMC Fam Pract 15 (2014): 177-180.
- Nili A, Tate M, Johnstone D, et al. A Framework for Qualitative Analysis of Focus Group Data in Information Systems (2014).
- Walker D, Myrick F. Grounded theory: An exploration of process and procedure. Qualitative health research 16 (2006): 547-559.
- Chapman A, Hadfield M, Chapman CJJotRCoPoE. Qualitative research in healthcare: an introduction to grounded theory using thematic analysis 45 (2015): 201-205.
- Mayer IJIJoS. Retailing, Marketing. Qualitative research with a focus on qualitative data analysis 4 (2015): 53-67.


 Impact Factor: * 4.2
Impact Factor: * 4.2 Acceptance Rate: 72.62%
Acceptance Rate: 72.62%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 