A study on significant prognostic factors in patients with stage IB Gastric Cancer
Keishiro Aoyagi1*, Yu Tanaka1, Hideaki Kaku1, Yuya Tanaka1, Taro Isobe1, Naotaka Murakami2, Seiya Ishibashi1, Fumihiko Fujita
1Department of Gastrointestinal Surgery, Faculty of Medicine, Kurume University, Kurume, Fukuoka 830-0011, Japan
2Department of Surgery, Omuta City Hospital, Omuta, Fukuoka 836-0861, Japan
*Corresponding Author: Keishiro Aoyagi, Department of Gastrointestinal Surgery, Faculty of Medicine, Kurume University, 67 Asahi-machi, Kurume city, Fukuoka 830-0011, Japan
Received: 01 September 2023; Accepted: 11 September 2023; Published: 03 October 2023
Article Information
Citation: Keishiro Aoyagi, Yu Tanaka, Hideaki Kaku, Yuya Tanaka, Taro Isobe, Naotaka Murakami, Seiya Ishibashi, Fumihiko Fujita. A study on Significant Prognostic factors in Patients with stage IB Gastric Cancer. Journal of Surgery and Research. 6 (2023): 332-342.
View / Download Pdf Share at FacebookAbstract
Objectives:
Aim: Most stage IB gastric cancers can undergo curative resection by surgery, but early stage and advanced cancers are mixed at this stage and little is known regarding their prognostic factors. In this study, factors involved in relapse-free and overall survival in stage IB gastric cancer were examined.
Methods: One hundred eighty-six patients with stage IB gastric cancer who underwent resection at our hospital between 1994 and 2018 were analyzed. Univariate analyses of factors involved in relapse-free survival and overall survival were performed using bivariate analyses, and multivariate analyses were undertaken using the Cox proportional hazards model.
Results: Five-year survival rate was 83.7% in stage IB (84.3% in T2N0 and 82.3% in T1N1). For relapse-free survival, a significant difference was observed in the degree of lymphatic vessel invasion and the number of lymph node metastases in the univariate analysis, and in the multivariate analysis, the degree of lymphatic vessel invasion was an independent prognostic factor. For overall survival, there were significant differences in age, clinical lymph node metastasis, tumor location, number of dissected lymph nodes, and preoperative co-existing disease in the univariate analysis. Age and clinical lymph node metastasis were independent prognosis factors in the multivariate analysis.
Conclusion: For relapse-free survival, the degree of lymphatic vessel invasion was an independent prognostic factor, and for overall survival, age and clinical lymph node metastasis were independent prognostic factors. We suggest that adjuvant chemotherapy should also be considered in cases with severe lymphatic invasion.
Keywords
<p>Gastric cancer, Stage IB, Prognostic factors, Relapse-free survival, Overall survival</p>
Article Details
Abbreviations:
CA19-9, carbohydrate antigen 19-9; CD, Clavien–Dindo; CEA; Carcinoembryonic antigen; CI, Confidence interval; Circ, Circumferential involvement; CT, computed tomography; D, extent of lymph node dissection; DG, distal gastrectomy; 5-FU, 5-fluorouracil; HCFU, 1-hexylcarbamoyl-5-fluorouracil; HR, hazard ratio; INFa, tumor displays expanding growth; INFb, tumor shows an intermediate pattern between INFa and INFc; INFc, tumor displays infiltrative growth; L; lower third; ly, lymphatic invasion; ly0, no lymphatic invasion; ly1, minimal lymphatic invasion; ly2, moderate lymphatic invasion; ly3, marked lymphatic invasion; M, middle third; N, lymph node metastasis; N0, no regional lymph node metastasis; N1, metastasis in 1-2 regional lymph nodes, N1(1), metastasis in 1 regional lymph node; N1(2), metastasis in 2 regional lymph nodes, N1(old classification), metastasis to Group 1 lymph nodes; N2 (old classification), metastasis to Group 2 lymph nodes; PET, positron emission tomography; PG, proximal gastrectomy; PPG, pylorus-preserving gastrectomy; SG, segmental gastrectomy; T, depth of tumor invasion; T1, Tumor confined to the mucosa or submucosa; T1a, tumor confined to the mucosa; T1b, tumor confined to the submucosa; T1b1, tumor invasion within 0.5 mm of the muscularis mucosae; T1b2, tumor invasion 0.5 mm or deeper into the muscularis mucosae; T2, tumor invades the muscularis propria; TG, total gastrectomy; U, upper third; UFT, Tegafur-uracil; v, venous invasion; v0, no venous invasion; v(+), venous invasion positive
Introduction
Gastric cancer remains one of the most common malignancies worldwide, with more than 1 million estimated new cases annually, and most cases are frequently diagnosed at an advanced stage, making it the third highest cause of cancer-associated death [1]. However, in Japan, many stomach cancers are detected at stage I, the frequency of which has increased by up to 50% over the past two decades due to improvements in the accuracy of endoscopy examinations and the expansion of screening systems [2]. Stage I gastric cancer, which is classified as stage IA and stage I B according to the Japanese Classification of Gastric Carcinoma [3], has a good prognosis and is not indicated for postoperative chemotherapy [4]. Stage IA does not involve lymph node metastasis and is confined to T1, and endoscopic submucosal dissection or reduction surgery less than D2 is often performed, showing a very good prognosis with a 5-year survival rate of 91.5% [5]. However, stage IB gastric cancer comprises tumor invasion of the muscularis propria with no regional lymph node metastasis (T2N0) or tumor confined to the mucosa or submucosa with metastasis in 1-2 regional lymph nodes (T1N1). Stage IB gastric cancer is a mixture of early (T1) (T1) and advanced cancers (T2) (T2), and a mixed stage of positive (N1) and negative lymph node metastases (N0). Stage IB gastric cancer is generally not subject to modified surgery and is cured by performing standard D2 dissection, and it has a relatively good prognosis with a 5-year survival rate of 83.6% [5]. Ahn et al. reported that 5-year survival was 95.1% for stage IA and 88.9% for stage IB following surgery alone (90.2% in T2N0 and 87.6% in T1N1) [6]. A small number of recurrent cases are also observed. Nonetheless, few reports have studied prognostic factors in stage IB. In this study, we examined the recurrence factors and prognostic factors of stage IB.
Materials and mMethods
Patients
Of 234 patients with stage IB gastric cancer according to the Japanese Classification of Gastric Carcinoma (3rd English edition) [3] who underwent curative gastrectomy at Kurume University Hospital from January 1994 to December 2018, 186 patients were included in this study, excluding residual gastric cancer (n=6), fewer than 16 lymph nodes dissected (n=26), neo-adjuvant chemotherapy (n=1), postoperative observation period less than 1 month (n=6), and double cancers (n=16). T1 is classified as mucosa (T1a) or submucosa (T1b), and T1b is subclassified as tumor invasion within 0.5 mm of the muscularis mucosae (T1b1) or tumor invasion 0.5 mm or deeper into the muscularis mucosae (T1b2) [3][3]. The number of patients with T1aN1, T1b1N1, T1b2N1, and T2N0 was 3, 10, 54, and 119, respectively (Figure 1). The period from 1984 to 2005 was considered the early phase, whereas that from 2006 to 2018 was considered the late phase. Patients who had complaints such as epigastric pain or epigastric discomfort were defined as symptom (+), and those in which gastric carcinoma was identified in a medical examination without complaint were defined as symptom (−).
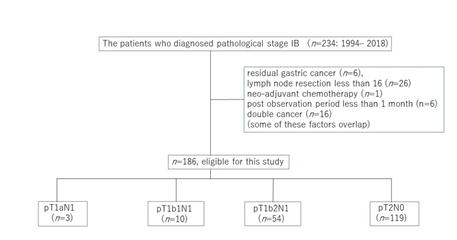
Figure 1: Flow diagrams and details of the 234 patients diagnosed with stage ?B cancer according to the third English edition of the Japanese Classification of Gastric Carcinoma.
Length of hospitalization, postoperative complications, and preoperative comorbidity
The length of hospitalization was defined as the period from the date of surgery to the date of discharge. Postoperative complications were evaluated on the basis of the Clavien–Dindo (CD) classification, and treatment was provided as necessary. CD grade 2 or higher was considered as postoperative complication (+). Preoperative comorbidity as listed in the Charlson comorbidity index was considered a preoperative comorbidity (+).
Tumor size and number of lesions
The major tumor axis was described as the tumor diameter. Where there were multiple lesions, the most advanced tumor (or the largest lesion where the T category or T stage was identical) was classified.
Clinicopathological terms
Clinicopathological terms such as main tumor portion (upper (U), middle (M), and lower (L)), main tumor part (lesser (Less), greater (Gre), anterior (Ant), posterior (Post), and circumferential involvement (Circ)), macroscopic type, histological classification, depth of tumor invasion (T), tumor infiltrative pattern (INF) (INFa, tumor displays expanding growth; INFb, tumor shows an intermediate pattern between INFa and INFc; and INFc, tumor displays infiltrative growth), lymphatic invasion (ly) (ly0, no lymphatic invasion; ly1, minimal lymphatic invasion; ly2, moderate lymphatic invasion; and ly3, marked lymphatic invasion), venous invasion (v) (v0, no venous invasion; and V(+), venous invasion-positive), and lymph node metastasis (N) were used in accordance with the Japanese Classification of Gastric Carcinoma (3rd English edition) [3]. Lymph node metastases were also considered by the old classification, which classified N number according to the site of lymph node metastasis [7].
Lymph node dissection was performed in all patients. The extent of lymph node dissection (D) was determined as defined in the Japanese gastric cancer treatment guidelines 2010 (ver. 3) [4].
Chemotherapy
Twelve patients underwent postoperative chemotherapy (1-hexylcarbamoyl-5 fluorouracil (HCFU), 6 patients (one patient was also given a combination of mitomycin C); 5-fluorouracil (5-FU), 4 patients; tegafur-uracil (UFT), 1 patient; and S-1, 1 patient).
Tumor markers
Cases in which preoperatively measured carcinoembryonic antigen (CEA) or carbohydrate antigen 19-9 (CA19-9) exceeded the reference value (CEA: 5.0 ng/mL; CA19-9: 37.0 U/mL) were considered to have tumor marker elevation.
Postoperative follow-up
All patients were examined postoperatively by ultrasound, computed tomography (CT), and gastroscopy, and tumor markers were measured at least once a year, while positron emission tomography/computed tomography (PET/CT) was performed as required.
Clinicopathological factors
To search for prognostic factors for stage IB gastric cancer, the following clinicopathological factors were examined: period (early (1984–2005) vs late (2006–2018)), age (<70 years vs ≥70 years), sex (male vs female), chief complaint ( + vs −), surgical method (total gastrectomy or proximal gastrectomy vs distal gastrectomy or pylorus-preserving gastrectomy or segmental gastrectomy), lymphadenectomy (D2 vs D1 or D1+), clinical depth of tumor invasion (cT1 vs ≥cT2), clinical lymph node metastasis (cN(−) vs cN(+)), macroscopic type (superficial type vs advanced type), main tumor portion (U vs M or L), main tumor part (Circ vs non-Circ), histological type (differentiated type vs undifferentiated type), pathological depth and lymph node metastasis (T1N1 vs T2N0), tumor infiltrative pattern (INFa or INFb vs INFc), lymphatic invasion (ly0 or ly1 vs ly2 or ly3), venous invasion (v0 vs v(+)), tumor size (≤40 mm vs ≥40 mm), number of lymph node metastases (N0 or metastasis in one regional lymph node (N1 (1)) vs metastasis in two regional lymph nodes (N1 (2)), lymph node metastases by the old convention classification (N0 or metastasis to group 1 lymph nodes (N1) vs metastasis to group 2 lymph nodes N2), number of dissected lymph nodes (≤40 vs >40), postoperative chemotherapy ((−) vs (+)), preoperative comorbidities ((−) vs (+)), postoperative complications ((−) vs (+)), multiple cancers ((−) vs (+)), tumor markers (elevated vs not elevated), and postoperative hospital stay (<20 days vs ≥20 days).
Statistical analyses
Relapse-free survival was defined as the period after the patient had undergone surgery, the disease had not recurred, and the patient remained alive. Overall survival was defined as the period between surgery and the occurrence of death. The data for patients who did not experience an event were treated as censored cases in the final observation data. The survival curves were calculated using the Kaplan–Meier method and compared using the log-rank test. Cox’s proportional hazards model was used to perform univariate and multivariate analyses. A P-value of <0.05 was defined as statistically significant. JMP software, version 16 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
Patients’ characteristics
Patients’ characteristics are shown in table patients’ characteristics are shown in table S1. Median follow up was 60 months, with the shortest observation period as 1 month and longest observation period as 245 months. Eighty-one patients were included in the early period and 105 patients were included in the late period. Median age was 69 years (minimum age, 32 years; maximum age, 89 years). One hundred twenty-nine patients were men and 57 were women. Tumor location was in the upper third of the stomach in 48 patients, the middle third in 55 patients, and the lower third in 83 patients. One hundred sixty-one patients underwent open surgery, and 25 patients underwent laparoscopic surgery. One hundred thirty patients underwent distal gastrectomy (DG), 32 patients underwent total gastrectomy (TG), 20 patients underwent proximal gastrectomy (PG), 3 patients underwent pylorus-preserving gastrectomy (PPG), and 1 patient underwent segmental gastrectomy (SG). The extent of lymph node dissection was D2 in 111 patients, D1+ in 37 patients, and D1 in 38 patients.
Prognosis of stage IB gastric cancer
The 3-year relapse-free survival rate of stage IB gastric cancer was 94.1% (T1N1: 90.3%; T2N0: 96.2%) and the 5-year relapse-free survival rate was 92.5% (T1N1: 88.3%; T2N0: 94.8%). There was no significant difference in relapse-free survival between T1N1 and T2N0 (P=0.1629) (Figureure 2A). The 3-year overall survival rate of stage IB gastric cancer was 93.0% (T1N1: 90.3%; T2N0: 94.4%) and the 5-year overall survival rate was 83.7% (T1N1: 82.3%; T2N0: 84.3%). There was no significant difference in overall survival between T1N1 and T2N0 (P=0.8318) (Figure 2B).
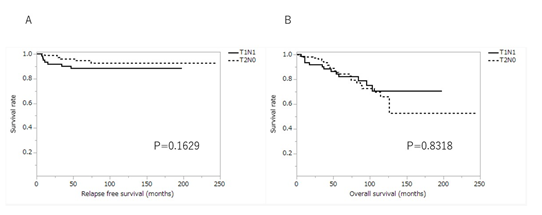
Figure 2: Kaplan-Meier curves for relapse free survival and overall survival of patients with stage IB between T1N1 and T2N0. (A) relapse free survival; (B) overall survival. T1N1, tumor confined to mucosa or submucosa with metastasis in 1-2 regional lymph nodes; T2N0, tumor invades the muscularis propria with no regional lymph node metastasis.
Recurrence
Recurrence was observed in 13 patients and the confirmed recurrence period was an average of 27.7 months (shortest period: 7 months; longest period: 74 months). The breakdown of recurrence was peritoneal recurrence in three patients, hepatic recurrence, in two, lymph node recurrence in one, remnant stomach in one, hepatic and peritoneal recurrence in one, hepatic and lymph node recurrence in one, lung and brain recurrence in one, remnant stomach, lymph node and peritoneal recurrence in one, and recurrence location unknown in two.
Relapse-free survival according to patients’ characteristics
Table 1 shows a comparison of relapse-free survival according to patients’ characteristics. A significant difference was observed in the degree of lymphatic vessel invasion and the number of lymph node metastases. Three- and 5-year survival rates of patients with no lymphatic invasion (ly0) or minimal lymphatic invasion (ly1) were 96.5% and 95.4%, while those of patients with moderate lymphatic invasion (ly2) or marked lymphatic invasion (ly3) were 89.0% and 86.5%, respectively. The survival rate of patients with ly0 or ly1 was significantly higher than that of patients with ly2 or ly3 (P=0.0158) (Figure 3). Relapse-free survival of T2N0 cases with ly2 or ly3 was significantly worse than that of cases with ly0 or ly1 (P=0.0129), but in T1N1 cases, there was no significant difference in relapse-free survival between cases with ly2 or ly3 and cases with ly0 or ly1 (P=0.4766). Three- and 5-year survival rates of patients with no regional lymph node metastasis (N0) or metastasis in one regional lymph node (N1(1)) were 95.1% and 94.2%, respectively, and those of patients with metastasis in two regional lymph nodes (N1(2)) were 86.4% and 80.2%, respectively. The survival rate of patients with N0 or N1(1) was significantly higher than that of patients with N1(2) (P=0.0295).
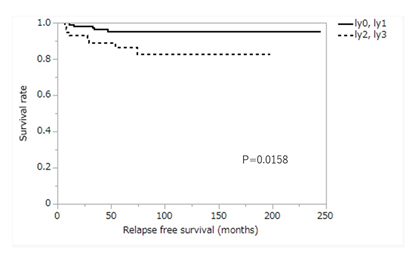
Figure 3: Kaplan-Meier curve for relapse free survival according to the degree of lymphatic vessel invasion. ly0, no lymphatic invasion; ly1, minimal lymphatic invasion; ly2, moderate lymphatic invasion; ly3, marked lymphatic invasion.
|
Characteristics |
No. of patients |
3-yr rate |
5-yr rate |
P-value |
|
Period |
0.1838 |
|||
|
Early |
81 |
96.2 |
96.2 |
|
|
Late |
105 |
92 |
88.6 |
|
|
Age |
0.2833 |
|||
|
<70 |
101 |
94.9 |
94.9 |
|
|
≥70 |
85 |
93.1 |
88.6 |
|
|
Gender |
0.9374 |
|||
|
Male |
129 |
93.3 |
93.3 |
|
|
Female |
57 |
95.9 |
91.1 |
|
|
Symptom |
0.2703 |
|||
|
Positive |
92 |
96.4 |
94.6 |
|
|
Negative |
76 |
90 |
88.4 |
|
|
Surgical method |
0.3501 |
|||
|
TG, PG |
52 |
95.5 |
95.5 |
|
|
DG, PPG, SG |
134 |
93.5 |
91.5 |
|
|
Lymph node dissection |
0.8963 |
|||
|
D2 |
111 |
93.4 |
93.4 |
|
|
D1, D1+ |
75 |
94.9 |
90.4 |
|
|
Clinical *T |
0.7424 |
|||
|
T1 |
89 |
95.1 |
93.7 |
|
|
>T1 |
97 |
93.1 |
91.2 |
|
|
Clinical N |
0.8547 |
|||
|
Negative |
142 |
93.1 |
93.1 |
|
|
Positive |
43 |
97.4 |
88.8 |
|
|
Macroscopic type |
0.5152 |
|||
|
Type 0 |
117 |
92.3 |
91.1 |
|
|
Type 1,2,3,4,5 |
69 |
96.8 |
94.4 |
|
|
Main tumor portion |
0.4198 |
|||
|
U |
48 |
95.3 |
95.3 |
|
|
M, L |
138 |
93.7 |
91.7 |
|
|
Main tumor part |
0.4103 |
|||
|
Non-circ |
175 |
93.7 |
92.1 |
|
|
circ |
11 |
100 |
100 |
|
|
Histology |
0.778 |
|||
|
Differentiated type |
95 |
96.6 |
93.3 |
|
|
Undifferentiated type |
90 |
91.5 |
91.5 |
|
|
T, N |
0.1629 |
|||
|
T1N1 |
67 |
90.3 |
88.3 |
|
|
T2N0 |
119 |
96.2 |
94.8 |
|
|
INF |
0.5711 |
|||
|
a, b |
143 |
95.4 |
94.3 |
|
|
c |
36 |
90.5 |
90.5 |
|
|
Ly |
0.0158 |
|||
|
ly0,1 |
126 |
96.5 |
95.4 |
|
|
ly2, 3 |
60 |
89 |
86.5 |
|
|
V |
0.5227 |
|||
|
v0 |
114 |
95.3 |
94.1 |
|
|
v (+) |
72 |
92 |
89.9 |
|
|
Length of tumor (mm) |
0.2049 |
|||
|
≤ 40 |
93 |
95.1 |
95.1 |
|
|
>40 |
84 |
93.6 |
90.2 |
|
|
N |
0.0295 |
|||
|
N0, N1(1) |
163 |
95.1 |
94.2 |
|
|
N1(2) |
23 |
86.4 |
80.2 |
|
|
N (old classification) |
0.5013 |
|||
|
N0, N1 |
178 |
94.4 |
92.7 |
|
|
N2 |
8 |
87.5 |
87.5 |
|
|
Dissected lymph nodes |
0.533 |
|||
|
≤ 40 |
89 |
92.6 |
92.6 |
|
|
>40 |
97 |
95.5 |
92.8 |
|
|
Chemotherapy |
0.2325 |
|||
|
– |
174 |
94.9 |
93.1 |
|
|
+ |
12 |
83.3 |
83.3 |
|
|
Preoperative comorbidity |
0.9965 |
|||
|
- |
124 |
94 |
92.9 |
|
|
+ |
64 |
94.2 |
91.2 |
|
|
Postoperative complication |
0.7764 |
|||
|
- |
146 |
94.6 |
92.7 |
|
|
+ |
40 |
91.7 |
91.7 |
|
|
Primary-lesion |
0.9069 |
|||
|
1 |
169 |
94.1 |
92.4 |
|
|
2 or 3 |
17 |
93.3 |
93.3 |
|
|
Tumor maker |
0.2671 |
|||
|
not elevated |
173 |
94.2 |
93.4 |
|
|
elevated |
13 |
92.3 |
80.8 |
|
|
Postoperative days |
0.4194 |
|||
|
< 20 days |
112 |
93.9 |
91.2 |
|
|
≥ 20 days |
71 |
94.1 |
94.1 |
|
|
Total |
186 |
94.1 |
92.5 |
TG, total gastrectomy; PG, proximal gastrectomy; DG, distal gastrectomy; PPG, pylorus-preserving gastrectomy; SG, segmental gastrectomy; T, depth of tumor invasion; T1, tumor confined to the mucosa or submucosa; N, lymph node metastasis; Type 0, superficial; Type 1, mass; Type 2, ulcerative; Type 3, infiltrative ulcerative; Type 4, diffuse infiltrative; Type 5, unclassifiable; U, Upper third; M, middle third; L, lower third; Circ, circumferential involvement; T1N1, tumor confined to mucosa or submucosa with metastasis in 1-2 regional lymph nodes; T2N0, tumor invades the muscularis propria with no regional lymph node metastasis; INFa, tumor displays expanding growth; INFb, tumor shows an intermediate pattern between INFa and INFc; INFc, tumor displays infiltrative growth; ly0, no lymphatic invasion; ly1, minimal lymphatic invasion; ly2, moderate lymphatic invasion; ly3, marked lymphatic invasion; v0, no venous invasion; v(+), venous invasion positive; N1(1), metastasis in 1 regional lymph node; N1(2), metastasis in 2 regional lymph nodes; N1(old classification), metastasis to Group 1 lymph nodes; N2 (old classification), metastasis to Group 2 lymph nodes.
Table 1: Comparison of relapse-free survival rate by patient’s characteristics
Overall survival according to patients’ characteristics
Table 2 shows a comparison of overall survival according to patients’ characteristics. There were significant differences in age, preoperative lymph node metastasis, tumor location, number of dissected lymph nodes, and preoperative comorbidity. Three- and 5-year survival rates in patients younger than 70 years were 96.9% and 91.2%, respectively, and those in patients 70 years or older were 87.6% and 72.5%, respectively. The survival rate of patients younger than 70 was significantly higher than that of patients aged 70 or older (P<0.0001) (Figure 4). Three- and 5-year survival of patients with no clinical lymph node metastasis (cN(−)) was 93.2% and 86.9%, respectively, and those of patients who were clinical lymph node metastasis-positive (cN(+)) were 92.0% and 70.4%, respectively. The survival rate of patients with cN(-) was significantly higher than that of patients with cN(+) (P=0.0273) (Figure 5). In T2N0 cases, the overall survival of clinical lymph node metastasis-positive cases was significantly worse than that of clinical lymph node metastasis-negative cases (P=0.0037), but in T1N1 cases, there was no significant difference in overall survival between clinical lymph node metastasis-positive cases and clinical lymph node metastasis-negative cases (P=0.8621). Three- and 5-year survival rates of patients with non-circumferential tumor involvement (non-Circ) were 93.3% and 84.3%, respectively, and those of patients with circumferential tumor involvement (Circ) were 85.7% and 71.4%, respectively. The survival rate of patients with non-Circ was significantly higher than that of patients with Circ (P=0.0385). Three- and 5-year survival rates of patients with ≤40 resected lymph nodes were 89.7% and 77.0%, respectively, and those of patients with >40 resected lymph nodes were 95.7% and 89.0%, respectively. The survival rate of patients with ≤40 resected lymph nodes was lower than that of patients with >40 resected lymph nodes (P=0.0082). Three- and 5-year survival rates of patients without preoperative comorbidity were 95.5% and 90.2%, respectively, and those of patients with preoperative comorbidity were 81.2% and 81.2%, respectively. The survival of patients without preoperative comorbidity was significantly higher than that of patients with preoperative comorbidity (P=0.0444).
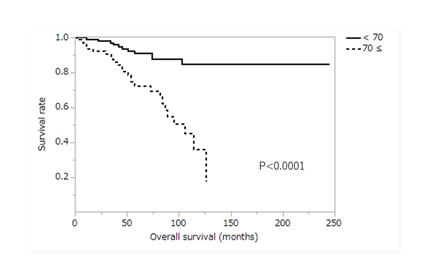
Figure 4: Kaplan-Meier curve for overall survival between patients aged 70 or older and those aged under 70 years. ≥70, patients aged 70 or older; <70, patients aged under 70 years.
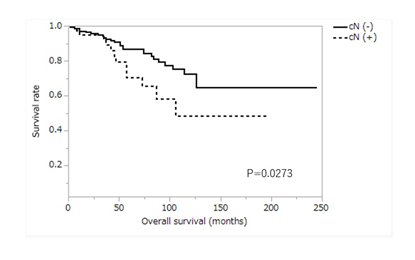
Figure 5: Kaplan-Meier curve for overall survival according to clinical lymph node metastasis. cN(-), no clinical lymph node metastasis; cN(+), clinical lymph node metastasis positive.
|
Characteristics |
No. of patients |
3-yr rate |
5-yr rate |
P-value |
|
Period |
0.4872 |
|||
|
Early |
81 |
94.9 |
86.5 |
|
|
Late |
105 |
91.3 |
80.9 |
|
|
Age |
<0.0001 |
|||
|
<70 |
101 |
96.9 |
91.2 |
|
|
≥70 |
85 |
87.6 |
72.5 |
|
|
Gender |
0.9114 |
|||
|
Male |
129 |
92.5 |
86.2 |
|
|
Female |
57 |
94.1 |
78.9 |
|
|
Symptom |
0.9042 |
|||
|
Positive |
92 |
95.1 |
85.8 |
|
|
Negative |
76 |
90.5 |
80.7 |
|
|
Surgical method |
0.9548 |
|||
|
TG, PG |
52 |
95.7 |
78.9 |
|
|
DG, PPG, SG |
134 |
92 |
85.3 |
|
|
Lymph node dissection |
0.0776 |
|||
|
D2 |
111 |
92.6 |
88.3 |
|
|
D1, D1+ |
75 |
93.4 |
75.5 |
|
|
Clinical T |
0.311 |
|||
|
T1 |
89 |
94.1 |
86 |
|
|
>T1 |
97 |
91.8 |
81.1 |
|
|
Clinical N |
0.0273 |
|||
|
Negative |
142 |
93.2 |
86.9 |
|
|
Positive |
43 |
92 |
70.4 |
|
|
Macroscopic type |
0.6429 |
|||
|
Type 0 |
117 |
91.5 |
82.4 |
|
|
Type 1,2,3,4,5 |
69 |
95.3 |
95.6 |
|
|
Main tumor portion |
0.7724 |
|||
|
U |
48 |
97.6 |
78.7 |
|
|
M, L |
138 |
91.4 |
85 |
|
|
Main tumor part |
0.0385 |
|||
|
Non-circ |
175 |
93.3 |
84.3 |
|
|
circ |
11 |
85.7 |
71.4 |
|
|
Histology |
0.9204 |
|||
|
Differentiated type |
95 |
92.8 |
83.5 |
|
|
Undifferentiated type |
90 |
93 |
83.3 |
|
|
T, N |
0.8318 |
|||
|
T1N1 |
67 |
90.3 |
82.3 |
|
|
T2N0 |
119 |
94.4 |
84.3 |
|
|
INF |
0.6593 |
|||
|
a, b |
143 |
94.5 |
85.8 |
|
|
c |
36 |
91.3 |
81 |
|
|
ly |
0.0567 |
|||
|
ly0,1 |
126 |
95.6 |
87.3 |
|
|
ly2, 3 |
60 |
87.5 |
76.1 |
|
|
v |
0.2365 |
|||
|
v0 |
114 |
93.2 |
86.3 |
|
|
v (+) |
72 |
92.7 |
79.4 |
|
|
Length of tumor (mm) |
0.9539 |
|||
|
≤ 40 |
93 |
90.5 |
82 |
|
|
>40 |
84 |
94.9 |
85.4 |
|
|
N |
0.056 |
|||
|
N0, N1(1) |
163 |
94 |
85.8 |
|
|
N1(2) |
23 |
85.9 |
68.8 |
|
|
N (old classification) |
0.7937 |
|||
|
N0, N1 |
178 |
92.7 |
83.8 |
|
|
N2 |
8 |
100 |
75 |
|
|
Dissected lymph nodes |
0.0082 |
|||
|
≤ 40 |
89 |
89.7 |
77 |
|
|
>40 |
97 |
95.7 |
89 |
|
|
Chemotherapy |
0.5766 |
|||
|
– |
174 |
93.1 |
82.9 |
|
|
+ |
12 |
91.7 |
91.7 |
|
|
Preoperative comorbidity |
0.0444 |
|||
|
- |
124 |
95.5 |
90.2 |
|
|
+ |
64 |
90.2 |
76.1 |
|
|
Postoperative complication |
0.5773 |
|||
|
- |
146 |
96.2 |
84.5 |
|
|
+ |
40 |
81.2 |
81.2 |
|
|
Primary-lesion |
0.5884 |
|||
|
1 |
169 |
93.4 |
84 |
|
|
2 or 3 |
17 |
86.2 |
79 |
|
|
Tumor maker |
0.4571 |
|||
|
not elevated |
173 |
92.4 |
84.7 |
|
|
elevated |
13 |
100 |
71.6 |
|
|
Postoperative days |
0.8076 |
|||
|
< 20 days |
112 |
94.1 |
81.7 |
|
|
≥ 20 days |
71 |
91.2 |
85.9 |
|
|
Total |
186 |
93 |
83.7 |
TG, total gastrectomy; PG, proximal gastrectomy; DG, distal gastrectomy; PPG, pylorus-preserving gastrectomy; SG, segmental gastrectomy; T, depth of tumor invasion; T1, tumor confined to the mucosa or submucosa; N, lymph node metastasis; Type 0, superficial; Type 1, mass; Type 2, ulcerative; Type 3, infiltrative ulcerative; Type 4, diffuse infiltrative; Type 5, unclassifiable; U, Upper third; M, middle third; L, lower third; Circ, circumferential involvement; T1N1, tumor confined to mucosa or submucosa with metastasis in 1-2 regional lymph nodes; T2N0, tumor invades the muscularis propria with no regional lymph node metastasis; INFa, tumor displays expanding growth; INFb, tumor shows an intermediate pattern between INFa and INFc; INFc, tumor displays infiltrative growth; ly0, no lymphatic invasion; ly1, minimal lymphatic invasion; ly2, moderate lymphatic invasion; ly3, marked lymphatic invasion; v0, no venous invasion; v(+), venous invasion positive; N1(1), metastasis in 1 regional lymph node; N1(2), metastasis in 2 regional lymph nodes; N1(old classification), metastasis to Group 1 lymph nodes; N2 (old classification), metastasis to Group 2 lymph nodes.
Table 2: Comparison of overall survival rate by patient’s characteristics
Multivariate analysis
Regarding factors involved in the prognosis of stage IB gastric cancer according to a comparison of relapse-free survival rate with patients’ characteristics, the degree of lymphatic vessel invasion and the number of lymph node metastases were significant prognostic factors for relapse-free survival. In the multivariate analysis, the degree of lymphatic vessel invasion [hazard ratio (HR): 3.224 confidence interval (CI): 1.060-10.790; P=0.0426] was an independent prognostic factor for relapse-free survival (Table 3). RegardingConsidering the factors involved in the prognosis of stage IB gastric cancer according tobased on a comparison of overall survival rate withand patient’s characteristics, age, clinical lymph node metastasis, tumor location, number of dissected lymph nodes, and preoperative comorbidity were significant prognostic factors for overall survival. In the multivariate analysis, age [HR: 3.738 CI: 1.793-7.796; P=0.0004] and clinical lymph node metastasis [HR: 2.153 CI: 1.021-4.314; P=0.0354] were independent prognostic factors for overall survival (Table 4).
|
Characteristics |
OR |
95%CI |
P value |
|
ly0, ly1 vs ly2, ly3 |
3.223667 |
[1.059585-10.7903] |
0.0426 |
|
N0, N1 (1) vs N1 (2) |
2.790634 |
[0.747596-8.710365] |
0.0917 |
ly0, no lymphatic invasion; ly1, minimal lymphatic invasion; ly2, moderate lymphatic invasion; ly3, marked lymphatic invasion; OR, odds ratio; CI, Confidence interval; N0, no regional lymph node metastasis; N1(1), metastasis in 1 regional lymph node; N1(2), metastasis in 2 regional lymph nodes.
Table 3: Multivariate analysis of relapse-free survival
|
Characteristics |
OR |
95%CI |
P value |
|
Age: <70 vs ≥70 |
3.7384573 |
[1.792824-7.7955574] |
0.0004 |
|
Clinical N: Negative vs Positive |
2.152854 |
[1.020564-4.314142] |
0.0354 |
|
Main tumor part: non-Circ vs Circ |
2.882294 |
[0.844744-7.48643] |
0.0508 |
|
Dissected lymph nodes: ≤ 40 vs >40 |
0.522156 |
[0.2561-1.069351] |
0.0805 |
|
Preoperative comorbidity: - vs + |
1.252941 |
[0.618531-2.515473] |
0.5256 |
OR, odds ratio; CI, confidence interval; N, lymph node metastasis; Circ, circumferential involvement.
Table 4: Multivariate analysis of overall survival
Discussion
With the increased awareness of cancer prevention and the improvement in detection rates for early gastric cancer by gastroscopy in recent years, the proportion of gastric cancer patients with stage I and II gastric cancer in Japan is 72.3% [8-10]. According to epidemiological statistics, the recurrence rates of patients with stage I and II gastric cancer are approximately 10% and 30%, respectively [11]. With the increase in the proportion of patients with stage I and II gastric cancer, more attention should be paid to improving the evaluation of these patients, along with appropriate treatment methods and the prognosis of patients with recurrence. The present study identified a subset of patients with stage IB gastric cancer who had unfavorable outcomes. We found that patients with stage T1N1 and T2N0 cancer had similar outcomes. Aoyama et al. [12] reported that the survival rates of patients with stage IB cancer were clearly associated with tumor location. Several authors reported the significance of tumor location in terms of the prognosis of gastric cancer. For example, Piso et al. [13] evaluated 532 patients with gastric cancer and reported that long-term survival was worse in patients with proximal disease than in those with distal tumors. The proximal stomach is a predominant site for undifferentiated-type tumors, which tend to have a poorer prognosis than differentiated-type tumors. Anatomically, the lymphatic drainage is complex, and tumors located in this region can metastasize to almost all lymph nodes except #5. Curative surgery for proximal tumors is D2 total gastrectomy with splenectomy, which is more invasive than that for distal cancer. Patients receiving total gastrectomy also have a poor prognosis due to 5%–19% weight loss and malnutrition [14,15]. Therefore, the selection of the surgical approach for gastric cancer will significantly affect the quality of life and survival rate, and thus needs to be carefully considered. However, in our study, there was no significant difference in survival among cases in the upper, middle, and lower stomach; moreover, there was no significant difference in survival regarding the surgical method. Yokoyama et al. [16] previously demonstrated that undifferentiated type adenocarcinoma was the only risk factor for the recurrence of stage IB gastric cancer. However, there are some differences between the present study and that of Yokoyama et al. First, the evaluation of staging was different. We classified stage using the third English edition of the Japanese Classification of Gastric Carcinoma [3], while Yokoyama et al. [16] used the 2nd English edition of the Japanese Classification of Gastric Carcinoma [7]. In addition, Yokoyama et al. [16] included T3N0 and T1N2 cancers, which are now classified as stage IIA. In our study, there was no significant difference in survival between patients with differentiated-type adenocarcinoma and undifferentiated-type adenocarcinoma. The degree of lymphatic vessel invasion was an independent prognostic factor for relapse-free survival in our study. Lymphatic channels play a pivotal role in the spread and recurrence of solid organ tumors. Lymphatic invasion of malignant tumors, acting as a micro-metastatic tumor focus, is a useful predictive marker of lymph node metastasis and cancer recurrence in gastric cancer [17-19]. Relapse-free survival of T2N0 cases with ly2 or ly3 was significantly worse than that of cases with ly0 or ly1, but in T1N1 cases, there was no significant difference in relapse-free survival between cases with ly2 or ly3 and those with ly0 or ly1. Choi et al. [20] reported that when comparing the survival rate on the basis of the status of lymphatic invasion for each stage, the lymphatic invasion-positive group had a significantly poor prognosis in stage II, and there was no difference between the two groups in stage III. When they compared 5-YSRs 5-year survival rates according to lymphatic invasion status in each N stage group, they concluded that the presence of lymphatic invasion may be the most important independent prognostic factor in N0 and N1 gastric cancer and might be an upstaging factor of N stage at any N stage. Therefore, in addition to the number of metastasized lymph nodes, the presence of lymphatic invasion should be included in N-stage determination. Ishigami et al. [21] performed a retrospective analysis of lymphatic invasion in 170 patients with early gastric cancer invading into the submucosa and reported that the overall survival rate of patients without lymph node metastases was not influenced by the presence of lymphatic invasion. However, patients with severe lymphatic invasion have a high risk of relapse and need careful postoperative follow-up, with consideration of adjuvant chemotherapy, just as in advanced gastric cancer. Therefore, the studies of Choi and Ishigami supported our findings that lymphatic vessel invasion was an independent prognostic factor for relapse-free survival [20,21]. Therefore, cases with advanced lymphatic invasion should be considered for postoperative chemotherapy, including stage IB disease. Age and clinical lymph node metastasis were independent prognostic factors of overall survival. In our study, the 5-year survival rate of patients younger than 70 years was 91.2%; however, that of patients aged 70 or older was only 72.5%. Ahn et al. [6] analyzed stage-specific survival using the third English edition of the Japanese Classification of Gastric Carcinoma [3] and reported that 5-year survival was 88.9% for stage IB (90.2% in T1N1 and 87.6% in T2N0, respectively) and 83.1% for stage IIA (84.0% in T1N2 and 82.1% in T3N0, respectively). Thus, survival was worse in the latter cases than in the former. Our reported 5-year survival rate is inferior to that reported by Ahn et al.[6], this is because the average age was 55.6 years in their study compared with 67.6 years in our study. The fact that our patients were more than 10 years older seemed to be a major cause. We previously reported that the 5-year overall survival rates of patients with stage II gastric cancer in the <65 years, 65-74 years, and >74 years groups were 80.4%, 74.5%, and 56.7%, respectively [22]. The overall survival of patients with stage II gastric cancer was significantly lower in the >74 years group than in the <65 years or 65-74 years group [22]. Nonetheless, there was no significant difference in relapse-free survival according to age distribution in patients with stage IB gastric cancer; however, the number of comorbidity-related deaths was significantly higher in patients aged 70 or older than in those aged under 70 years. In the current study, the number of preoperative comorbidity cases was 38 cases (44.7%) in patients under 70 years or older, and 24 cases (23.8%) in patients under 70 years. The number of patients with preoperative comorbidity in the 70 years or older group was significantly higher than that in the under 70 years group. However, there was no significant difference in the number of postoperative complications between patients aged 70 or older and those aged under 70 years. This result suggested that patient age alone is not a valid reason to avoid gastrectomy; rather, comprehensive assessment is necessary to determine the optimum treatment strategy for older patients with gastric cancer. Lymph node metastasis (N stage) is one of the factors that most affects the survival of patients with gastric cancer [23-27]. The 7th edition TNM classification of N stages of gastric cancer states that ‘‘histological examination of a regional lymphadenectomy specimen will ordinarily include 16 or more lymph nodes’’. In this classification, it is only a recommendation and no longer a prerequisite that no fewer than 16 dissected lymph nodes are needed for adequate N stage classification. In 2012, Xu et al. [28] demonstrated that it is necessary to examine at least 16 lymph nodes for accurate pathological examination of gastric cancer, even in node-negative gastric cancer patients. Therefore, we excluded patients who underwent lymph node dissection of fewer than 16 lymph nodes from our study. In our study, clinical lymph node metastasis was not a prognostic factor in relapse-free survival, but it was an independent prognostic factor in overall survival. In T2N0 cases, the overall survival of clinical lymph node metastasis-positive cases was significantly worse than that of clinical lymph node metastasis-negative cases, but in T1N1 cases, there was no significant difference in overall survival between clinical lymph node metastasis-positive cases and clinical lymph node metastasis-negative cases. It seems that careful postoperative observation is necessary for clinical lymph node metastasis-positive T2N0 cases. There are some limitations to the present study. First, this was a retrospective single-institution study with a small sample size. Moreover, age or lymphatic invasion, which were marginally significant in the present study, might become more significant by increasing the number of patients or by extending the follow-up period. Second, there is bias regarding the time period in this study. The data were collected between 1994 and 2018, and surgical procedures and perioperative care have changed over this time. These factors might have affected our results.
Conclusion
We examined factors involved in relapse-free and overall survival in stage IB gastric cancer. For relapse-free survival, the degree of lymphatic vessel invasion was an independent prognostic factor, and for overall survival, age and clinical lymph node metastasis were independent prognostic factors. In particular, in T2N0 cases, despite the absence of lymph node metastasis, lymphatic vessel invasion was a strong prognostic factor in relapse-free survival. Therefore, in addition to the number of metastasized lymph nodes, the presence of severe lymphatic invasion should be included in N-stage determination, and adjuvant chemotherapy should also be considered in cases with severe lymphatic invasion.
Acknowledgments
We thank H. Nikki March, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
No funding was received.
Conflict of interest
The authors have no conflicts of interest to report.
References
- Smyth EC, Nilsson M, Grabsch HI, et al. Gastric cancer. Lancet 396 (2020): 635-648.
- Saka M, Katai H, Fukagawa T, et al. Recurrence in early gastric cancer with lymph node metastasis. Gastric Cancer 11 (2008): 214-218.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14 (2011): 101-112.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 13 (2011): 113-123.
- Katai H, Ishikawa T, Akazawa K, et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: A retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001-2007). Gastric Cancer 21 (2018): 144-154.
- Ahn HS, Lee HJ, Hahn S, et al. Evaluation of the seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer 116 (2010): 5592-5598.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 2nd English edition. Gastric Cancer 1 (1998): 10-24.
- Kim JW, Hwang I, Kim MJ, et al. Clinicopathological characteristics and predictive markers of early gastric cancer with recurrence. J Korean Med Sci 24 (2009): 1158-1164.
- Jeong O, Park YK. Clinicopathological features and surgical treatment of gastric cancer in South Korea: The results of 2009 nationwide survey on surgically treated gastric cancer patients. J Gastric Cancer 11 (2011): 69-77.
- Park JH, Ryu MH, Kim HJ, et al. Risk factors for selection of patients at high risk of recurrence or death after complete surgical resection in stage I gastric cancer. Gastric Cancer 19 (2016): 226-233.
- Kao YC, Fang WL, Wang RF, et al. Clinicopathological differences in signet ring cell adenocarcinoma between early and advanced gastric cancer. Gastric Cancer 22 (2019): 255-263.
- Aoyama T, Yoshikawa T, Fujikawa H, et al. Prognostic factors in stage IB gastric cancer. World J Gastroenterol 20 (2014): 6580-6585.
- Piso P, Werner U, Lang H, et al. Proximal versus distal gastric carcinoma--what are the differences? Ann Surg Oncol 7 (2000): 520-525.
- Tokunaga M, Hiki N, Fukunaga T, et al. Better 5-year survival rate following curative gastrectomy in overweight patients. Ann Surg Oncol 16 (2009): 3245-3251.
- Feng F, Zheng G, Guo X, et al. Impact of body mass index on surgical outcomes of gastric cancer. BMC Cancer 18 (2018): 151.
- Yokoyama T, Kamada K, Tsurui Y, et al. Clinicopathological analysis for recurrence of stage Ib gastric cancer (according to the second English edition of the Japanese classification of gastric carcinoma). Gastric Cancer 14 (2011): 372-377.
- Del Casar JM, Corte MD, Álvarez A, et al. Lymphatic and/or blood vessel invasion in gastric cancer: Relationship with clinicopathological parameters, biological factors and prognostic significance. J Cancer Res Clin Oncol 134 (2007): 153-161.
- Yoo CH, Noh SH, Shin DW, et al. Recurrence following curative resection for gastric carcinoma. Br J Surg 87 (2000): 236-242.
- Kim JH, Park SS, Park SH, et al. Clinical significance of immunohistochemically-identified lymphatic and/or blood vessel tumor invasion in gastric cancer. J Surg Res 162 (2010): 177-183.
- Choi WH, Kim MJ, Park JH, et al. Lymphatic invasion might be considered as an upstaging factor in N0 and N1 gastric cancer. J Clin Med 9 (2020): 1275.
- Ishigami S, Natsugoe S, Hokita S, et al. Carcinomatous lymphatic invasion in early gastric cancer invading into the submucosa. Ann Surg Oncol 6 (1999): 286-289.
- Aoyagi K, Murakami N, Isobe T, et al. Prognosis of older patients with stage II and III gastric cancer. Open J Surg 4 (2020): 8-14.
- Randle RW, Swords DS, Levine EA, et al. Optimal extent of lymphadenectomy for gastric adenocarcinoma: A 7-institution study of the U.S. gastric cancer collaborative. J Surg Oncol 113 (2016): 750-755.
- Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: Data from a large US-population database. J Clin Oncol 23 (2005): 7114-7124.
- Yokota T, Ishiyama S, Saito T, et al. Lymph node metastasis as a significant prognostic factor in gastric cancer: A multiple logistic regression analysis. Scand J Gastroenterol 39 (2004): 380-384.
- Spolverato G, Ejaz A, Kim Y, et al. Prognostic performance of different lymph node staging systems after curative intent resection for gastric adenocarcinoma. Ann Surg 262 (2015): 991-998.
- Gholami S, Janson L, Worhunsky DJ, et al. Number of lymph nodes removed and survival after gastric cancer resection: An analysis from the US gastric cancer collaborative. J Am Coll Surg 221 (2015): 291-299.
- Xu D, Huang Y, Geng Q, et al. Effect of lymph node number on survival of patients with lymph node-negative gastric cancer according to the 7thedition UICC TNM system. Plos One 7 (2012): e38681.


 Impact Factor: * 4.2
Impact Factor: * 4.2 Acceptance Rate: 72.62%
Acceptance Rate: 72.62%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 