Application of BloodSTOP iX Wound Heal Nanocellulose Matrix for Burn Wound Care
Shiying Ju1, Kun Wang1, Liang Qiao1, Yingde Xu2, Amanda Reed-Maldonado3, Lia Banie3, Dongyi Peng3, Tianshu Liu3, Guifang Wang3, Zhongcheng Xin2, Guiting Lin3*
1Weifang People’s Hospital, Weifang Medical University, Weifang, ShanDong, China.
2Department of Urology, Peking University First Hospital and the Institute of Urology, Peking University, Beijing, P.R. China
3Knuppe Molecular Urology Laboratory, Department of Urology, School of Medicine, University of California, San Francisco, California, USA
*Corresponding Author: Guiting Lin, MD, PhD, Knuppe Molecular Urology Laboratory, Department of Urology, School of Medicine, University of California, 533 Parnassus Ave., San Francisco, CA 94143-0738, USA
Received: 14 December 2020; Accepted: 04 January 2021; Published: 12 January 2021
Article Information
Citation: Shiying Ju, Kun Wang, Liang Qiao, Yingde Xu, Amanda Reed-Maldonado, Lia Banie, Dongyi Peng, Tianshu Liu, Guifang Wang, Zhongcheng Xin, Guiting Lin. Application of BloodSTOP iX Wound Heal Nanocellulose Matrix for Burn Wound Care. Journal of Surgery and Research 4 (2021): 14-31.
View / Download Pdf Share at FacebookAbstract
Objectives: Better dressings are needed for both receiver and donor sites in clinical management of burn wounds. Current study aims to check the efficacy and safety of BloodSTOP iX Wound Heal Nanocellulose Matrix (BSM) in managing burn wound.
Materials and methods: Characterization of BloodSTOP iX Nanocellulose Matrix was assayed with scanning electron microscope (SEM) and Fourier Transform Infrared Spectroscopy (FT-IR). For the preclinical study, two burn injury animal models were established: a contusion model and partial-thickness burn model. A total of 12 rabbits (male/female; 2.0-2.5 kg) were used in this initial experiment. Assessments were made at two time points: 1 week and 2 weeks post-injury. Six rabbits underwent skin contusion and six partial-thickness skin burn. At 1 week and two weeks post-injury, gross pathologic and histologic observations of changes and scarring were conducted. For clinical trial, during September 2015 to September 2016, a total 46 patients from two medical centers were enrolled in this study, including control group 16 cases and BSM 30 case. Each group has been sub-grouped into skin donor site and transplanted site. At 7, 14 and 20 days post treatment, clinical outcome were assayed.
Results: The microstructure of Nanocellulose and nano-structure of BloodSTOP iX Wound Heal Nanocellulose Matrix was confirmed. Preclinical results demonstrated that BSM promoted the burn wound healing in both animal models. In the clinical experiment, BSM decreased recurrence of bleeding post application and burning/pain feeling, while an anti-infection effect was also noted. More strikingly, BSM promoted skin epithelialization and improved scar tissue. Those effects were observed in both donor and receiver sites.
Conclusion: BloodSTOP iX Wound Heal Nanocellulose Matrix (BSM) is safe and effective in burn wound c
Keywords
<p>BloodSTOP iX; Wound Heal; Nanocellulose; Burn care; Water Soluble Etherified Sodium Carboxymethyl Cellulose Matrix</p>
Article Details
1. Introduction
Currently, both dry and wet burn injuries are still very prevalent and burdensome clinical care issues that affect patient life quality [1]. Burn injuries are still among the most common household and civilian injuries, especially in children, which are characterized by severe skin damage and tissue cell death [2,3]. Though many people can recover from burns without serious health consequences, the most serious burns require immediate and proper emergency medical care to prevent complications such as hypovolemic shock, sepsis, and even death. Also, following the acute treatment, multiple surgical procedures are generally needed [4]. During these procedures, the wound area treatment is a big challenge for both patients and physicians [5]. The initial priority is to stabilize the patient, prevent infection, and optimize functional recovery. Following that, wound care is a critical task for successful burn treatment. In general, the standard is early excision and grafting, and recent research has demonstrated that excision within 24 or 48 hours can lower blood loss, decrease infection rate, shorten hospital stays, and decrease mortality [6,7]. Therefore, excision of the eschar at an early stage followed by covering of the wound as early as possible are both very important to successful burn care. To cover the burn wound, a split-thickness skin graft from an uninjured donor site on the same patient (autograft) is the gold standard, which provides sufficient coverage without immunological rejection [8,9].
However, better dressings are needed for both receiver and donor sites. The split-thickness skin grafts are in general meshed with variable expansion ratios in order to cover more of the injured area, but the movement of graft post-seeding is very bothersome issue for clinicians and patients. Meanwhile, the pain and bleeding on the donor sites is another wound-healing burden for the patient [10].
Nanocellulose matrix, a unique and promising natural material extracted from native cellulose, has gained much attention for its use as biomedical material, because of its remarkable physical properties, special surface chemistry and excellent biological properties with biocompatibility, biodegradability and low toxicity [11]. With the emergence and development of nanotechnology, cellulose, the most ancient and important natural polymer on earth revives and attracts more attention in the new form of ‘‘nanocellulose’’ to be used as novel and advanced material [12,13]. Nanocellulose matrix is described as the products or extracts from native cellulose (found in plants, animals, and bacteria) composed of the nanoscaled structure material. Generally, the family of nanocellulose can be divided in three types, (i) cellulose nanocrystals (CNC), with other designations such as nanocrystalline cellulose, cellulose (nano) whiskers, rod-like cellulose microcrystals; (ii) cellulose nanofibrils (CNF), with the synonyms of nanofibrillated cellulose (NFC), microfibrillated cellulose (MFC), cellulose nanofibers; and (iii) bacterial cellulose (BC), also referred to as microbial cellulose. It was reported that electrospun maleic anhydride-grafted poly (lactic acid) nanofibers reinforced with CNC can be the supporting scaffolds to culture the human adult adipose derived stem cells (hADSCs) and promote cell proliferation [14]. Research has demonstrated that the nanofibrous scaffolds play a pivotal role in tissue healing and regeneration processes by providing structurally 3-D environment to mimic the architecture of natural human extracellular matrix (ECM), and this promotes stem cells proliferation [11,15].
With advanced high technologies, water soluble etherified sodium carboxymethyl cellulose matrix (CMC) was used to generate spinning nanofibers and formed a well-organized membrane, and named as BloodSTOP iX Wound Heal Nanocellulose Matrix (BSM) for general wound management. Nanocellulose matrix has diameter of less than 100 nm, several thousand times smaller than that of a human hair. In wound management, nanocellulose matrix has such a large surface area providing biological interface with cells and tissue [16,17]. This nanocellulose matrix is a promising biomaterial for applications in tissue healing and engineering of skin, cartilage, bone, and others [18].
To cover the donor site in burn injury care, different dressings have been used. But, there is no standardized dressing in the clinic currently. BloodSTOP iX Wound Heal Nanocellulose Matrix (BSM, LifeScience PLUS, Mountain View, CA, USA) is a biocompatible, water soluble woven fiber matrix made from etherified regenerated viscose nanocellulose [16,17]. In our preliminary study, when BSM contacts a burn wound with liquid, BSM adheres to the wound and transforms to gel, stops bleeding, and forms a protective layer, thereby creating an optimal wound healing environment. Its composition provides certain advantages for natural dressing as well as improved wound management and patient comfort as compared to traditional Vaseline gauze.
In the current study, a pre-clinical animal experiment and pilot clinical trial were conducted to check the efficacy and safety of BSM as compared to standard Vaseline gauze, which has been used extensively worldwide in burn wound management for a long time.
Materials and Methods
1. Characterization of BloodSTOP iX Wound Heal Nanocellulose Matrix (BSM)
The morphology of the samples was studied using a Retiga Q Image digital still camera and ACT-1 software (Nikon Instruments Inc., Melville, NY, USA). For image analysis, the nanocellulose fibers were viewed under fluorescence light at 488 nm; four fields per sample were randomly selected, photographed, and recorded. Meanwhile, scanning electron microscope (SEM) was used to identify the microstructure of Nanocellulose, and Fourier Transform Infrared Spectroscopy (FT-IR) was also used for identification of materials in EAG Laboratories. In FT-IR, the infrared absorption bands are assigned to characteristic functional groups. Based on the presence of a number of such bands, materials under consideration were identified. Availability of spectra of known compounds increases the probability of making a positive identification.
2. Animal experiment
All experimental protocols were approved by the Institutional Animal Care and Use Committee at our institution. Two burn related injury animal models were used in the pre-clinical experiment: a contusion model and partial-thickness burn model. A total of 12 rabbits (male/female; 2.0-2.5 kg) were used in this initial experiment. Assessments were made at two time points: 1 week and 2 weeks post-injury. Six rabbits underwent skin contusion and six partial-thickness skin burn.
For the skin contusion model, each rabbit was shaved on the bilateral back. A skin contusion (20x80 mm, 0.2mm thickness) was made using a file brush. Immediately after modeling, the injury site was covered with saline-soaked gauze; the injury sites were randomly divided into groups A and B; the injuries in group A were dressed with 2 layers of the control dressing (Vaseline gauze); the injuries in group B were dressed with 2 layers of BSM. All wounds were covered with gauze, which was sutured to the skin. The injury sites were observed and photographed every day. The dressings were changed every day starting on day 2. At 1 week and two weeks post-injury, gross pathologic and histologic observations of changes and scarring were conducted.
For the partial-thickness burn model, each rabbit was shaved on the bilateral back. A burn injury (20x80 mm, 0.2mm thickness) was made using a 100°C water bag for 8 s. Immediately after modeling, the injury site covered with saline-soaked gauze; the injury sites were randomly divided into groups A and part B; the injuries in group A were dressed with 2 layers of the control dressing (Vaseline gauze); the injuries in group B were dressed with 2 layers of BSM. All wounds were covered with gauze, which was sutured to the skin. The injury sites were observed and photographed every day. The dressings were changed every day starting on day 2. At 1 week and two weeks post-injury, gross pathologic and histologic observations of changes and scarring were conducted.
3. Clinical trial: Safety and effectiveness of BSM for burn wound care in clinic
Following internal departmental and hospital ethics approval, patients were prospectively enrolled from September 2015 to September 2016. All patients received and signed the informed consent forms. A total 46 patients from two medical centers were enrolled in this study, including control group 16 cases and BSM 30 case. Each group has been sub-grouped into skin donor site and transplanted site. The clinical trial was conducted following the protocol below.
As per this protocol BSM is to be used in non-infected burn wounds post the acute stage, especially for the skin graft transplantation utilized after tangential excision of burns to decrease blood loss at the donor site (DS), aid in providing a moist wound environment, and enhancing tissue healing at the transplanted site (TS). After cleaning of burn wounds or after tangential debridement of burn wounds the product is to be applied on both DS and TS. The patients are to be on appropriate antibiotic coverage based on their condition. BSM does not contain antimicrobial treatment. Wounds covered with BSM will continue to produce exudate and will be moist. Careful monitoring of the site(s) is to be done. As per institutional guidelines dressing sites are to be kept clean or sterile and monitored. Dressing changes are to be performed by the appropriate certified practitioner. In this stage-I initial informal clinical trial, a total 22 patients were recruited, including 16 patients to be treated with BSM on both DS and TS site, 6 patients to be treated without BSM as control. Treatment procedure Clinical Therapeutic Protocol is to be followed.
Debridement of wounds, tangential excisions and skin grafting is to be done at the discretion of the practitioner with sedation or anesthesia as per policy. Once clean wounds are obtained, BSM is to be applied.
3.1 Debridement of burn wounds non-tangential excisions.
After wounds are debrided as best as possible, 2 layers of BSM are to be placed over open wounds that are not in pressure bearing or dependent areas. After this, placement of nonstick dressing, for example: Telfa, Adaptic, or a reasonable facsimile, is to be done. Then wrapping with gauze bandage roll is recommended with the possible placement of additional overlying dressings as per the practitioner’s discretion. In areas of pressure where the patient is in a supine position, for example, the posterior aspect of the head, the elbows, or similar areas, a different technique is to be used. In these areas 4-6 layers of BSM are to be used followed by the appropriate nonstick dressing and wrapped. It is recommended that dressings be changed daily or every other day based on the exudate production and the judgment of the practitioner.
3.2 Intraoperative burn debridement, tangential excisions, and skin grafting
During intraoperative treatment of burns where bleeding is a concern, BSM is to be placed. After creation of the appropriate wound bed by debridement BSM may be placed initially as a double layer to help decrease bleeding. After the skin graft is obtained, BSM is to be placed initially as a double layer to help decrease bleeding on the donor site (DS). With the placement of this initial double layer pressure should be maintained. After 2-5 minutes of pressure and attaining adequate hemostasis additional layers of BSM should be placed. For skin grafting: after skin graft stamp is transplanted to area after tangential excisions, BSM is to be placed above skin grafts. Multiple layers are appropriate secondary to the exudative property of the wound site, amount of bleeding, wound location, and condition of the patient. Current guideline for non-dependent (non-weight bearing) sites is the application of 1-2 layers in addition to the initial double layer placed for hemostasis. Additional layers are recommended in wounds with significant blood loss or significant exudate or dependent positions. Once all layers of BSM have been placed, there should be placement of nonstick dressing, for example: Telfa, Adaptic, or a reasonable facsimile, followed by placement of gauze bandage roll. After placement of gauze bandage role practitioners may decide on additional reinforcing dressings. A modification in technique which is appropriate for more extensive areas with bleeding, BSM may be placed in 2-4 layers on top of a nonstick dressing on top of gauze and applying this “sandwich” to the area being treated. This will allow for pressure to be applied to the area of concern without disruption of the wound site and ability to wrap the site without disturbing the BSM. Therefore there will be no disruption of the hemostasis attained with the dressing.
3.3 Post BSM placement care
During the post-debridement, post BSM placement period, wound should be monitored according to practitioner standard of care. During this time if significant bleeding is still noted intervention as per the practitioner is appropriate.
During the post-placement period if significant exudate is noted, reinforcement of the dressings as per the practitioner is appropriate. As per practitioner protocol and institutional protocol, patient should have vital signs monitored as their condition warrants. Practitioners should be notified of any change in the dressings as per usual policy and procedure.
3.4 Reapplication of BSM
In patients with burns and severe wounds there may be need to reapply BSM. This may be done several ways depending on the conditions and location of the dressing change. As conditions allow, BSM is to be changed under sterile or clean conditions as per the practitioner. The preferred technique is removal of outer dressings to the level of BSM. Depending on the bleeding of the wound or the exudative level there will be some BSM present on the wound. If additional tangential debridement is required then proceed as above. If wounds appear clean, facilitate removal of BSM with Saline or Sterile Water. The BSM will have become gel. Wipe away gel. It is not necessary to wipe all gel away as long as wounds are noted to be clean. With clean and stable wounds reapply the BSM as outlined above taking care to place additional layers on pressure bearing areas, such as posterior scalp, elbows, heels, etc. The more frequent the dressing changes, the fewer layers of BSM are required (in non-weight bearing areas). For example a forearm burn/wound may require one layer of BSM with nonstick dressing and wrap, as long as it is monitored daily.
Results
Part I: Characterization of BSM Nanocellulose
The materials characterization of BSM Nanosellulose were firstly assayed with fluorescence microscope, the materials are nanocellulose fibers with autofluorescence signaling (Figure 1). The scanning electron microscope (SEM) was used to identify the microstructure of Nanocellulose and confirmed nano-structure of BSM Nanocellulose (Figure 2). The Nanofibrillated cellulose/water soluble etherified carboxymethyl nanocellulose matrix were analyzed in EAG Laboratories, the results confirmed the purity of etherified carboxymethyl nanocellulose (Figure 3).
Part II: Animal experiment
1. Skin contusion animal model
In the control group, scabbing occurred done day after contusion; redness and swelling lasted at least five days. In the BSM group, no significant scarring and swelling were observed; three days later, the appearance returned to normal (Figure 4).
After 6 days, HE staining found that most of the injury site in the control group has returned to normal. However, some areas still showed excoriation. In the BSM group at 6 days, we found that the pathological changes were completely back to normal. After 12 days, abnormalities were not found either in the control group or the BSM group (Figure 5).
2. Partial-thickness skin burn animal model
In the control group, the most common symptom was redness and swelling at 1 day after injury; we observed small blisters and scarring under the naked eyes by the next day; significant skin damage lasted at least ten days; twelve days later, the appearance returned to normal. BSM group, significant scarring and swelling were also observed; however, the appearance returned to normal by the eighth day (Figure 6).
HE staining showed that typical blisters formed by the sixth day and disappeared at the twelfth day in the control group. In the BSM group, we also found that blisters formed at day 6. However, the skin damage in the BSM group was less than that of the control group in the corresponding time point. After 12 days, blisters were also not found in the BSM group (Figure 7).
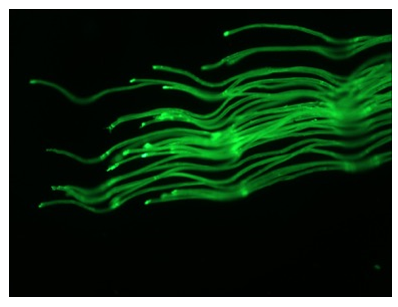
Figure 1: Characters of Nanocellulose under fluorescent microscope (NFC/CMC:Nanofibrillated cellulose/water soluble etherified carboxymethyl nanocellulose)
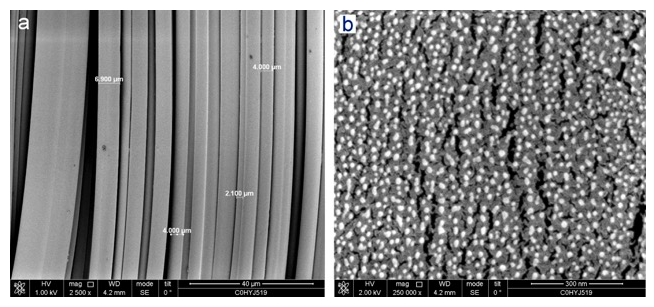
Figure 2: SEM structure of \BloodSTOP iX Wound Heal Nanocellulose Matrix
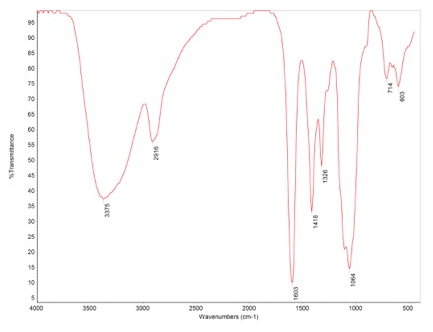
Figure 3: Nanofibrillated cellulose/water soluble etherified carboxymethyl nanocellulose analyzed with Fourier Transform Infrared Spectroscopy (FT-IR)
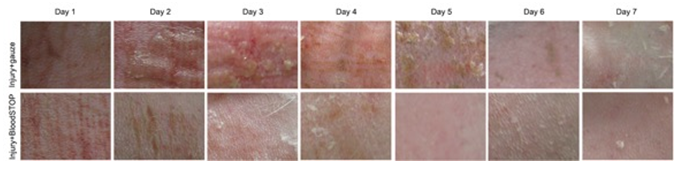
Figure 4: Gross performance of skin contusion model. The BSM (bottom panel) retained more moisture than the control group (top panel) and promoted skin regeneration.
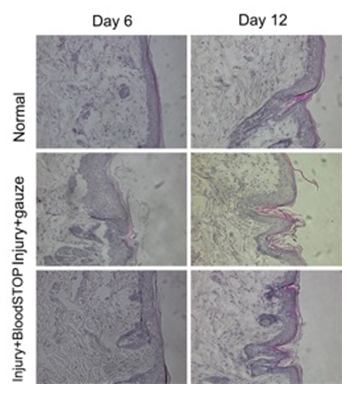
Figure 5: HE staining of skin contusion model. There is no obvious scar were formed in both control and BSM group, while the thickness of skin in BSM group (bottom panel) is better than control group (middle panel).
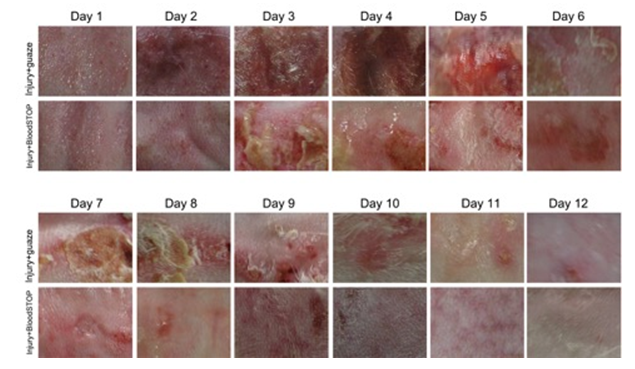
Figure 6: Gross performance of partial-thickness skin burn model: BSM promoted burn healing and skin regeneration.
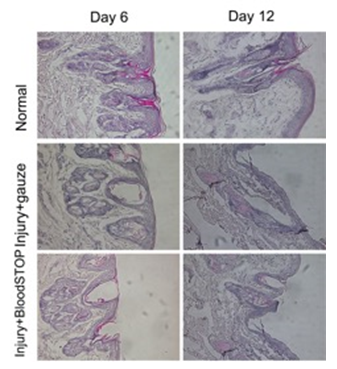
Figure 7: HE staining of partial-thickness skin burn model
Part III: Clinical trial
1. Comparable between control and BSM
A total 46 patients from two medical centers were enrolled in this study, including control group (16 cases) and BSM (30 cases). Each group was sub-grouped into skin donor site and transplanted site. In the control group, there were 4 skin donor sites and 12 transplanted sites, while there were 14 skin donor sites and 16 transplanted sites in BSM group. The general biological parameters are listed in Table 1. No significant difference was noted between the control (ctrl) and BSM. The clinical data is comparable.
Meanwhile, the patients’ general conditions, including diseases, were also similar and comparable.
Table 1: Comparison of general biological parameter
|
ctrl |
BSM |
P |
|
|
Age (yrs) |
40.25+13.05 |
41.41+10.35 |
0.7782 |
|
Body weight (lbs) |
147.52+28.6 |
157.06+13.41 |
0.2247 |
|
Height (cm) |
1669.06+8.028 |
177.53+4.5 |
0.2802 |
2. Clinical outcome
The clinical outcomes were presented as proportion in percentage and analyzed with Fisher's exact test to find the significance.
2.1 BSM decreased recurrence of bleeding post application
In the clinic data, BSM demonstrated better hemostatic effect compared to control device. There were 31.25% of patients with rebleeding in control group, while there were no patients with rebleeding in BSB group; the difference is statistically significant (P<0.001), which implies BSB has a better and longer hemostatic effect.
2.2 No allergy occurred in BSM group
There were 43.75% of patients who experienced allergic response in control group, while there was no allergic response in the BSM group. The difference is statistically significant (P<0.001).
2.3 Less irritation in BSM group
There were 18.75% of patients experiencing irritation in control group, while no irritation occurred in BSM group. However, the difference is not statistically significant (P<0.001).
2.4 BSM significantly decreased burning feeling
In the clinical observation, the burning feeling is very important for the patients. Burning feeling was experienced by 12.5% of patients in the control group, and by none in the BSM group. The difference is statistically significant (P<0.001).
2.5 BSM significantly decreased pain feeling
It has been reported previously that BSM has an anti-pain effect. In current data, there were 25% of patients experiencing pain post the application in the control group, while none felt pain in the BSM group. The difference is statistically significant (P<0.001).
2.6 Anti-infection of BSM
Very interesting, in current data, BSM demonstrated an anti-infection effect. There are 31.25% of patients for which infection occurred in the control group, while there were no patients for which infection occurred in BSM group. However, the difference is statistically significant (P<0.001). Above result is presented in Table 2.
Table 2: Clinical outcome assayed with proportion.
|
ctrl |
BSM |
P |
|
|
Recurrence of Bleeding |
31.25 |
0 |
<0.001 |
|
Allergy |
43.75 |
0 |
<0.002 |
|
Irritation |
18.75 |
0 |
<0.003 |
|
Burning |
12.5 |
0 |
<0.004 |
|
Pain |
25 |
0 |
<0.005 |
|
Infection |
31.25 |
0 |
<0.006 |
- BSM promote skin epithelialization and improve tissue scar
There were 29.4% patients in the BSM group for which occurred minor skin epithelialization. Scar tissue improvement occurred in 76.4% patients, including 23.5% reduction of scar and 52.9% significant inhibition of scar, while those effects is not noted in the control group.
3. Application of BSM on skin graft transplanted site in the clinic
Post the extensive tangential excisions, the stamp-like skin grafts were transplanted on the fresh surface of wound area where minor bleeding continued. The BSM was directly applied over the skin grafts and wound area with continue pressure for 3 minutes until BSM transformed a gel, and then Telfa and regular gauze were applied to form a “sandwich” dressing (Figure 8). The transplanted grafts were stably localized on the transplanted site. The transplanted grafts in the control group were covered with regular gaze.
In patients treated with BSM, the BSM became a gel, overlapping on the transplanted skin grafts and anchoring them in situ. Importantly, BSM promoted the skin cell proliferation and regeneration; at 8-14 days post the transplantation, the entire wound area was covered by new skin (Figure 9), while in the control group, still 40% of the wound area was still granulation tissue (Figure 10). Strikingly, 20 days post skin graft transplantation, there was still 10% area of granulation tissue in the control group, while a matured skin was formed in BSM treated group (Figure 11).
4. Application of BSM on Donor site during skin grafting
During skin grafting, after skin was taken from the donor site, fresh wounds were formed and bleeding occurred immediately. The BSM was applied onto the wound surface to stop the bleeding (Figure 12) followed by Telfa and regular gauze to form a “sandwich” dressing. The bleeding was stopped in 30 seconds post the application of BSM. The step-by-step procedures were photographed (Figure 13). In the control site (left panel), the wound surface was covered with Vaseline gauze (Right) while treatment group covered with BSM.
The hemostatic effect on the skin graft donor site was very significant, achieved in 30 seconds post application onto fresh wound surface; the effect lasted for 7 days (Figure 14). Fourteen days later, the wound healing in BSM groups is much better than those in control (Figure 15).
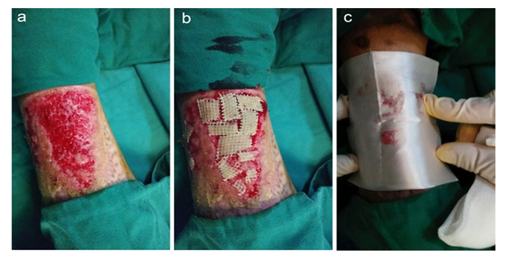
Figure 8: Application of BSM on transplanted site during skin grafting (a). Wound area post tangential excisions; (b). Skin grafting on wound area, (c). Application of BSM over the skin grafting wound area.
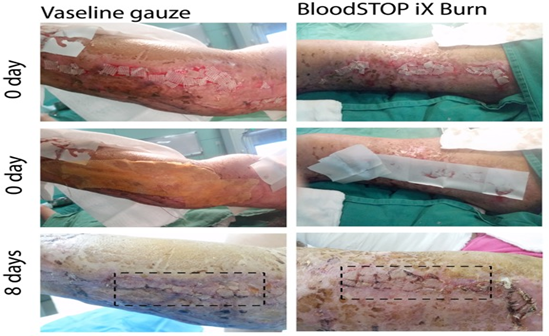
Figure 9: BSM promoted grafted skin regeneration 8 days post transplantation compare with Vaseline gauze control.

Figure 10: BSM promoted grafted skin regeneration 14 days post transplantation (a). Control; (b). BSB treated.
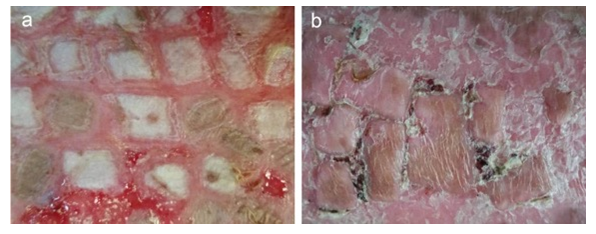
Figure 11: BSM promoted grafted skin regeneration 20 days post transplantation. (a). control; (b). BSB treated.
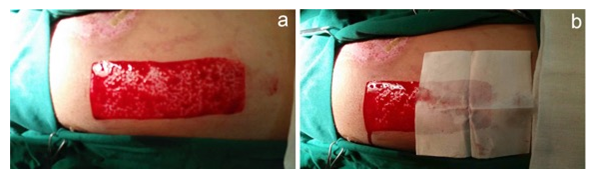
Figure 12: Application of BSM on donor site during skin grafting. (a). Wound area after taking skin graft; (b). BSB on wound area stopped bleeding in 30 seconds
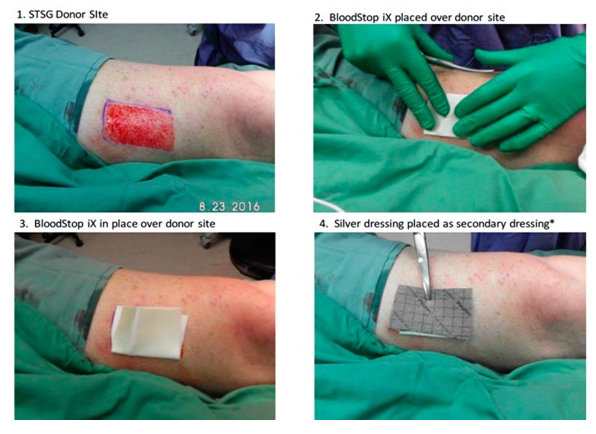
Figure 13: Application of BSM on donor site during skin grafting (Step-by-step).
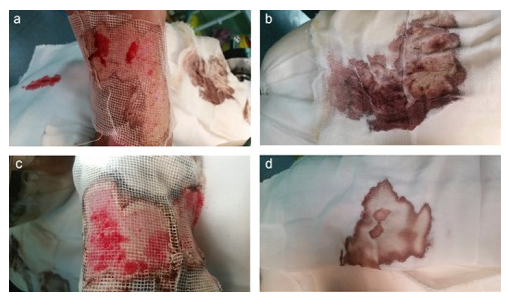
Figure 14: BSM decreased wound surface bleeding on donor site 7 days post surgery (a)&(b) control; (c)&(d) BSM treated group
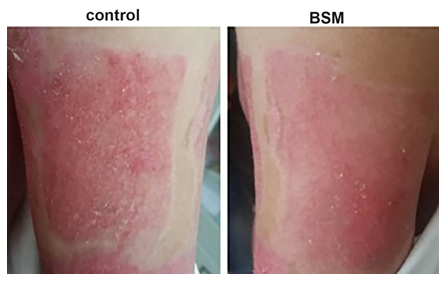
Figure 15: Wound healing in donor site 14 days post-surgery. In the control site (left panel), the wound surface was covered with Vaseline gauze, while the BSM treated site (Right) was covered with BSM. There are more micro-granules in the control site; the wound surface treated with BSM is smoother (Right panel).
Discussion
Burn injury wound care is a critical clinical issue, which is related to bleeding, infection, scarring and delay of healing [19]. To cover the donor site and graft transplanted site, many different dressings have been used [10]. Clinically, several factors affect appropriate dressing selection, such as depth of burn, condition of the wound bed, wound location, desired moisture retention and drainage, required frequency of dressing changes, and cost. The main purpose of dressing is to provide protection from bacterial and other contaminations, good gas exchange, moisture retention, and enhancement of functional recovery [16,20].
In general, alternation of mafenide acetate cream in the morning and silver sulfadiazine cream in the evening with gauze dressings used over the creams is the traditional approach for burn wound care [8]. The major classes of dressings include: alginate, for example Comfeel (Coloplast, Minneapolis, MN, USA), Aquacel (ConvaTec, Bridgewater, NJ, USA), or Sorbsan (Mylan, Morgantown, WV, USA); antimicrobial, for example Acticoat (Smith & Nephew, London, UK) or Silverlon (Argentum, Geneva, IL, USA); collagen, for example Fibracol (Johnson & Johnson, NewBrunswick, NJ) or Puracol (Medline, Mundelein, IL,USA); hydrocolloid, for example Duoderm (ConvaTec, Bridgewater, NJ, USA), Granuflex (ConvaTec, Bridgewater, NJ, USA), or Tegaderm (3M, Maplewood, MN, USA); hydrogel, for example Dermagel (Maximilian Zenho & Co, Brussels, Belgium), SilvaSorb (Medline, Mundelein, IL, USA), or Skintegrity (Medline, Mundelein, IL, USA);and polyurethane foam, for example Allevyn (Smith &Nephew, London, UK) or Lyofoa (Molnycke, Gothenburg, Sweden).
Many of these dressings exhibit antimicrobial properties through silver impregnation [9], however, very recent research demonstrated that silver may delay wound healing and should not be routinely used on uninfected donor skin even though silver dressings may reduce wound pain. Therefore, a new dressing is clearly needed.
Based on animal experimental results, we found that BloodSTOP iX Wound Heal Nanocellulose Matrix (BSM) could significantly repair pathological changes both in the skin contusion model and partial-thickness skin burn model. The results suggest that BSM might be used for healing of skin damage in the future.
In our clinical trial, we also found that BloodSTOP iX Wound Heal Nanocellulose Matrix (BSM) is safe for burn wound care for skin grafting on both donor site and transplanted site. BSM benefitted skin grafting by anchoring transplanted skin grafts in-situ, promoting skin regenerating and tissue healing, stopping bleeding on donor sites effectively, enhancing wound healing, and contributing to a very smooth regenerated new skin. Results suggest that BSM is a reliable and effective medical device for burn wound care.
References
- Lee KC, Joory K, Moiemen NS. History of burns: The past, present and the future. Burns & Trauma 2 (2014): 169-180.
- ter Horst B, Chouhan G, Moiemen NS, et al. Advances in keratinocyte delivery in burn wound care. Advanced Drug Delivery Reviews 123 (2018): 18-32.
- Rowan MP, Cancio LC, Elster EA, et al. Burn wound healing and treatment: review and advancements. Critical Care 19 (2015): 243.
- Wall S, Allorto N, Weale R, et al. Ethics of burn wound care in a low-middle income country. AMA Journal of Ethics 20 (2018): 575-580.
- Martineau L, Shek PN. Evaluation of a bi-layer wound dressing for burn care: I. Cooling and wound healing properties. Burns 32 (2006): 70-76.
- Dhaliwal K, Lopez N. Hydrogel dressings and their application in burn wound care. British Journal of Community Nursing 23 (2018): S24-S27.
- Brennan PG, Landry JK, Miles MV, et al. Intravenous ketamine as an adjunct to procedural sedation during burn wound care and dressing changes. Journal of Burn Care & Research 40 (2019): 246-250.
- Selig HF, Lumenta DB, Giretzlehner M, et al. The properties of an “ideal” burn wound dressing–what do we need in daily clinical practice? Results of a worldwide online survey among burn care specialists. Burns 38 (2012): 960-966.
- Fong J, Wood F, Fowler B. A silver coated dressing reduces the incidence of early burn wound cellulitis and associated costs of inpatient treatment: comparative patient care audits. Burns 31 (2005): 562-567.
- Jeschke MG, Shahrokhi S, Finnerty CC, et al. Wound coverage technologies in burn care: established techniques. Journal of Burn Care & Research 39 (2018): 313-318.
- Halib N, Perrone F, Cemazar M, et al. Potential applications of nanocellulose-containing materials in the biomedical field. Materials 10 (2017): 977.
- Poonguzhali R, Basha SK, Kumari VS. Novel asymmetric chitosan/PVP/nanocellulose wound dressing: In vitro and in vivo evaluation. International Journal of Biological Macromolecules 112 (2018): 1300-1309.
- Liu Y, Sui Y, Liu C, et al. A physically crosslinked polydopamine/nanocellulose hydrogel as potential versatile vehicles for drug delivery and wound healing. Carbohydrate Polymers 188 (2018): 27-36.
- Rodrigues C, de Assis AM, Moura DJ, et al. New therapy of skin repair combining adipose-derived mesenchymal stem cells with sodium carboxymethylcellulose scaffold in a pre-clinical rat model. PLoS One 9 (2014): e96241.
- Wagenhäuser MU, Mulorz J, Ibing W, et al. Oxidized (non)-regenerated cellulose affects fundamental cellular processes of wound healing. Scientific Reports 6 (2016): 32238.
- Li H, Wang L, Alwaal A, et al. Comparison of topical hemostatic agents in a swine model of extremity arterial hemorrhage: BloodSTOP iX Battle Matrix vs. QuikClot Combat Gauze. International Journal of Molecular Sciences 17 (2016): 545.
- Ferretti L, Qiu X, Villalta J, et al. Efficacy of BloodSTOP iX, surgicel, and gelfoam in rat models of active bleeding from partial nephrectomy and aortic needle injury. Urology 80 (2012): 1161-e1.
- Basu A, Strømme M, Ferraz N. Towards tunable protein-carrier wound dressings based on nanocellulose hydrogels crosslinked with calcium ions. Nanomaterials 8 (2018): 550.
- Friedman BC, Mendez-Vigo L, Wilson J, et al. A retrospective review of clinical experience with daptomycin for a variety of wound types in a burn and wound care facility. Southern Medical Journal 103 (2010): 748-752.
- Delaplain PT, Joe VC. Problems and Costs That Could Be Addressed by Improved Burn and Wound Care Training in Health Professions Education. AMA Journal of Ethics 20 (2018): 560-566.


 Impact Factor: * 4.2
Impact Factor: * 4.2 Acceptance Rate: 72.62%
Acceptance Rate: 72.62%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 