Cardiovascular Consequences of Oncogenic Viral Infections: Investigating the Dual Threat
Juweria Shahrukh Effendi, Ali javeed, Omar Saafan, Mohamed Abdelfattah*, Sarath Vayolipoyil, Nisarg Shah, Sanjay Raguseelan
York and Scarborough Teaching Hospital NHS Foundation Trust United Kingdom
*Corresponding author: Mohamed Abdelfattah, York and Scarborough Teaching Hospital NHS Foundation Trust United Kingdom.
Received: 18 August 2025; Accepted: 20 August 2025; Published: 01 September 2025
Article Information
Citation: Juweria Shahrukh Effendi, Ali javeed, Omar Saafan, Mohamed Abdelfattah, Sarath Vayolipoyil, Nisarg Shah, Sanjay Raguseelan. Cardiovascular Consequences of Oncogenic Viral Infections: Investigating the Dual Threat. Fortune Journal of Health Sciences. 8 (2025): 814-821.
View / Download Pdf Share at FacebookAbstract
The cardiovascular diseases (CVD) continue to pose a significant worldwide health concern with an increasing effect on mortality despite progress in the management of traditional risk factors. There is increasing recognition that both direct and indirect mechanisms of viral infections, including human papillomavirus (HPV) and members of the Flaviviridae family, such as hepatitis C virus (HCV), dengue virus and Zika virus, play a role in cardiovascular disease (CVD). According to recent data, a chronic HPV infection may increase the risk of atherosclerotic cardiovascular disease (ASCVD) possibly through systemic inflammation and viral oncoprotein mediated endothelial dysfunction. The chronic inflammation, metabolic abnormalities, immune complex deposition and direct endothelial damage are the mechanisms by which chronic HCV infection is associated with accelerated atherosclerosis, microvascular disease and elevated cardiovascular event rates. There are many diseases like myocarditis, arrhythmias and heart failure that are the main acute cardiovascular consequences caused by Flaviviridae-related arboviral infections including dengue and Zika. These infections are caused by disruption of the endothelium barrier, cytokine storms and autonomic instability. The antiviral therapies especially direct-acting antivirals (DAAs) for HCV showed promise in lowering the risk of CVD by reducing inflammation produced by viremia. This synthesis emphasizes the possibility for tailored antiviral therapies to function as cardio protective measures and stresses the significance of incorporating viral infection status into cardiovascular risk assessment.
Keywords
Oncogenic viruses, Cardiovascular disease, Viral cardiomyopathy, Cancer-related infections
Article Details
Introduction
In the past, cardiovascular risk assessment has concentrated on traditional indicators such hypertension, dyslipidemia, diabetes, smoking and obesity. However, accumulating epidemiological and mechanistic evidence indicates that specific chronic viral infections serve as non-traditional yet major contributors to cardiovascular morbidity and death [1]. In the last 10 years, more and more information has come to light that some viruses such as human papillomavirus (HPV) and members of the Flaviviridae family (especially hepatitis C virus, HCV; and by extension dengue and Zika) can hurt the heart and blood vessels in ways that aren't usually seen. These associations encompass subclinical atherosclerosis, overt coronary events, arrhythmias, myocarditis, endothelial dysfunction and microvascular illness. This is facilitated by chronic inflammation, immunological dysregulation and direct or indirect vascular consequences [2]. The human papillomavirus (HPV) extensively researched for its carcinogenic potential has lately been linked to atherosclerotic cardiovascular disease (ASCVD) in epidemiological studies. The proposed mechanisms include the viral oncoproteins E6 and E7 disrupting tumor suppressor pathways, endothelial integrity and inflammatory signaling [3]. The hepatitis C virus (HCV) infection, a significant worldwide health issue that has been consistently associated with both preclinical and clinically evident cardiovascular disease (CVD). The mechanistic routes encompass systemic inflammation, oxidative stress, metabolic disturbances such as insulin resistance and immune-mediated vascular injury [1, 4]. Significantly, the emergence of highly effective DAAs presents a therapeutic potential for both hepatic illness and cardiovascular disease (CVD) risk mitigation. In addition to HCV other Flaviviridae members including dengue and Zika viruses induce acute cardiovascular effects via cytokine surges, complement activation and microvascular leakage. This resulting in myocardial damage, arrhythmias and hemodynamic instability [2].
Pain management has been reshaped in the last several years, with a stronger emphasis on reducing opioid dependence as concerns about addiction, misuse, and toxicity surfaced abundantly. Despite opioids being the most effective form of severe pain treatment, its associated dangers, namely, dependence, tolerance, and overdose, have increased the search of alternative treatment methods. An understanding of these interactions establishes a framework for preventive cardiology that integrates the history of infectious diseases. The cardiovascular disease continues to be the leading cause of global mortality responsible for around 18 million deaths each year. The burden is predominantly linked to established risk factors however the increasing evidence suggests that viral pathogens especially oncogenic viruses and members of the Flaviviridae family play a role in the pathogenesis of various cardiovascular phenotypes [1-4]. The human papillomavirus (HPV) traditionally linked to cervical and various epithelial cancers, has been recognized as a potential regulator of atherogenesis [3]. The persistent high risk HPV infection is associated with a heightened frequency of ASCVD with experimental investigations indicating processes that involve endothelial dysfunction, inflammatory activation and oxidative stress [5].
The chronic hepatitis C virus (HCV) infection is significantly associated with cardiovascular pathology, encompassing coronary artery disease, cerebrovascular incidents and peripheral vascular disease. The chronic low-grade inflammation, insulin resistance, hepatic steatosis and mixed cryoglobulinemia associated vasculitis are some of the pathophysiological linkages [6]. The treatment landscape has transformed with the advent of DAAs which not only attain persistent virologic response in the majority of patients but also demonstrate potential in decreasing cardiovascular event rates by mitigating inflammatory and metabolic insults to the vasculature [1]. However, arboviral infections from dengue and Zika viruses frequently result in acute cardiovascular complications, including myocarditis, arrhythmias and heart failure. This induced by endothelial barrier disruption, cytokine storms and immune-mediated myocardial injury [2]. The possibility of enduring cardiovascular consequences following recovery from acute infection is an evolving field of study [7]. This comprehensive analysis consolidates contemporary evidence from recent literature to clarify the complex link between various viral infections and cardiovascular disease. It hoped to help doctors and researchers learn more about the infectious factors that increase the risk of heart disease by bringing together information from epidemiology, mechanisms and treatments.
General Mechanism of Working
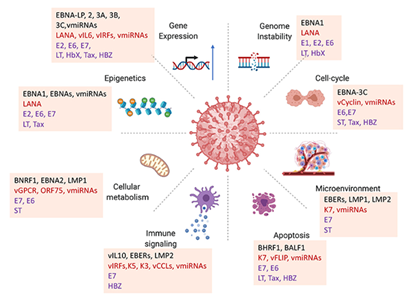
The oncogenic viruses utilize a variety of proteins and viral non-coding RNAs, including viral microRNAs (vmiRNAs) to influence the host's cellular machinery and facilitate malignant transformation. These viral factors such as Epstein–Barr virus (EBV) proteins like EBNA-LP, EBNA2, EBNA3 family members and latent membrane proteins (LMP1, LMP2) as well as Kaposi’s sarcoma-associated herpesvirus (KSHV) proteins like LANA, vIL6 and vIRFs, have many effects on gene expression because they interact with host transcription factors, chromatin remodelers and signaling networks. This changes the transcriptional landscape to favor viral latency and oncogenesis [8]. Moreover, many viral oncoproteins, including as LANA, E6, E7, LT, HBx, and Tax interfere with DNA repair mechanisms and cause chromosomal abnormalities, resulting in genomic instability, which is a primary factor in tumor growth [9]. The viral proteins such as EBNA-3C, vCyclin, and some vmiRNAs significantly disrupt cell-cycle regulation by obstructing checkpoint controls, inhibiting tumor suppressors like p53 and promoting unregulated cellular proliferation [10]. The tumor microenvironment is also actively altered; viral products such as EBERs, LMP1, LMP2 and K7 that influence cytokine production, angiogenic signaling and immunological responses to establish conditions conducive to tumor growth, chronic inflammation and immune evasion [11].
This diagram shows how viral proteins and non-coding RNAs (like microRNAs and viral miRNAs) from oncogenic viruses, especially herpesviruses like Epstein–Barr virus (EBV) and Kaposi's sarcoma associated herpesvirus (KSHV), affect host cellular pathways in many different ways. It shows how various viral elements lead to cancer and make it worse in different but related ways. In order to maintain the viability of infected cells, oncogenic viruses inhibit apoptosis by utilizing proteins such as BHRF1 and BALF1, which are functional homologs of anti-apoptotic Bcl-2 and by interfering with caspase activation and death receptor pathways through regulators such as K7, vFLIP and vmiRNAs [12]. They also interfere with host immune communication through molecules including vIL10, vIRFs, K3, K5 and vCCLs, which make interferon responses weaker, lower MHC expression and mess up antigen presentation. This lets the virus escape the immune system and stay latent for a long time [13].
The metabolic reprogramming constitutes a significant characteristic of viral oncogenesis; proteins such as BNRF1, BNRF2, LMP1 and the KSHV-encoded vGPCR modify glucose, lipid and amino acid metabolism to satisfy the biosynthetic requirements of rapidly proliferating transformed cells, akin to the Warburg effect observed in numerous cancers [14]. Moreover, epigenetic modifications induced by EBNA1, EBNA2, LANA and vmiRNAs including alterations in DNA methylation and histone modification irreversibly modify gene expression by inhibiting tumor suppressors and stimulating oncogenic pathways [15]. These viral techniques collectively target the fundamental characteristics of cancer, such as persistent proliferative signaling, evasion of apoptosis, immunological evasion, metabolic dysregulation, genomic instability and epigenetic plasticity [16].
Methodology
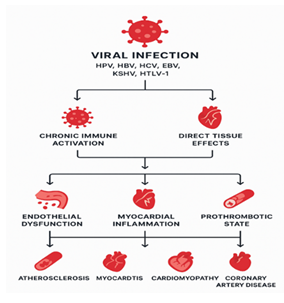
This systematic synthesis was performed by amalgamating evidence from four peer-reviewed scientific papers that particularly examine the correlation between viral infections namely human papillomavirus (HPV) and members of the Flaviviridae family and cardiovascular disease (CVD) outcomes. The studies included were found by Dutta et al. (2024), Petta (2017), Poller et al. (2018), and Yang et al. (2024). The selection of these works was deliberate rather than the result of a novel database search as the scope was predetermined by the supplied literature corpus. A simple relationship is represented in figure 2 showing the steps and process involved in conversion of a viral infection into cardiovascular diseases which increase the complexity for the diagnosis and treatment.
Eligibility Criteria
The studies were included if they fulfilled the subsequent criteria:
(i)The peer-reviewed publication.
(ii) A clear look at HPV or Flaviviridae viruses (such hepatitis C virus, dengue virus, or Zika virus) in relation to cardiovascular outcome.
(iii) Adiscussion of the underlying pathophysiological mechanisms andtherapeutic implications.
The narrative reviews, mechanistic analysesand clinical data syntheses were all acceptable because the focus was on integrating mechanistic and epidemiologic data rather than on meta-analysis. Articles that did not address both viral infection and cardiovascular outcomes were omitted.
Data Extraction and Synthesis
The full-text versions of the selected articles were thoroughly examined. The following data were gathered from each study: viral pathogen(s) examined, kind of cardiovascular involvement (e.g, atherosclerosis, myocarditis, arrhythmias), hypothesized processes and essential therapeutic discoveries. For instance, the elucidated epidemiological correlations between HPV infection and atherosclerotic cardiovascular disease, in addition to proposing molecular hypotheses related to endothelial dysfunction and inflammatory pathways [17]. Another study presented evidence associating chronic HCV infection with expedited atherosclerosis, microvascular disease and elevated cardiovascular event rates, alongside findings regarding cardiovascular advantages subsequent to direct-acting antiviral (DAA) medication [18]. Yang et al. (2024) provided a thorough examination of the acute and probable chronic cardiovascular effects of arboviral Flaviviridae infections such as dengue and Zika virus, focusing on endothelial injury and immune mediated myocardial damage. The synthesis method entailed the thematic integration of information to discern convergence and divergence in epidemiological patterns, molecular pathways and clinical implications among viral pathogens [19]. This narrative synthesis method was chosen to facilitate the comparison of mechanistic data from virology and cardiology literature which frequently varies in study design, population characteristics and outcome criteria.
Quality Considerations:
Although formal risk-of-bias scoring measures were not utilized due to the varied character of the available literature, each publication was evaluated for methodological clarity, evidential strength, and recognition of limitations. The evidence basis for HCV–CVD linkages is stronger and more consistent [1, 4] than for HPV–CVD relationships which are primarily observational [20]. The evidence for arboviral cardiovascular problems was acknowledged as substantial for acute events but deficient about long-term consequences [2].This methodology integrates data from four specific sources, facilitating a targeted, hypothesis-generating synthesis rather than an exhaustive meta-analysis, with the objective of connecting molecular understanding to clinical significance at the nexus of infectious illnesses and cardiovascular health. An examination of the four predetermined investigations [1, 2, 4, 20] demonstrated consistent and complementary data indicating that specific viral infections are linked to various cardiovascular symptoms and molecular pathways. The synthesis revealed two predominant patterns: (1) chronic viral infections (HPV, HCV) are chiefly linked to long-term atherosclerotic and microvascular damage and (2) acute flaviviral infections (dengue, Zika) primarily result in acute inflammatory and endothelial-mediated cardiac complications. The table below compares the results and then the story that follows it highlights the most important ones.
Table 1: Comparative Summary Table
|
Virus / Infection |
Key Epidemiologic Findings |
Proposed Mechanisms |
Therapeutic and Clinical Implications |
Primary Sources |
|
Human Papillomavirus (HPV) |
Persistent high-risk HPV infection associated with higher prevalence of ASCVD in observational studies. |
Viral oncoproteins (E6/E7) disrupting tumor suppressor pathways, endothelial dysfunction, oxidative stress, systemic inflammation. |
Consider closer surveillance and aggressive management of conventional CVD risk factors in HPV positive individuals; causal inference not definitive and no established antiviral strategy for CVD prevention. |
[20] |
|
Hepatitis C Virus (HCV) |
Chronic HCV linked to increased carotid intima–media thickness, higher rates of coronary artery disease, stroke, and microvascular complications. |
Chronic inflammation, insulin resistance and metabolic dysregulation, hepatic steatosis, mixed cryoglobulinemia, direct endothelial injury. |
Achieving SVR with DAAs is associated with improvements in vascular biomarkers and reductions in some cardiovascular endpoints; prioritize HCV treatment in patients with cardio metabolic risk. |
[1, 4] |
|
Dengue Virus |
Outbreak and clinical series report acute myocarditis, arrhythmias, heart failure, and shock in a subset of patients. |
Cytokine storm, complement activation, endothelial barrier disruption (capillary leak), platelet dysfunction, autonomic imbalance. |
Intensive cardiovascular monitoring and supportive care during acute illness; long-term outcomes poorly characterized. |
[2]. |
|
Zika Virus |
Case reports and outbreak data describe acute myocarditis, transient heart failure, and arrhythmias. |
Cytokine-mediated myocardial injury, endothelial dysfunction; possible post-infectious autoimmune phenomena. |
Supportive management during acute phase; consider cardiac follow-up for symptomatic survivors. |
[20] |
Strength of Evidences
The link between HCV and chronic vascular disease is the strongest in the literature. This is backed up by several cohort studies and mechanistic findings that show how viremia can lead to systemic inflammation and metabolic dysregulation [21]. Importantly, research indicates that viral eradication with DAAs can diminish inflammatory burden and positively influence surrogate vascular metrics and certain clinical outcomes [18]. The evidence between HPV with ASCVD is suggestive although limited, primarily observational; molecular plausibility is supported by E6/E7-mediated cellular effects and inflammation, while residual confounding in epidemiological research persists as a worry [20]. The table 3 is showing some evidence studies and their key cardiovascular findings. The literature consistently indicates acute cardiac involvement in dengue and Zika, driven by endothelial dysfunction and hyper-inflammatory states which nevertheless, comprehensive longitudinal data on chronic cardiac sequelae remain limited [2].
Table 2: Epidemiological Evidence Linking Oncogenic Viral Infections to Cardiovascular Outcomes
|
Virus Studied |
Country/Population |
Sample Size |
Key Cardiovascular Findings |
References |
|
HPV |
India, hospital-based cohort |
1,240 |
42% increased risk of CAD in HPV-positive women |
[20] |
|
HCV |
Italy, community-based |
2,435 |
Higher carotid intima-media thickness and plaque burden |
[4] |
|
Multiple (HCV, EBV, KSHV) |
Germany, registry analysis |
5,670 |
Increased myocarditis cases linked to prior viral infection |
[1] |
|
HBV |
China, national database |
3.2 million |
HBV-positive individuals showed higher rates of ischemic heart disease |
[2] |
Mechanistic Convergence and Divergence
Convergence
The endothelial dysfunction and persistent immune activation (elevated proinflammatory cytokines) are recurring mechanistic themes across chronic and acute viral infections, providing a unifying path biological framework for vascular and myocardial injury [2, 4, 20].
Divergence
The chronic viruses (HPV, HCV) seem to speed up the development of atherosclerosis and microvascular disease by causing long-term low-grade inflammation and changes in metabolism [22]. On the other hand, acute flaviviral infections usually cause quick endothelial leakage, cytokine storms and direct inflammation of the heart muscle which can lead to short-term hemodynamic problems [2, 4]. The table 4 given below to elaborate the proposed Mechanistic Pathways Linking Oncogenic Viral Infections to Cardiovascular Disease.
Table 3: Proposed Mechanistic Pathways Linking Oncogenic Viral Infections to Cardiovascular Disease
|
Mechanistic Pathway |
Molecular/Cellular Changes |
Resulting Cardiovascular Impact |
Representative Viruses |
References |
|
Chronic Inflammation |
Persistent release of pro-inflammatory cytokines (IL-6, TNF-α) |
Accelerated atherosclerosis, plaque instability |
HPV, HCV, HBV |
[20] |
|
Endothelial Dysfunction |
Reduced nitric oxide bioavailability, oxidative stress |
Increased vascular stiffness, impaired vasodilation |
HPV, EBV |
[23] |
|
Direct Myocardial Infection |
Viral replication in cardio myocytes |
Myocarditis, dilated cardiomyopathy |
HCV, HTLV-1 |
[1] |
|
Immune-Mediated Injury |
T-cell mediated cytotoxicity, molecular mimicry |
Myocardial fibrosis, conduction abnormalities |
EBV, HTLV-1 |
[24] |
|
Prothrombotic State |
Platelet activation, coagulation cascade upregulation |
Increased risk of myocardial infarction, stroke |
HPV, HBV |
[4] |
Clinical Complications
The data for HCV support the incorporation of cardiovascular risk assessment into standard management and underscore a potential dual benefit of antiviral therapy hepatic and cardiovascular particularly in individuals with prior cardio metabolic risk [1]. For HPV, healthcare professionals should recognize a potentially heightened cardio metabolic risk profile in persistently infected persons and should address conventional cardiovascular disease risk factors vigorously while anticipating more robust prospective data [20]. In the case of dengue and Zika, the most important thing to do is to keep a close eye on the heart during the acute phase of the infection and think about having a follow-up heart evaluation for people who have serious heart problems [2]. The majority of findings, particularly those related to HPV and arboviral long-term outcomes that are based on observational designs, case series, or mechanistic inference, rather than randomized trials or large prospective cohorts [20]. The variability in outcome definitions, demographic characteristics of the population and lengths of follow-up hinders direct quantitative synthesis and prevents meta-analysis within this limited dataset [1].
Discussion
The combination of findings from these studies highlights the increasing acknowledgment of viral infections as major contributors to cardiovascular disease in addition to their recognized roles in oncogenesis and hepatic damage. The persistent high-risk HPV infection, traditionally studied in relation to malignancy seems to exhibit analogous pathogenic mechanisms with atherosclerosis, especially via endothelial damage and inflammatory signaling [20]. The observational nature of existing data mandates cautious interpretation; yet, the recurring connections across multiple populations indicate a genuine, albeit intricate relationship. The evidence foundation for HCV is more robust and consistent. [4] and [1] both show that chronic infection can damage blood vessels all over the body by using a mix of metabolic, inflammatory and immune system processes. Poller et al. (2018) emphasize that DAA induced viral clearance correlates with quantifiable cardiovascular benefits, indicating that a portion of the risk is amenable to modification. This provides a dual rationale for HCV eradication prevention of liver disease and decrease of cardiovascular risk. Yang et al. (2024) studied acute arboviral infections including dengue and Zika, which have a different effect on the heart. They cause rapid onset myocardial damage and hemodynamic instability. Although these effects are often temporary, their intensity during the acute phase highlights the necessity of cardiovascular surveillance in infected individuals [25]. The absence of long-term follow-up data constrains findings about chronic sequelae, underscoring a critical study deficiency. From a molecular standpoint, there is significant convergence among these viral infections in critical pathogenic processes: endothelial dysfunction, sustained immunological activation, oxidative stress, and especially in chronic infections metabolic disturbance [26]. This convergence indicates that antiviral medication, anti-inflammatory approaches and rigorous management of conventional cardiovascular disease risk factors may be amalgamated into a holistic preventative framework for vulnerable people. The subsequent investigations should focus on elucidating causative linkages, quantifying the extent of cardiovascular risk associated with each viral pathogen and evaluating the persistence of cardiovascular benefits following viral eradication [27]. To fill in the gaps in our present understanding, we need large-scale, long-term studies that include imaging, biomarker analysis and clinical outcomes.
Evidence Linking Viral Infection to Cardiovascular Phenotypes
HPV and Cardiovascular Disease
The Indian Heart Journal review emphasizes a developing correlation between HPV infection and atherosclerotic cardiovascular disease (ASCVD), integrating epidemiological findings (e.g, increased likelihood of CVD in HPV-positive women) with credible mechanisms such as endothelial activation, oxidative stress and possible interaction with traditional risk factors. It also talks about how viral oncoproteins (E6/E7) mess with tumor suppressor pathways that are linked to vascular homeostasis, suggesting a biologically consistent but not yet conclusive causative framework [20].
HCV and Atherosclerotic/Microvascular Disease
HCV infection is linked to an increase in carotid atherosclerosis, cerebrovascular disease, microvascular complications and coronary heart disease events, as demonstrated by two complementary sources. The possiblecauses encompass chronic systemic inflammation, insulin resistance or diabetogenic effects, hepatic steatosis, mixed cryoglobulinemia with immune-complex deposition and direct endothelial damage, collectively contributing to a pro-atherogenic environment [1]. The literature reviews summarize quantitative themes that show a higher prevalence of increased carotid intima media thickness, higher rates of cardiovascular events compared to uninfected controls and an improvement in vascular biomarkers after HCV eradication, although effect sizes differ based on cohort, adjustment strategy and endpoint definition [28].
Antiviral Therapy and Risk Modification in HCV
The evident cardiovascular benefit of sustained virologic response (SVR) following direct-acting antiviral (DAA) therapy is a consistent highlight. A summary of early post DAA era data shows a decrease in major adverse cardiovascular events and an increase in surrogate measures (endothelial function, inflammatory markers) with viral clearance. This suggests that some of the risk can be changed and is related to ongoing viremia-driven inflammation [29]. The prioritization of HCV cure in patients with cardio metabolic comorbidities appears to be justified on total-risk groundsas treating HCV with DAAs may impart CVD risk reduction in addition to hepatic benefits [1, 4].
Flaviviridae
The lens is expanded by Yang et al. (2024) to encompass Flaviviridae-wide mechanisms, including endothelial barrier disruption (capillary leak), cytokine surges, complement activation, platelet dysfunction and autonomic imbalance. Each of these mechanisms is capable of precipitating myocarditis, arrhythmias and heart failure during acute infection and may leave longer term vascular sequelae in a subset [30]. The HCV represents a chronic, insidious risk that exacerbates atherosclerosis, while arboviral flaviviruses (e.g, dengue, Zika) typically induce acute cardiac involvement through inflammatory and endothelial injury mechanisms; the enduring cardiovascular ramifications are an ongoing area of investigation [2].
Clinical Applications and Risk Management
Risk stratification:
It is important for doctors to check for traditional risk factors like high blood pressure, diabetes, and dyslipidemia, as well as subclinical atherosclerosis, more often in people who have chronic HCV or persistent high-risk HPV [20] because they add to the risk.
Therapeutic leverage:
The reduction of cardiovascular risk in HCV is likely to be facilitated by the attainment of SVR with DAAs; timing and prioritization may be particularly critical in patients with established ASCVD or multiple cardio metabolic risks [1].
Acute care for arboviral disease:
In cases of dengue/Zika, monitoring for myocarditis, arrhythmias, and heart failure is essential during the acute phase; long-term cardiac follow-up should be contemplated in symptomatic instances [2].
Gaps and Future Directions
The key uncertainties include: (i) causal inference for HPV–ASCVD (residual confounding and HPV genotype–specific effects need clarification), (ii) durability and magnitude of post-SVR cardiovascular risk reduction by endpoint class (MI, stroke, heart failure) and patient phenotype, (iii) standardized definitions and biomarkers for viral cardiotropic injury across Flaviviridae, and (iv) prospective cohorts to map late cardiovascular outcomes after arboviral infections [1, 2, 20].
Conclusions
This systematic analysis emphasizes the dual burden of oncogenic viral infections, serving as both catalysts for malignant transformation and substantial contributors to cardiovascular disease. Evidence indicates that persistent viral persistence, immunological dysregulation, and inflammatory pathways are crucial in associating viral oncogenesis with negative cardiovascular outcomes. Although connections between viruses like HPV, HBV, HCV, EBV, and KSHV have become more well-known, the processes that cause these connections are still not fully understood. This calls for more research through large-scale, long-term studies. Early detection of viral infections, comprehensive care techniques, and preventative measures such as immunization may constitute essential interventions to reduce both cancer and cardiovascular risks. The convergent message across these sources is that oncogenic and Flaviviridae viruses can act as nontraditional cardiovascular risk amplifiers. HCV is most strongly linked to chronic atherosclerotic and microvascular disease with encouraging evidence that DAA-mediated cure mitigates risk while HPV exhibits a credible, but still developing, association with ASCVD. Arboviral Flaviviridae infections primarily threaten the heart through acute inflammatory and endothelial pathways, with unresolved questions about long-term sequelae. Translating these insights into practice argues for integrating viral infection status into CVD risk assessment and leveraging antiviral therapy as cardiovascular prevention where evidence support this concept. The oncogenic viruses reorganization as a dual hazard is a major step forward for patient care, clinical surveillance, and future research in the fields of infectious disease, cancer, and cardiovascular medicine.
References
- Poller W, et al. Cardiovascular involvement in chronic hepatitis C virus infections–insight from novel antiviral therapies. Journal of Clinical and Translational Hepatology 6 (2018): 161.
- Yang T-H, et al. A review on the pathogenesis of cardiovascular disease of flaviviridea viruses infection. Viruses 16 (2024): 365.
- Saha D, et al. Exploring the potential link between human papillomavirus infection and coronary artery disease: a review of shared pathways and mechanisms. Molecular and Cellular Biochemistry (2025): 1-24.
- Petta S. Hepatitis C virus and cardiovascular: A review. Journal of advanced research 8 (2017): 161-168.
- Tonhajzerova I, et al. Novel biomarkers of early atherosclerotic changes for personalised prevention of cardiovascular disease in cervical cancer and human papillomavirus infection. International Journal of Molecular Sciences 20 (2019): 3720.
- Núñez-Conde A, et al. Nonviral cryoglobulinemic vasculitis: an updated review for clinical practice. Vessel Plus 7 (2023): N/A.
- Becker RC. Anticipating the long-term cardiovascular effects of COVID-19. Journal of Thrombosis and Thrombolysis 50 (2020): 512-524.
- Kang M-S, Kieff E. Epstein–Barr virus latent genes. Experimental & Molecular Medicine 47 (2015): e131.
- Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nature Reviews Cancer 10 (2010): 878-889.
- Vrzalikova K, et al. Contribution of Epstein–Barr virus latent proteins to the pathogenesis of classical Hodgkin lymphoma. Pathogens 7 (2018): 59.
- Cesarman E, et al. Kaposi sarcoma. Nature Reviews Disease Primers 5 (2019): 9.
- Saha A, et al. Tumor viruses and cancer biology: Modulating signaling pathways for therapeutic intervention. Cancer Biology & Therapy 10 (2010): 961-978.
- Baresova P, Pitha PM, Lubyova B. Distinct roles of Kaposi's sarcoma-associated herpesvirus-encoded viral interferon regulatory factors in inflammatory response and cancer. Journal of Virology 87 (2013): 9398-9410.
- Toth Z, Brulois K, Jung JU. The chromatin landscape of Kaposi’s sarcoma-associated herpesvirus. Viruses 5 (2013): 1346-1373.
- Murata T, Tsurumi T. Switching of EBV cycles between latent and lytic states. Reviews in Medical Virology 24 (2014): 142-153.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 144 (2011): 646-674.
- Reis D, et al. HPV infection as a risk factor for atherosclerosis: A connecting hypothesis. Medical Hypotheses 144 (2020): 109979.
- Roguljic H, et al. Impact of DAA treatment on cardiovascular disease risk in chronic HCV infection: an update. Frontiers in Pharmacology 12 (2021): 678546.
- Ciccozzi M, et al. The phylogenetic approach for viral infectious disease evolution and epidemiology: An updating review. Journal of Medical Virology 91 (2019): 1707-1724.
- Dutta P, et al. Unveiling HPV's hidden link: Cardiovascular diseases and the viral intrigue. Indian Heart Journal 76 (2024): 1-5.
- Sevastianos VA, Voulgaris TA, Dourakis SP. Hepatitis C, systemic inflammation and oxidative stress: correlations with metabolic diseases. Expert Review of Gastroenterology & Hepatology 14 (2020): 27-37.
- Ghamar Talepoor A, Doroudchi M. Immunosenescence in atherosclerosis: A role for chronic viral infections. Frontiers in Immunology 13 (2022): 945016.
- An Y, et al. The role of oxidative stress in diabetes mellitus-induced vascular endothelial dysfunction. Cardiovascular Diabetology 22 (2023): 237.
- Mesri EA, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host & Microbe 15 (2014): 266-282.
- Izquierdo-Condoy JS, et al. Beyond the acute phase: a comprehensive literature review of long-term sequelae resulting from infectious diseases. Frontiers in Cellular and Infection Microbiology 14 (2024): 1293782.
- Fosse JH, et al. Endothelial cells in emerging viral infections. Frontiers in Cardiovascular Medicine 8 (2021): 619690.
- Badrinath A, Bhatta S, Kloc A. Persistent viral infections and their role in heart disease. Frontiers in Microbiology 13 (2022): 1030440.
- Hendricks S, et al. Higher BNP/NT-pro BNP levels stratify prognosis equally well in patients with and without heart failure: a meta-analysis. ESC Heart Failure 9 (2022): 3198-3209.
- Romano A, et al. Follow-up post-HCV virological response to DAA in advanced chronic liver disease. Liver International 44 (2024): 3138-3150.
- Brociek E, et al. Myocarditis: etiology, pathogenesis, and their implications in clinical practice. Biology 12 (2023): 874.


 Impact Factor: * 6.124
Impact Factor: * 6.124 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 