Facilitated Self-Assembling Technology (FAST) for the Preparation of Nanoparticles to Increase the Solubility and Bioavailability of Hydrophobic Molecules
Nicolette Frank1, Douglas Dickinson1, Yutao Liu2, Hongfang Yu2, Jingwen Cai2, and Stephen Hsu*,1,3
1Camellix Research Laboratory, Augusta, GA 30912, USA.
2Department of Cellular Biology & Anatomy, Medical College of Georgia, Augusta University, Augusta, GA 30912, USA.
3Department of Oral Biology & Diagnostic Sciences, Dental College of Georgia, Augusta University, Augusta, GA 30912, USA
*Corresponding author: Stephen Hsu, Department of Oral Biology & Diagnostic Sciences, Dental College of Georgia, Augusta University, Augusta, GA 30912, USA.
Received: 03 April 2025; Accepted: 07 April 2025; Published: 19 April 2025
Article Information
Citation:
Nicolette Frank, Douglas Dickinson, Yutao Liu, Hongfang Yu, Jingwen Cai and Stephen Hsu. Facilitated Self-Assembling Technology (FAST) for the Preparation of Nanoparticles to Increase the Solubility and Bioavailability of Hydrophobic Molecules. Fortune Journal of Health Sciences 8 (2025): 283-295.
View / Download Pdf Share at FacebookAbstract
Approximately 70–90% of drug candidates at the development stage are hydrophobic. These compounds often exhibit poor water solubility. Examples of hydrophobic drug candidates include quercetin, cannabidiol (CBD), tetrahydrocannabinol (THC), retinoic acid, tocotrienols, paclitaxel, and ivermectin. This physical property can limit the development of hydrophobic molecules for new drug use, and may be associated with low bioavailability, reduced therapeutic effects, and increased dosage that could cause unwanted adverse effects.
Background: In previous work, we developed a novel Facilitated Self-assembling Technology (FAST for short) with several specific practical methods. The major advantage of FAST is that the hydrophobic compound is not engineered or encapsulated, and there is no addition of lipid, surfactant, or metal component. The nanoparticles prepared using this technology are highly hydrophilic and stable. This nanotechnology would allow many drug candidates to be developed with increased solubility and bioavailability in their own nanoparticle form.
Objectives: The purpose of the current study is to use three practical methods of FAST to prepare water nanosuspensions of several known poorly soluble drug candidates including CBD, quercetin and paclitaxel.
Methods: ZetaView nanoparticle tracking analysis was used to characterize nanoparticle suspensions by size distribution and Zeta potential, and electron microscopy imaging was used to examine the shape of the particles.
Results: FAST generated stable nanoparticle suspensions of these compounds, enabling new drug development and improved solubility and bioavailability of existing drugs.
Keywords
<p>Nanoparticles, EGCG-palmitate, CBD, THC, Quercetin, Ivermectin, Retinoic Acid, Paclitaxel, Synthetic steroids, Azole antifungals, Bioavailability, Solubility</p>
Article Details
Introduction
Various attempts have been made to increase the solubility and bioavailability of drug candidates, including methods employing nanotechnology. The first FDA-approved medication using nanotechnology was Rapamune (sirolimus), which was solubilized through a wet milling method [1]. Since then, various nanotechnologies have been developed, including solid lipid nanoparticles and nanostructured lipid carriers, which require solid or solid/liquid fats as carriers. Advanced nanotechnologies, such as supercritical antisolvent (SAS) methods, can increase the solubility of certain molecules, but it is a complicated process with limited use. Other nanotechnology methods include nano emulsions, nanogels, with either oil and surfactant, or crosslinked polymers. In addition, engineered nanoparticles can be made through metal organic frameworks, carbon nanotubes, and mesoporous silica [2, 3]. The recently emerged nanocrystal technology using methods such as wet milling and high-pressure homogenization allows drugs with poor solubility to form nanocrystal particles. An increased number of FDA-approved drugs are associated with nanocrystal technology [4]. However, these nanocrystals were not self-assembled crystals, and therefore often require a stabilizer such as surfactants or polymers that coat the nanoparticles to maintain stability of the suspension formulations. In summary, current nanotechnology is often involved in time-consuming processes with additional ingredients/components and/or specific engineering methods. Specifically, nanoparticles generated by wet milling or high-pressure homogenization do not involve self-assembly of the molecules according to the amphipathic molecular structures.
To address these limitations, we developed a novel approach called Facilitated Self-Assembling Technology (FAST) in prior research, to convert lipid-soluble green tea polyphenols into nanoparticles easily suspended in aqueous formulations. FAST methods are simple and easy to perform, and has been used to generate nanoparticles comprised of epigallacatechin-3-gallate-palmitates (EC16) with consistent size range, and high stability [5, 6]. FAST was developed in a project using an EC16m (epigallacatechin-3-gallate-mono-palmitate) nanoformulation for nasal application against human respiratory viruses. We were able to prepare nasal nanoformulations with high efficacy against human coronavirus without mucociliary toxicity [5, 6]. The virucidal efficacy of EC16 nanoparticles is more than 100-fold higher than epigallacatechin-3-gallate (EGCG) dissolved in DMSO [5, 7, 8]. These particles can be seen under electron microscopes as tightly packed self-assembled structures [9]. Methods derived from FAST to generate EC16/EC16m nanoparticles/nanocrystals are easy, economical, and rapid, with self-assembled nanoparticles/nanocrystals consist of amphipathic molecules with a hydrophilic (negatively charged in water) moiety facing the aqueous phase, therefore the surface charge is sufficient to maintain stability by repulsion force in aqueous suspensions. These nanoparticles are easy to suspend in water and other aqueous solutions such as normal saline or phosphate buffer saline and are stable in room temperature. The major advantage of FAST is that it is not engineered or associated with other components. The EC16 nanoparticles can be dried or in liquid form. Thus, EC16 nanoparticles can be used in various formulations, drugs, and consumer products for antiviral/virucidal, anti-biofilm, anti-inflammatory, anti-neurodegeneration, antiaging, and sporicidal purposes.
Based on the data generated from our laboratories, we hypothesize that FAST can be applied to many hydrophobic compounds with poor water solubility and/or bioavailability, to generate nanoparticles/nanocrystals in stable aqueous nanosuspension or dry form to improve effectiveness and/or delivery efficiency. Examples of medications for improvement include azole antifungal drugs and insoluble glucocorticoids. The current study is aimed at testing our hypothesis by generating nanoparticles from a list of representative compounds with poor water-solubility and bioavailability, including cannabis (Cannabidiol and Tetrahydrocannabinol), synthetic steroid drugs (medroxyprogesterone acetate and triamcinolone acetonide), azole antifungal drug (Fluconazole), cancer drug (Paclitaxel), flavonoids (quercetin, EGCG-mono-palmitate [EC16m]), antiparasitic drug (ivermectin), and lipid-soluble vitamin (retinoic acid). Structures of these hydrophobic compounds shown below are from Wikipedia, except for EC16m, which was provided by Camellix, LLC. USA. If successful, FAST could lead to a wide range of applications for hydrophobic compounds to be developed for effective delivery to specific targets by oral, topical, nasal, inhalational, injectable, and other applications.

Materials and Methods
Compounds and oral rinse
Epigallocatechin-3-Gallate-Palmitates (EC16) and Epigallocatechin-3-Gallate-Mono-Palmitate (EC16m) were obtained from Camellix, LLC (Evans, GA, USA). Delta-9-Tetrahydrocannabinol (THC-9, 06-722-453), Medroxyprogesterone acetate (AC461120010), Quercetin hydrate, 95% (AC174070100), Ivermectin (AAJ6277703), and Retinoic Acid, all trans isomer (MP021902695) were purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA). Cannabidiol (CBD) isolate powder was purchased from Crescent Canna (New Orleans, LA). Paclitaxel was purchased from MedKoo Biosciences (Durham, NC. USA). Triamcinolone Acetonide (PHR1701) was obtained from Sigma-Aldrich (St. Louis, MO. USA). Fluconazole powder was purchased from Everything Aquatic (Medford, OR. USA). Both unflavored and peppermint flavored oral rinse were provided by International Nutrition, Inc. (Middle River, MD, USA).
Preparation of nanoparticles
The nanoparticles of the compounds were prepared using FAST (proprietary, patent pending) with three specific practical methods (A, A1 and A2). Method A was the main method that was used for nanoparticle preparations from EC16m [7] and other compounds except EC16 in this study, from which were formed by simplified methods with reduced workflow Method A1 and Method A2. Briefly, hydrophobic compound samples were initially dissolved in a compound-specific organic solvent as single molecules in solution. The dissolved single molecules then formed organized nanoparticles in a specific facilitating environment/medium in a container with isothermal or polythermal conditions according to the chemical property of a specific compound. The resulting nanoparticles are hydrophilic with a high surface charge, with hydrophilic groups oriented outward and hydrophobic groups at the core. All nanoparticle stocks at 1% were stored in glycerol for further dilution with sterilized double-distilled water. In addition, a food grade dispersing agent was used in the water nanosuspensions of some nanoparticles as described [7]. The oral rinse formulations were mixed directly with the EC16 nanoparticle stock to 0.01% w/v. EC16 nanoparticle powder was obtained by centrifugation of 0.1% EC16 water nanosuspension at 13,400 RPM for 45 minutes, followed by washing with purified water twice before final centrifugation. Subsequently, pellets were collected and dried. The EC16 nanoparticle powder was then reconstituted with water for analysis.
Evaluation of Particle Size Distribution and Zeta potential
ZetaView nanoparticle tracking analysis was performed according to a method described previously [6 ,7]. The particle size distribution and concentration were measured using the ZetaView X20 (Particle Metrix, Meerbusch, Germany) and corresponding software. The measuring range for particle diameter is 10–2000 nm. All samples were diluted by the same volume of 1× PBS and then loaded into the cell. Particle information was collected from the instrument at 11 different positions across the cell, with two cycles of readings. Standard operating procedure was set to a temperature of 23 °C, a sensitivity of 70, a frame rate of 30 frames per second, and a shutter speed of 100. The post-acquisition parameters were set to a minimum brightness of 20, a maximum area of 1000, a minimum area of 10, and a trace length of 15 [6 ,7].
Electron microscopy imaging of EC16 nanoparticles.
The 1% EC16 nanoparticle stock was diluted with PBS to 0.01% and fixed in 4% paraformaldehyde and 2% glutaraldehyde. After mixing, 5 µl of the sample was removed and transferred to a Formvar/Copper 200 mesh grid and allowed to dry for 15 minutes. Excess solution was then removed using filter paper particles and was negatively stained by addition of 5 µl of 2% aqueous uranyl acetate. Multiple images were captured from each sample in a JEM 1400 Flash Transmission Electron Microscope (JEOL, Peabody, MA) at 120kV, using a Gatan OneView Digital Camera (Gatan Inc., Pleasanton, CA).
Results
EC16 and EC16m nanoparticles.
Three different methods based on FAST were used for preparing the nanoparticles. Method A is the main method that was used for nanoparticle preparations, except EC16 nanoparticles, which were formed by simplified methods Method A1 and Method A2. In addition, a food grade dispersing agent was used in the water nanosuspensions of some nanoparticles as described [6, 7]. Figure 1 shows the size distribution of EC16 nanosuspensions prepared using Method A1 and with a food grade dispersing agent. The median size of the EC16 nanoparticles was 152.5 ± 78.8 nm, with a range of 95 to 218 nm. At 0.01% w/v EC16, the density of the nanoparticles was 3.2x109 particles/ml, and the Zeta potential was -60.11 ± 0.59 mV (Fig 1A). When the dispersing agent was added to the water suspension, the median size of the nanoparticles became 163.8 ± 104.2 nm, ranging from 74 to 435 nm. At 0.01% w/v EC16, the density of the nanoparticles was 2.4x109 particles/ml, and the Zeta potential was -58.08 ± 0.55 mV (Fig 1B). When EC16 nanoparticles were prepared by Method A2, the median size of the nanoparticles was 128.1 ± 65.9 nm, ranging from 66 to 143.9 nm. At 0.01% EC16, the density of the nanoparticles was 1x1010 particles/ml and the Zeta potential was -56.65 ± 0.65 mV (Fig 2A). With the addition of the dispersing agent, the median size of the nanoparticles was 147.7 ± 63.8 nm, ranging from 57.8 to 162.1 nm. At 0.01% EC16, the density of the nanoparticles is 6.5x109 particles/ml, and the Zeta potential was -55.22 ± 0.88 mV (Fig 2B).
Method A was used in all previously published data [5-7, 9] and for EC16m preparation (Fig 3). The median size of EC16m nanoparticles was 115.9 ± 57.5 nm, ranging from 30 to 120.9 nm. At 0.02% EC16m, the density of the nanoparticles was 2.3x1010 particles/ml, with Zeta potential of -50.33 ± 0.98 mV (Fig 3A). With the addition of the dispersing agent, the median size of the nanoparticles was 154.9 ± 77.7 nm, ranging from 65 to 182.5 nm. At 0.03% EC16m, the density of nanoparticles was 4.8x109 particles/ml, and the Zeta potential was -60.56 ± 0.73 mV (Fig 3B).
To test if the nanoparticle powder produced by a FAST method cpuld be easily reconstituted in water, an EC16 water nanosuspension at 0.1% prepared by Method A2 was condensed and dried, producing a white powder. The dry EC16 nanoparticles were then reconstituted in double distilled water as a nanosuspension. At 0.01% EC16, the density of the particles reached 1x1010 particles/ml, with median size of 141.4 ± 105.4 nm and size range from 49 to 178.9 nm (Fig 5B). The size distribution was comparable to that of EC16 nanoparticles prepared by Method A2 (Fig 2), as was the Zeta potential.
CBD nanoparticles.
The median size of CBD nanoparticles was 206 ± 103.4 nm, ranging from 147 to 438 nm. At 0.06% CBD, the density of the nanoparticles was 4.7x108 particles/ml and the Zeta potential was -51.64 ± 0.85 mV (Fig 4A). With inclusion of 1% w/v of the dispersing agent, the median size of nanoparticles was 222.7 ± 135.3 nm, ranging from 69 to 271.9 nm. At 0.06% CBD, the density of nanoparticles was 4.7x108 particles/ml and the Zeta potential was -48.09 ± 0.14 mV (Fig 4B).
THC-9 and reconstituted EC16 nanoparticles.
The median size of the THC-9 nanoparticles was 232.3 ± 151.3 nm, ranging from 149 to 605 nm. At 0.01% THC-9 the density of the nanoparticles was 2.2x108 particles/ml, and the Zeta potential was -38 ± 0.51 mV (Fig 5A). The median size of the water-reconstituted EC16 nanoparticles was 141.4 ± 105.4 nm, ranging from 49 to 178.9 nm. At 0.01% EC16 the density of nanoparticles was 1010 particles/ml and Zeta potential was -56 ± 0.58 mV (Fig 5B).
Quercetin nanoparticles
The median size of quercetin nanoparticles was 163.8 ± 102.2 nm, ranging from 40 to 269.8 nm. At 0.02% w/v quercetin the density of the nanoparticles was 3.2x109 particles/ml, and the Zeta potential was -62 ± 1.0 mV (Fig 6A). With 1% w/v of the dispersing agent the median size of nanoparticles was 184.9 ± 119.6 nm, ranging from 69.6 to 256 nm. At 0.02% w/v quercetin the density of nanoparticles was 1.9x109 particles/ml and the Zeta potential was -38.4 ± 0.78 mV (Fig 6B).
Ivermectin nanoparticles
The median size of ivermectin nanoparticles was 176 ± 112.1 nm, ranging from 115 to 420.7 nm. At 0.02% w/v ivermectin the density of the nanoparticles is 3.5x108 particles/ml with Zeta potential of -52.32 ± 0.69 mV (Fig 7A). With 1% w/v of the dispersing agent the median size of nanoparticles was 160.6 ± 90.4 nm, ranging from 98 to 344.5 nm. At 0.02% w/v ivermectin, the density of nanoparticles was 5x108 particles/ml and the Zeta potential was -53.92 ± 1.49 mV (Fig 7B).
Retinoic acid nanoparticles
The median size of retinoic acid nanoparticles was 147 ± 155.4 nm, ranging from 75.5 to 341.5 nm. At 0.02% w/v retinoic acid, the density of nanoparticles was 3.7x108 particles/ml, and the Zeta potential was -48.34 ± 1.07 mV (Fig 8A). With 1% w/v of the dispersing agent the median size of nanoparticles was 162.8 ± 107.8 nm, ranging from 120.3 to 407.3 nm. At 0.02% w/v retinoic acid the density of nanoparticles was 7.2x108 particles/ml and the Zeta potential was -53.99 ± 1.50 mV (Fig 8B).
Paclitaxel nanoparticles
The median size of paclitaxel nanoparticles was 119 ± 111.0 nm, ranging from 117.3 to 265.8 nm. At 0.01% w/v paclitaxel, the density of nanoparticles was 3.2x108 particles/ml, and the Zeta potential was -49.5 ± 2.13 mV (Fig 9A). With 1% w/v of the dispersing agent the median size of nanoparticles was 130.1 ± 138.2 nm, ranging from 42.3 to 432.6 nm. At 0.01% w/v paclitaxel the density of nanoparticles was 3.9x108 particles/ml and the Zeta potential was -42.27 ± 1.40 mV (Fig 9B).
Fluconazole nanoparticles
The median size of fluconazole nanoparticles was 150.3 ± 290.5 nm, ranging from 84.9 to 390 nm. At 0.01% fluconazole w/v, the density of the nanoparticles was 2.0x108 particles/ml (Fif 10A). The Zeta potential of the nanoparticles was -44.50 ± 0.69 mV (Fig 10B).
Medroxyprogesterone acetate and triamcinolone acetonide nanoparticles
The median size of medroxyprogesterone acetate nanoparticles was 187.9 ± 80.9 nm, ranging from 105.7 to 303.6 nm. At 0.01% w/v medroxyprogesterone acetate, the density of nanoparticles was 4.65x108 particles/ml, and the Zeta potential was -53.21 ± 0.99 mV (Fig 11A). The median size of triamcinolone acetonide nanoparticles was 217.8 ± 152.8 nm, ranging from 152.7 to 315.2 nm. At 0.01% w/v triamcinolone acetonide the density of nanoparticles was 3.9x107 particles/ml and the Zeta potential was -47.28 ± 0.23 mV (Fig 11B).
EC16 nanoparticles in two water-based oral rinse formulations
The median size of the nanoparticles in an unflavored oral rinse formulation was 176.7 ± 184.4 nm, ranging from 81 to 268 nm. At 0.01% w/v EC16 the density of the nanoparticles was 1.6x109 particles/ml and the Zeta potential was -39 ± 1.35 mV (Fig 12A). In the peppermint flavored oral rinse formulation, the median size of nanoparticles was 121.6 ± 63.6 nm, ranging from 103 to 126.9 nm. At 0.01% w/v EC16, the density of nanoparticles was 8.6x108 particles/ml with Zeta potential of -51.54 ± 0.01 mV (Fig 12B).
Transmission electron microscopy image of EC16 nanoparticles
Figure 11 shows a representative TEM image of an EC16 nanoparticle suspension fixed in 4% paraformaldehyde and 2% glutaraldehyde. The particle sizes ranged from approximately 100 nm to >300 nm, which was consistent with the EC16 nanoparticles prepared by Method A [4-6].

Figure 1: Size and distribution of EC16 nanoparticles. A. Preparation Method A1. The median size of the nanoparticles was 152.5 ± 78.8 nm, with a range from 95 to 218 nm. At 0.01% EC16, the density of the nanoparticles was 3.2x 109 particles/ml. The Zeta potential was -60.11 ± 0.59 mV. B. Preparation Method A1 with addition of a food-grade dispersing agent. The median size of the nanoparticles was 163.8 ± 104.2 nm, with a range from 74 to 435 nm. At 0.01% EC16, the density of the nanoparticles was 2.4x109 particles/ml. The Zeta potential was -58.08 ± 0.55 mV.

Figure 2: Size and distribution of EC16 nanoparticles. A. Preparation Method A2. The median size of the nanoparticles was 128.1 ± 65.9 nm, with a range from 66 to 143.9 nm. At 0.01% EC16, the density of the nanoparticles was 1x1010 particles/ml. The Zeta potential was -56.65 ± 0.65 mV. B. Preparation Method A2 with addition of a food-grade dispersing agent. The median size of the nanoparticles was 147.7 ± 63.8 nm, with a range from 57.8 to 162.1 nm. At 0.01% EC16, the density of the nanoparticles was 6.5x109 particles/ml. The Zeta potential was -55.22 ± 0.88 mV.

Figure 3: Size and distribution of EC16m nanoparticles. A. Preparation Method A. The median size of the nanoparticles was 115.9 ± 57.5 nm, ranging from 30 to 120.9 nm. At 0.02% EC16m, the density of the nanoparticles was 2.3x1010 particles/ml. The Zeta potential was -50.33 ± 0.98 mV. B. Preparation Method A with addition of a food-grade dispersing agent. The median size of the nanoparticles was 154.9 ± 77.7 nm, ranging from 65 to 182.5 nm. At 0.03% EC16m, the density of nanoparticles was 4.8x109 particles/ml. The Zeta potential was -60.56 ± 0.73 mV.

Figure 4: Size and distribution of CBD nanoparticles. A. Preparation Method A. The median size of the nanoparticles was 206 ± 103.4 nm, ranging from 147 to 438 nm. At 0.06% CBD, the density of the nanoparticles was 4.7x108 particles/ml. The Zeta potential was -51 ± 0.85 mV. B. Preparation Method A with addition of a food-grade dispersing agent. The median size of the nanoparticles was 222.7 ± 135.3 nm, ranging from 69 to 271.9 nm. At 0.06% CBD, the density of nanoparticles was 4.7x108 particles/ml. The Zeta potential was -48.09 ± 0.14 mV.

Figure 5: Size and distribution of THC-9 nanoparticles and EC16 nanoparticles reconstituted from died powder. A. Preparation Method A. The median size of the THC-9 nanoparticles was 232.3 ± 151.3 nm, ranging from 149 to 605 nm. At 0.01% w/v THC-9, the density of the nanoparticles was 2.2x108 particles/ml. The Zeta potential was -38 ± 0.51 mV. B. Preparation Method A. The median size of the water-reconstituted EC16 nanoparticles was 141.4 ± 105.4 nm, ranging from 49 to 178.9 nm. At 0.01% EC16, the density of nanoparticles was 1x1010 particles/ml. The Zeta potential was -56 ± 0.58 mV.

Figure 6: Size and distribution of quercetin nanoparticles. A. Preparation Method A. The median size of the nanoparticles was 163.8 ± 102.2 nm, ranging from 40 to 269.8 nm. At 0.02% quercetin, the density of the nanoparticles was 3.2x109 particles/ml. The Zeta potential was -62 ± 1.0 mV. B. Preparation Method A with addition of a food-grade dispersing agent. The median size of the nanoparticles was 184.9 ± 119.6 nm, ranging from 69.6 to 256 nm. At 0.02% quercetin, the density of nanoparticles was 1.9x109 particles/ml. The Zeta potential was -38.4 ± 0.78 mV.

Figure 7: Size and distribution of Ivermectin nanoparticles. A. Preparation Method A. The median size of the nanoparticles is 176 ± 112.1 nm, ranging from 115 to 420.7 nm. At 0.02% ivermectin, the density of the nanoparticles is 3.5x108 particles/ml. Zeta potential is -52.32 ± 0.69 mV. B. Preparation Method A with addition of a food-grade dispersing agent. The median size of nanoparticles is 160.6 ± 90.4 nm, ranging from 98 to 344.5 nm. At 0.02% ivermectin, the density of nanoparticles is 5x108 particles/ml. Zeta potential is -53.92 ± 1.49 mV.

Figure 8: Size and distribution of retinoic acid nanoparticles. A. Preparation Method A. The median size of the nanoparticles was 147 ± 155.4 nm, ranging from 75.5 to 341.5 nm. At 0.02% retinoic acid, the density of the nanoparticles was 3.7x108 particles/ml. The Zeta potential was -48.34 ± 1.07 mV. B. Preparation Method A with addition of a food-grade dispersing agent. The median size of the nanoparticles was 162.8 ± 107.8 nm, ranging from 120.3 to 407.3 nm. At 0.02% retinoic acid, the density of the nanoparticles was 7.2x108 particles/ml. The Zeta potential was -53.99 ± 1.50 mV.

Figure 9: Size and distribution of paclitaxel nanoparticles. A. Preparation Method A. The median size of paclitaxel nanoparticles was 119 ± 111.0 nm, ranging from 117.3 to 265.8 nm. At 0.01% w/v paclitaxel, the density of nanoparticles was 3.2x108 particles/ml, and the Zeta potential was -49.55 ± 2.13 mV. B. Preparation Method A with addition of a food-grade dispersing agent. The median size of nanoparticles was 130.1 ± 138.2 nm, ranging from 42.3 to 432.6 nm. At 0.01% w/v paclitaxel the density of nanoparticles was 3.9x108 particles/ml and the Zeta potential was -42.27 ± 1.40 mV.
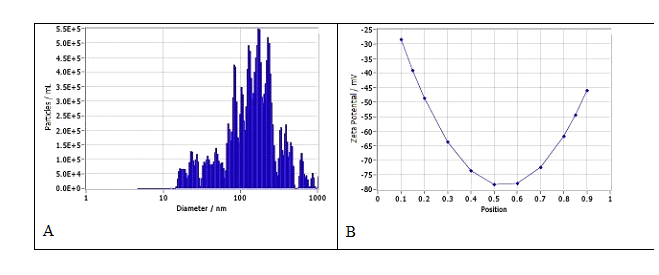
Figure 10: Size, distribution, and Zeta potential of fluconazole nanoparticles in water suspension. A. Size and distribution. The median size of the nanoparticles was 150.3 ± 290.5 nm, ranging from 84.9 to 390 nm. At 0.01% fluconazole w/v, the density of the nanoparticles was 2.0x108 particles/ml. B. The Zeta potential of the nanoparticles was -44.50 ± 0.69 mV.

Figure 11: Size and distribution of medroxyprogesterone acetate and triamcinolone acetonide nanoparticles in water suspension. A. The median size of medroxyprogesterone acetate nanoparticles was 187.9 ± 80.9 nm, ranging from 105.7 to 303.6 nm. At 0.01% w/v medroxyprogesterone acetate, the density of nanoparticles was 4.65x108 particles/ml, and the Zeta potential was -53.21 ± 0.99 mV. B. The median size of triamcinolone acetonide nanoparticles was 217.8 ± 152.8 nm, ranging from 152.7 to 315.2 nm. At 0.01% w/v triamcinolone acetonide the density of nanoparticles was 3.9x107 particles/ml and the Zeta potential was -47.28 ± 0.23 mV.

Figure 12: Size and distribution of EC16 nanoparticles in two water-based oral rinse formulations. A. Unflavored oral rinse. The median size of the nanoparticles was 176.7 ± 184.4 nm, ranging from 81 to 268 nm. At 0.01% EC16 w/v, the density of the nanoparticles was 1.6x109 particles/ml. The Zeta potential was -39 ± 1.35 mV. B. Peppermint oral rinse. The median size of nanoparticles was 121.6 ± 63.6 nm, ranging from 103 to 126.9 nm. At 0.01% EC16 nanoparticles, the density of nanoparticles was 8.6x108 particles/ml. The Zeta potential was -51.54 ± 0.01 mV.

Figure 13: Representative transmission electron microscopy image of EC16 nanoparticles.
Discussion
FAST is a relatively simple tool to prepare nanoparticles/nanocrystals without the use of sophisticated technology or agents that could cause adverse effects. We previously reported the use of FAST Method A to generate EC16 and EC16m nanoparticles for nasal aqueous formulations [5-7]. To explore approaches to further simplify the method, specific proprietary Methods A1 and A2 were used for EC16 nanoparticle preparation. In addition, an FDA approved, commonly used food additive dispersing agent (proprietary, patent pending) was tested with the suspensions. Fig 1A shows the size distribution of EC16 nanoparticles in water suspension; Fig 1B is the size distribution of the water suspension with the dispersing agent at 1% w/v. There was no statistical difference in median size between the two formulations. However, the dispersing agent altered the size range, producing smaller particles (~74 nm, 10.1%) and some larger particles (~435 nm, 3.6%), compared to the EC16 nanosuspension without the dispersing agent (95–218 nm). Both nanosuspensions had excellent stability with Zeta potential at about -60 mV (a Zeta Potential value above +30 mV or below −30 mV is generally considered stable). Therefore, FAST Method A1 was a suitable method to prepare EC16 nanoparticles. Method A2 is another simplified method derived from Method A. As shown in Fig 2, the EC16 nanoparticles have a median size of 128.1 ± 65.9 nm, with a range of 66 to 143.9 nm (Fig 2A), while the dispersing agent resulted in a slightly larger median size of 147.7 ± 63.8 nm, with similar size range 57.8 to 162.1 nm (Fig 2B). It appears that the dispersing agent had little effect on the nanoparticle size, and the charges measured by Zeta potentials were similar to each other (-56.65 ± 0.65 mV vs. -55.22 ± 0.88 mV), although slightly lower than those for particles produced by Method A1. Also, both Method A2 suspensions had a narrower size range than that of EC16 nanosuspensions made with Method A1 (Fig 1), suggesting Method A2 can be used for producing EC16 nanoparticles with smaller range in size.
In summary, both Methods A1 and A2, simplified versions of Method A, were capable of producing EC16 nanoparticles with high stability in terms of high surface charges, consistent with previously published data using Method A. It is important to note that all three methods are simple, economical, require a short time (<30 min), and little equipment.
Method A was used to prepare EC16m nanoparticles, as well nanosuspensions for all other compounds. As shown in Fig 3, Method A was able to produce EC16m nanoparticles with a narrow range (30 to 120.9 nm) (Fig 3A), compared to the nanoparticles in dispersing agent suspension (65 to 182.5 nm) (Fig 3B). The median size of particles was smaller in the suspension without dispersing agent (115.9 ± 57.5 nm) in comparison to the suspension with the dispersing agent (154.9 ± 77.7 nm). Interestingly, the EC16 nanosuspension without dispersing agent had substantially more nanoparticles than the nanosuspension with the dispersing agent (2.3x1010 vs. 4.6x109/ml). However, it appears that the EC16m nanosuspension with the dispersing agent was potentially more stable, a with Zeta potential exceeding -60 mV. On the other hand, both nanosuspensions were potentially very stable, with Zeta potential greater than -50 mV. We chose 0.02% EC16m and EC16 based on the previously tested nasal application formulations [5], and two ongoing animal studies, all of which have this concentration of EC16m or EC16, and which did not show mucociliary toxicity [5] of adverse effect in experimental mice (data not shown, study ongoing).
For CBD nanoparticles (Fig 4), the dispersing agent reduced the range of particle size (69 to 271 nm vs. 147 to 438 nm) but somewhat increased the median size, although without statistical difference (232.3 ± 135.3 vs. 206 ± 103.4 nm). Interestingly, the two CBD nanosuspensions with 0.06% CBD have identical particle density of 4.7x108 particles/ml, and a potentially high stability with a Zeta potential around -50 mV (Fig 4). Compared to the EC16 nanoparticles, the CBD nanoparticles were larger in diameter, leading to a lower particle density. The stability of the two compounds in terms of surface charges in the nanosuspensions are similarly high. A noticeable difference was found in the nanosuspension of THC-9 without the dispersing agent. As shown in Fig 5A, the median size of the particles (232.3 ± 151.3 nm) was similar to that of CBD. However, unlike the other compounds tested, the size distribution was discontinued, with four discrete subpopulations (Fig 5A). Although the nanosuspension was stable with a Zeta potential at -38 ± 0.51, this charge was significantly lower than that of other compounds. However, it is important to note that the THC-9 sample was in a methanol solution at a very low concentration, which was different from other compounds that were obtained in powdered form. It is known that there are different nanotechnology methods to produce nanoparticles of both CBD and THC [10]. The FAST-generated nanoparticles could provide an alternative approach.
The properties of the reconstituted suspension prepared from dried EC16 nanoparticles were similar to those of the original suspension. This result demonstrates that EC16 nanoparticles can be condensed to a powder form and reconstituted in aqueous suspensions or in dry delivery forms. This process could be used for other compounds if dry powder form is preferred. A number of nanotechnologies have been applied to generate nanoparticles of quercetin, a flavonoid with poor solubility but the potential to benefit human health. Previous studies used lipid-based nanocarriers, polymer-based nanocarriers, micelles and hydrogels composed of natural or synthetic polymers to produce nanoparticles of quercetin [11]. Fig 6 demonstrates that quercetin is suitable for nanosuspension preparation using Method A. The results from the two nanosuspensions indicate that the dispersing agent caused the suspension to have a significantly reduced surface charge, potentially decreasing stability (Zeta potential of -38.4 mV vs. -62 mV), and associated with decreased particle density (1.9x109 vs. 3.2x109/ml at 0.02%) in comparison to the nanosuspension without the dispersing agent. These differences suggest that the dispersing agent may not be beneficial to every compound in terms of stability and particle size.
Ivermectin was initially used as a veterinary medicine for treating parasite infections, but consistently faced limitations due to its poor water solubility and low bioavailability. Various strategies have been applied to increase the solubility of this drug, including lipid-based, polymer-based, drug-loaded nanoparticles, and nanostructured carriers [12]. In the current study, the results demonstrate that both tested nanosuspensions of ivermectin had identical Zeta potential (Fig 7). The particle size distributions appeared different. In the nanosuspension with the dispersing agent only 14.2% of particles had diameters greater than 200 nm, while the counterpart has more than 35% particles with diameters greater than 200 nm. This effect of the dispersing agent resulted in significantly more particles (5x108/ml) in the presence of the dispersing agent compared to the suspension without the agent (3.5x108/ml) at 0.02% w/v ivermectin (Fig 7). Therefore, the addition of the dispersing agent is dependent on the specific compound for its effect. Retinoids present considerable potential to treat multiple conditions, but one of the major challenges to their use is their low solubility. Attempts to produce nanoparticles of retinoic acid have been reported, including encapsulation in other nanoparticles, micelles, liposomes, films, or by attaching to a carrier [13]. Retinoic acid has a hydrocarbon chain and a 6-carbin ring, as an amphipathic compound similar to other compounds tested. Despite the carboxylic acid group is charged at pH 7, retinoic acid is hydrophobic with poor water solubility. The current study demonstrates that these chemical properties did not prevent retinoic acid from being self-assembled into nanoparticles using FAST Method A (Fig 8). The dispersing agent gave a similar Zeta potential (-54 mV) to that of nanosuspension without the dispersing agent (-48.34 mV). Another advantage of using the dispersing agent is that the particle density almost doubled (7.2x108 vs. 3.7x108/ml) at 0.02% concentration, by shifting the distribution from larger to smaller particles as seen in Fig 8.
Paclitaxel, a cancer drug with poor solubility, is able to self-assemble to form nanoparticles using Method A (Fig 9). In water suspension, the median size of paclitaxel nanoparticles was 119 nm, ranging from 117.3 to 265.8 nm. At 0.01% w/v paclitaxel, the density of nanoparticles was 3.2x108 particles/ml, and the Zeta potential was -49.55 mV (Fig 9A). An interesting observation is that the size distribution of nanoparticles is narrow, with approximately 90% particles around the major peak of 117.3 nm (Fig 9A). The Zeta potential of the suspension indicates the surface of the surface of the particles are also negatively charged, and provides strong electrostatic repulsion among the particles, leading to higher stability of the suspension. The addition of the food-grade dispersing agent has a slightly lower Zeta potential (-42.27 mV) but higher particle density (3.9x108). Currently used FDA approved Paclitaxel formulations include a formulation of 50:50 mix of ethanol and a polyoxyethylated castor oil (Taxol), a formulation involving human serum albumin (Abraxane), and a liposome formulation containing lecithin and cholesterol (Lipusu) [14]. Our result suggests that FAST technology could provide another option for paclitaxel (and docetaxel) formulations with self-assembled nanoparticles associated with strong surface charge.
Fluconazole is the first of a new subclass of synthetic triazole antifungal drugs with relatively low water solubility. Fluconazole is used to treat serious fungal or yeast infections, such as oropharyngeal candidiasis (thrush, oral thrush) and vaginal candidiasis through oral or intravenous routes. It is also used in children on life support called extracorporeal membrane oxygenation (ECMO). Nanoparticle fluconazole prepared via FAST exhibit potential advantages over tablets, solutions and suspensions, including enhanced solubility, possibly improved bioavailability, lower dosing requirements, versatile delivery options (e.g., topical, nasal), and simpler manufacturing. Its stable nanosuspension (150.3 nm, -44.50 mV Zeta potential, Fig 10) could enable faster dissolution and better tissue penetration, particularly for localized or mucosal infections, while reducing gastrointestinal side effects. Especially, the nanoparticle form of fluconazole may be used in novel applications (e.g., topical treatment of resistant fungal strains) or in patients with absorption challenges.
Both medroxyprogesterone acetate (MPA) and triamcinolone acetonide (TA) are hydrophobic steroids with poor water solubility. FAST allows enhanced solubility and penetration could achieve therapeutic effects at lower doses, reducing risks from side effects. Systemic delivery of MPA via tablets (Provera) or injections (Depo-Provera) leads to off-target effects (e.g., bone density loss with long-term use). Nanoparticle form of MPA with the small size and hydrophilic surface (187.9 ± 80.9 nm, -53.21 ± 0.99 mV, Fig 11A) could enable targeted delivery to reproductive tissues or tumors (e.g., endometrial cancer) if combined with ligands or carriers. The localized nanosuspensions could reduce systemic side effects, offering a safer profile for long-term use compared to tablets. In addition, MPA nanoparticles allow it for oral nanosuspensions, topical gels, transdermal patches, or nasal sprays. TA is currently available as injectable suspensions, topical creams/ointments, nasal sprays, and intra-articular injections for inflammation, arthritis, or allergies. Its poor water solubility (~80 µg/mL) limits formulation flexibility and dissolution rates, making them ideal candidates for FAST modifications, as demonstrated by their stable nanosuspension (217.8 ± 152.8 nm, -47.28 ± 0.23 mV; Figure 11B). TA nanoparticles’ small size and hydrophilic surface could improve penetration through skin, mucosa, or joint tissues, increasing local bioavailability. For example, topical nanosuspensions might achieve higher dermal concentrations than creams, reducing application frequency and injections could offer tunable release (vs. microcrystals), balancing acute and chronic needs. Other potential benefits localized delivery, including ocular nanosuspensions, which could treat uveitis with higher corneal penetration than creams, while inhaled forms might target lung inflammation, offering alternatives to systemic injections. Localized delivery could reduce systemic exposure compared to current forms.
To examine the feasibility of using EC16 nanoparticles in oral care products we initially tested 0.05% and 0.005% w/v of EC16 nanoparticles in an unflavored oral rinse product containing erythritol, provided by International Nutrition, Inc. Both concentrations were stable and compatible with the oral rinse with a particle size range of 40.7 to 251.4 nm, and median size of 170.6 ± 97.3 nm (data not shown). To further investigate the feasibility of EC16 nanoparticles, the 1% EC16 stock was directly added to two oral rinse products containing xylitol. One oral rinse also contains natural peppermint flavor. As shown in Fig 12A, the unflavored oral rinse with EC16 nanoparticles has a similar size distribution to the previously tested unflavored product, with more than 60% particles under 200 nm, resulting in a high density of 1.6x109 particles/ml at 0.01% EC16. In contrast, the peppermint oral rinse with EC16 nanoparticles had significantly higher surface charges, with Zeta potential of -51.54 mV vs. -39 mV of the unflavored oral rinse. In addition, the particle range was narrow, with most particles at around 100 to 130 nm range (Fig 12B). These results demonstrate that EC16 nanoparticles can be easily incorporated into aqueous products with high surface charges. Transmission electron microscopy was performed to investigate the EC16 nanoparticle structure, shape, and size, after the self-assembling process. As shown in Fig 13, the rounded nanoparticles showed high polydispersity, with diameters ranging from approximately 100 nm to >300 nm, consistent with the ZetaView results. The characteristics of EC16 nanoparticles were described in a recent publication [9]. It is postulated that other compounds in the current study would have similar particle structures and characteristics, pending future studies. The limitations of FAST include 1: It is only for hydrophobic and poorly soluble molecules with chemical structures suitable to self-assembly into nanoparticles/nanocrystals; 2: it may not be suitable for large compounds that are soluble in aqueous solutions.
Conclusions
The invention of the Facilitated Self-assembling Technology (FAST) would allow compounds with poor solubility and bioavailability to form nanoparticles by themselves to either suspend in an aqueous formulation or in a dried powder form for a variety of delivery methods such as oral, nasal, topical, injectable, inhalable, etc. Nanoparticles produced by methods of FAST are easy, rapid, low cost, stable with high surface charges, and highly organized without addition of surfactant or stabilizer. FAST can be used in drug development and drug improvement, as well as for healthcare, disease control and prevention, cosmetic, and consumer products, pending future studies.
Author Contributions: Conceptualization, S.H., D.D.; methodology, S.H., N.F., Zeta View validation, Y.L., H.Y., J.C. and N.F.; data analysis, S.H., D.D., N.F.; investigation, N.F., D.D., and S.H.; re-sources, Y.L.; data curation, S.H.; writing—original draft preparation, S.H.; writing—review and editing, N.F.; D.D.; visualization, N.F.; supervision, S.H. and D.D.; project administration, S.H.; funding acquisition, S.H., D.D. All authors have read and agreed to the published version of the manuscript.
Funding: This work was funded by a grant from the National Institute on Deafness and Other Communication Disorders (NIDCD) (1R41DC020678-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments: The authors want to thank Dr. Brenden Marshal for TEM work and support from Augusta University Research Institute and Office of Innovation Commercialization.
Conflicts of Interest: The authors declare no conflicts of interest.
References
- Ogden J, Parry-Billings M. Nanotechnology approaches to solving the problems of poorly water-soluble drugs. Drug Discov 6 (2005): 71–76.
- Kumari L, Choudhari Y, Patel P, et al. Advancement in Solubilization Approaches: A Step towards Bioavailability Enhancement of Poorly Soluble Drugs. Life (Basel) 13 (2023): 1099.
- Bhalani DV, Nutan B, Kumar A, et al. Bioavailability Enhancement Techniques for Poorly Aqueous Soluble Drugs and Therapeutics. Biomedicines 10 (2022): 2055.
- Liu J, Liangxing Tu L, Cheng M, et al. Mechanisms for oral absorption enhancement of drugs by nanocrystals Journal of Drug Delivery Science and Technology 56 (2020).
- Frank N, Dickinson D, Garcia W, et al. Feasibility Study of Developing a Saline-Based Antiviral Nanoformulation Containing Lipid-Soluble EGCG: A Potential Nasal Drug to Treat Long COVID. Viruses 16 (2024): 196.
- Frank N, Dickinson D, Lovett G, et al. Evaluation of Novel Nasal Mucoadhesive Nanoformulations Containing Lipid-Soluble EGCG for Long COVID Treatment. Pharmaceutics 16 (2024): 791.
- Frank N, Dickinson D, Garcia W, et al. Evaluation of Aqueous Nanoformulations of Epigallocatechin-3-Gallate-Palmitate (EC16) Against Human Coronavirus as a Potential Intervention Drug. Biomed J Sci & Tech Res 50 (2023): 2023.
- Hurst BL, Dickinson D, Hsu S. Epigallocatechin-3-Gallate (EGCG) Inhibits Sars-Cov-2 Infection in Primate Epithelial Cells. Microbiol Infect Dis 5 (2021): 1-6.
- Frank N, Dickinson D, Dudish D, et al. Potential Therapeutic Use of EGCG-Palmitate Nanoparticles for Norovirus Infection. Biomed J Sci & Tech Res 59 (2024): 2024.
- Rebelatto ERL, Rauber GS, Caon T. An update of nano-based drug delivery systems for cannabinoids: Biopharmaceutical aspects & therapeutic applications. International Journal of Pharmaceutics 635 (2023): 122727.
- Tomou EM, Papakyriakopoulou P, Elmina-Marina Saitani EM, et al. Recent Advances in Nanoformulations for Quercetin Delivery. Pharmaceutics 15 (2023): 1656.
- Maiara Callegaro Velho MC, Funk NL, Deon M, et al. Ivermectin-Loaded Mesoporous Silica and Polymeric Nanocapsules: Impact on Drug Loading, In Vitro Solubility Enhancement, and Release Performance. Pharmaceutics 16 (2024): 325.
- Napoli RFJ, Tariq Enver, Liliana Bernardino L, et al. Advances and challenges in retinoid delivery systems in regenerative and therapeutic medicine. Nature Communications 11 (2020): 4265.
- Haddad, R.; Alrabadi, N.; Altaani, B.; Li, T. Paclitaxel Drug Delivery Systems: Focus on Nanocrystals' Surface Modifications. Polymers (Basel) 14 (2022): 658.


 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 