Mito-Nutraceutical Potential of Plant Secondary Metabolites
Subramani Paranthaman Balasubramani*, Skyler A. Armstead, Adreanna T. Buggs, Myla S. Bell
Department of Natural Sciences, Albany State University, Albany, GA 31705 USA
*Corresponding author: Subramani Paranthaman Balasubramani, Department of Natural Sciences, Albany State University, Albany, GA 31705 USA
Received: 22 February 2025; Accepted: 03 February 2025; Published: 20 March 2025
Article Information
Citation: Subramani Paranthaman Balasubramani, Skyler A. Armstead, Adreanna T. Buggs, Myla S. Bell. Mito-Nutraceutical Potential of Plant Secondary Metabolites Fortune Journal of Health Sciences, 8 (2025): 217-244.
View / Download Pdf Share at FacebookAbstract
Mitochondria, the cellular powerhouses, are essential for energy production and various metabolic processes. This review explores the potential of plant secondary metabolites (PSMs) as nutraceuticals to modulate mitochondrial structure and function. Mitochondrial dynamics, including fission, fusion, biogenesis, and mitophagy, are crucial for maintaining cellular homeostasis. Dysregulation of these processes is linked to diseases such as neurodegenerative disorders, metabolic syndromes, cancer and aging. PSMs, including polyphenols, alkaloids, terpenoids, and flavonoids, form a part of our nutrition and can influence mitochondrial function through multiple pathways. PSMs enhance mitochondrial efficiency, stimulate biogenesis, and reduce oxidative stress by activating key signaling pathways like AMP-activated protein kinase (AMPK), sirtuins, and nuclear factor erythroid 2-related factor 2 (Nrf2). These compounds also stabilize mitochondrial membranes, protect against oxidative damage, and support mitochondrial quality control mechanisms. By modulating mitochondrial dynamics and reducing oxidative stress, PSMs offer promising avenues for improving metabolic health and combating degenerative diseases. However, challenges such as variability in PSM content, dosage for targeted delivery and interactions with other dietary components must be addressed. Future research should focus on developing targeted delivery systems, such as PSM-loaded nanoparticles, to enhance bioavailability and to maximize therapeutic efficacy. In summary, PSMs represent a valuable resource for mitochondrial therapeutics, with the potential to revolutionize approaches to health and disease management. Understanding the molecular mechanisms by which PSMs modulate mitochondrial function can lead to innovative strategies for enhancing cellular resilience and metabolic efficiency.
Keywords
<p>Plant Secondary Metabolites; Nutraceuticals; Mitochondrial dynamics; Aging; Chronic diseases; Nanoparticles</p>
Article Details
Introduction
Mitochondria, often referred to as the "powerhouses of the cell," are vital cellular organelles found in nearly all eukaryotic organisms. These tiny, double-membraned structures play a central role in cellular energy production by generating adenosine triphosphate (ATP), the primary energy currency of the cell. Their presence is crucial for a variety of metabolic processes that sustain life, from muscle contraction to cell division and beyond. Structurally, mitochondria are characterized by an outer membrane that encapsulates the organelle and an intricately folded inner membrane, forming structures called cristae. These cristae increase the surface area available for chemical reactions, particularly those involved in the electron transport chain and oxidative phosphorylation, the processes that drive ATP production. The matrix, the innermost compartment, houses enzymes, mitochondrial DNA (mtDNA), and ribosomes, enabling mitochondria to produce some of their own proteins.
Interestingly, mitochondria are unique among organelles because they possess their own DNA, inherited maternally, and are capable of reproducing independently within the cell. This autonomy hints at their evolutionary origins as free-living prokaryotes that entered into a symbiotic relationship with ancestral eukaryotic cells. This theory, known as the endosymbiotic theory, explains their distinct genetic and structural characteristics.Beyond energy production, mitochondria are involved in various essential cellular functions. They regulate calcium signaling, contribute to the synthesis of certain biomolecules, and play a pivotal role in programmed cell death, or apoptosis, which is crucial for development and disease prevention [1]. Understanding mitochondria is fundamental to exploring health and disease, as mitochondrial dysfunction has been implicated in a range of conditions, from neurodegenerative disorders like Parkinson's and Alzheimer's to metabolic syndromes and aging [2]. This multifaceted organelle continues to captivate researchers, underscoring its importance in biology and medicine.
MITOCHONDRIAL DYNAMICS: A BALANCING ACT OF CELLULAR LIFE
Mitochondrial dynamics encompass the processes of fission, fusion, biogenesis, and mitophagy, which collectively maintain mitochondrial structure, function, and distribution [2]. These dynamic processes are essential for cellular homeostasis, enabling mitochondria to adapt to physiological demands, repair damage, and respond to stress. Their balance is crucial for health, and dysregulation is implicated in various diseases [3, 4, 5]. Fusion allows mitochondria to merge, forming networks that enhance their functional capacity and facilitate the exchange of mitochondrial DNA, proteins, and metabolites. This process mitigates the effects of mitochondrial DNA mutations and ensures the uniform distribution of metabolic components. Key proteins involved in fusion include mitofusins (Mfn1, Mfn2) on the outer membrane and optic atrophy protein 1 (OPA1) on the inner membrane [6]. Fission, conversely, enables mitochondrial division, critical for proper distribution during cell division, isolation of damaged regions, and mitophagy. The protein dynamin-related protein 1 (Drp1) orchestrates fission by recruiting to mitochondrial sites to mediate division. Disruptions in the balance of fusion and fission can lead to dysfunctional mitochondrial networks, impairing cellular function [6].
Biogenesis, regulated by the master activator PGC-1α, ensures the formation of new mitochondria to meet energy demands and replace damaged ones. It involves the replication of mitochondrial DNA, synthesis of proteins, and integration of lipids [4]. Mitophagy, a quality control mechanism, eliminates dysfunctional mitochondria. Proteins such as PINK1 and Parkin signal for the autophagic degradation of damaged organelles, preventing the accumulation of reactive oxygen species (ROS) and inflammation [7]. Impaired mitochondrial dynamics contribute to neurodegenerative diseases, metabolic syndromes, cardiovascular disorders, and aging [6]. Targeting the proteins involved in these processes offers therapeutic potential for correcting mitochondrial dysfunction. Mitochondrial dynamics represent a finely tuned regulatory system critical to cellular health and disease intervention strategies.
THE ROLE OF MITOCHONDRIA IN AGING AND DEGENERATIVE DISEASES
Mitochondria are central to aging, degenerative diseases, and cancer progression due to their roles in energy production, cellular homeostasis, and oxidative stress regulation. Aging is associated with mitochondrial dysfunction, characterized by reduced ATP production, increased reactive oxygen species (ROS), and mitochondrial DNA (mtDNA) mutations [8]. This contributes to cellular damage, tissue dysfunction, and age-related diseases. Oxidative stress, driven by excess ROS, damages proteins, lipids, and DNA, creating a cycle of mitochondrial damage and ROS production that accelerates aging and increases vulnerability to degenerative conditions [7, 9]. Mitochondrial dysfunction is a hallmark of neurodegenerative diseases, such as Alzheimer’s and Parkinson’s, where impaired mitophagy, mitochondrial fragmentation, and defective energy metabolism lead to neuronal death [2, 7].
For example, mutations in PINK1 and Parkin, key regulators of mitophagy, are linked to mitochondrial accumulation in Parkinson’s disease. Similarly, metabolic syndromes, cardiovascular diseases, and sarcopenia are associated with mitochondrial dysfunction in aging [2, 10]. Therapies targeting mitophagy, mitochondrial biogenesis, and ROS reduction are being explored to slow aging and mitigate degenerative diseases. In cancer, mitochondria play a multifaceted role by supporting energy metabolism, signaling, and cell survival. Cancer cells exhibit metabolic reprogramming, often shifting to aerobic glycolysis (Warburg effect) while retaining mitochondrial activity to sustain ATP production and biosynthesis [3, 4]. Mitochondria also produce ROS, which at moderate levels promote genetic instability, survival, and angiogenesis [2]. Cancer cells balance ROS by upregulating antioxidant systems [8, 11]. Mitochondria regulate apoptosis, but cancer cells evade this by altering mitochondrial pathways, such as overexpressing anti-apoptotic proteins like Bcl-2 [11]. Altered mitochondrial dynamics, including fusion, fission, and mitophagy, enhance adaptability, drug resistance, and metastasis. Targeting mitochondrial metabolism, ROS regulation, and dynamics holds therapeutic potential for aging-related diseases and cancer, offering new avenues for intervention [8].
NUTRITIONAL FACTORS IMPACTING MITOCHONDRIAL STRUCTURE AND FUNCTION
Nutritional factors significantly influence mitochondrial structure and function, shaping cellular energy production, oxidative stress management, and overall metabolic health [12]. The quality and quantity of nutrients can directly affect mitochondrial biogenesis, dynamics, and efficiency, impacting health and susceptibility to disease [13]. Macronutrients such as carbohydrates, fats, and proteins are primary sources of substrates for mitochondrial energy production through oxidative phosphorylation. An excess of nutrients, particularly fats and refined carbohydrates, can lead to mitochondrial overload, promoting the production of ROS and impairing mitochondrial function [14, 15]. Conversely, caloric restriction or intermittent fasting has been shown to enhance mitochondrial efficiency, stimulate biogenesis, and reduce oxidative stress [16]. Micronutrients are also essential for mitochondrial function. Vitamins like B-complex (e.g., B1, B2, B3, and B5) are cofactors in key mitochondrial enzymes, while minerals such as magnesium and iron are crucial for ATP synthesis and electron transport chain activity [17].
Deficiencies in these nutrients can impair mitochondrial energy production and increase susceptibility to dysfunction. Antioxidants, including vitamins C and E, and compounds like coenzyme Q10 and polyphenols, play a protective role by neutralizing excess ROS and preventing oxidative damage to mitochondrial membranes, proteins, and DNA [18]. Diets rich in fruits, vegetables, and antioxidant compounds can support mitochondrial health. Omega-3 fatty acids, found in fish oil, enhance mitochondrial membrane fluidity and function, improving energy efficiency and reducing inflammation. In contrast, excessive intake of saturated and trans fats can impair membrane integrity and promote dysfunction [13]. Plant-derived bioactive compounds, such as resveratrol and curcumin, can activate signaling pathways like AMP-activated protein kinase (AMPK) and sirtuins, promoting mitochondrial biogenesis and resistance to stress [19]. Balanced nutrition, rich in essential micronutrients, antioxidants, and healthy fats, supports mitochondrial health, while overnutrition or deficiencies can impair structure and function, contributing to metabolic and degenerative diseases.
Plant Secondary Metabolites and Their Impact on Mitochondrial Structure and Function
Plant secondary metabolites (PSMs) are bioactive compounds produced by plants that serve various ecological functions, such as defense against herbivores and pathogens. These compounds, including polyphenols, alkaloids, terpenoids, and flavonoids, have significant impacts on mitochondrial structure and function in human cells, influencing energy production, oxidative stress, and cellular signaling pathways. Figure 1 gives a brief classification of different types of nutrients provided by plant foods with a special emphasis on secondary metabolites. Polyphenols, such as resveratrol, quercetin, and catechins, are among the most studied PSMs. They modulate mitochondrial function by enhancing oxidative phosphorylation efficiency, stimulating mitochondrial biogenesis through pathways like AMP-activated protein kinase (AMPK) and sirtuins, and reducing reactive oxygen species (ROS) levels. Polyphenols also stabilize mitochondrial membranes, protecting against damage induced by oxidative stress [20].
Alkaloids, including berberine, have demonstrated the ability to regulate mitochondrial energy metabolism. Berberine, for instance, improves mitochondrial function by activating AMPK, thereby enhancing energy efficiency and reducing mitochondrial dysfunction in metabolic disorders [21]. Terpenoids, such as carotenoids and ginsenosides, support mitochondrial health by protecting against oxidative damage and modulating mitochondrial dynamics [20, 22]. Carotenoids, for example, enhance mitochondrial membrane integrity and reduce ROS production, while ginsenosides promote mitochondrial energy production and biogenesis [23]. Flavonoids, such as hesperidin and kaempferol, impact mitochondrial function by modulating apoptosis, reducing oxidative damage, and supporting mitochondrial DNA integrity [24]. These effects are particularly valuable in mitigating age-related mitochondrial decline and preventing degenerative diseases. Additionally, some PSMs exhibit protective effects against mitochondrial dysfunction associated with diseases. For instance, curcumin and epigallocatechin gallate (EGCG) have shown potential in reducing mitochondrial damage in neurodegenerative and metabolic disorders by improving antioxidant defenses and enhancing mitochondrial quality control mechanisms, such as mitophagy [25, 26]. In summary, plant secondary metabolites profoundly influence mitochondrial structure and function by enhancing energy efficiency, reducing oxidative stress, and protecting against dysfunction. Their potential therapeutic applications offer promising avenues for improving metabolic health and combating mitochondrial-related diseases.
MOLECULAR PATHWAYS TARGETED BY PSMS TO MODULATE MITOCHONDRIAL STRUCTURE AND FUNCTION
Plant secondary metabolites (PSMs) modulate mitochondrial structure and function through various molecular pathways, influencing energy production, oxidative stress management, and cellular signaling. These pathways involve direct interactions with mitochondrial components as well as regulation of key signaling cascades, which collectively enhance mitochondrial health and resilience. Table 1 provides a comprehensive list of PSMs identified to have mitochondrial structure and function modulating potential.
Table 1: List of PSMs identified to have mitochondrial dynamics and function modulating potential
|
Secondary Metabolite |
Chemical Formula |
Chemical structure |
Source of compound |
Function |
|
Acacetin |
C16H12O5 |
|
Black locust, Damiana, Silver birch, Rainforest spleenwort |
Mitophagy |
|
Allin |
C6H11NO3S |
|
Garlic |
Mitophagy |
|
Andrographolide |
C20H30O5 |
|
Bitter weed |
Antioxidant, Mitochondrial integrity, Mitophagy |
|
Anthocyanin |
C15H11O+ |
|
Strawberries, Blueberry, Raspberry, Black rice, and Black soybean, among many others that are red, blue, purple, or black. |
Basal respiration |
|
Apigenin |
C15H10O5 |
|
Parsley, Celery, Celeriac, and Chamomile tea |
Mitochondrial energetics |
|
Apocynin |
C9H10O3 |
|
Dogbane and Kutki |
Respiration |
|
Asiatic acid |
C30H48O5 |
|
Gotu kola plant, Hardy kiwi, Dune Koko tree, Guava, Chilean guava berry, and Craibiodendron |
Mitochondrial dynamics, ATP generation |
|
Astaxanthin |
C40H52O4 |
|
Adonis plants |
Antioxidant |
|
Astragaloside IV |
C41H68O14 |
|
Mongolian milkvetch |
Antioxidant, ETC activity, Mitocondrial regeneration |
|
Bacosides and Bacopasides |
C41H68O13 & C47H76O18 |
|
Water hyssop |
Respiration, Mitochondrial regeneration |
|
Baicalin |
C21H18O11 |
|
Chinese skullcap, Blue skullcap in marsh skullcap, and Indian trumpet tree |
Optimize mitochondrial regeneration, Antioxidant |
|
Berberine |
C20H18NO4+ |
|
Barberry, Tree turmeric, Oregon grape, Goldenseal, yellow-root, Amur cork tree, Chinese goldthread, Prickly poppy, California poppy |
Mitophagy, Antioxidant, SIRT3 activation, ATP generation, Mitochondrial integrity |
|
Boswellic acids |
C30H48O3 |
|
Indian Frankincense |
Mitochondrial respiration |
|
Bouchardatine |
C22H19N3O2 |
|
Union nut plant |
Mitochondrial biogenesis |
|
Caffeic acid |
C9H8O4 |
|
Apples, Artichokes, Berries, Pears |
Antioxidant, Modulate mitochondrial dynamics |
|
Caffeoylquinic acid derivatives |
C16H18O9 |
|
Sweet potatoes, Coffee bean, |
Respiration, Fatty acid oxidation |
|
Capsaicin |
C18H27NO3 |
|
Chili pepper |
Antioxidant, Ionic balance |
|
Carnosic acid |
C20H28O |
|
Rosemary and Common sage |
Antioxidant, Mitochondrial dynamics, Respiration |
|
Catalpol |
C15H22O10 |
|
Rehmannia glutionsa, |
Enhanced ATP and membrane potential, improved mitochondrial dynamics |
|
Chlorogenic acid |
C16H18O9 |
|
Sweet potatoes, Green coffee beans, Apples, pears, Berries, carrots, Potatoes, Tomatoes, and Eggplants, Artichoke, Betel, Burdock, Wormwood, and Rosemary, Honeysuckle, Eucommia, Tobacco leaves, and Mulberry leaves. |
Fatty acid oxidation, Antioxidant |
|
Chrysoeriol |
C16H12O6 |
|
Artemisia |
Suppress protein processing |
|
Cinnamic acid |
C9H8O2 |
|
Spinach, Cinnamon, Citrus fruits, Wheat |
Fatty acid oxidation, Antioxidant |
|
Crocin |
C44H64O24 |
|
Saffron, flowers of Crocus and Gardenia |
Respiration |
|
Curcumin |
C21H20O6 |
|
Turmeric |
Mitophagy, modulate antioxidant activity, Membrane potential |
|
Cyanidin |
C15H11O6+ |
|
Red-fleshed apples, Bilberries, Cranberries, Chokeberries, Grapes, Hawthorn, Lingonberries, Loganberries, Raspberries, Açai berries, Red cabbage and Red onions |
Antioxidant, Mitochindrial dynamics |
|
Delphinidin |
C15H11O7+ |
|
Maqui berries, Cranberries, Concord grapes, Bilberries, Strawberries, Blueberries, Blackberries, Blackcurrant, Redcurrant, Raspberries, Eggplant, Roselle, and Wine. |
Respiration |
|
Dihydroartemisnin |
C15H24O5 |
|
Sweet wormwood plant, and Artemisia annua |
Ionic balance |
|
Dihydromyricetin |
C15H12O8 |
|
Vine tea, Oriental raisin tree, Deodar cedar, Moyeam, Katsura tree, and Japanese fantail willow |
Antioxidant, Mitochondrial biogenesis, Mitochondrial gene expression |
|
Dioscin |
C27H42O3 |
|
Wild yam, Fenugreek, Yam, Himalayan trillium, Ginger, Smilax china, and Rhizoma polygonati |
Antoxidant, Mitochondrial integrity |
|
Ellagic acid |
C14H6O8 |
|
Goji berries, Blackberries, Raspberries, Strawberries, and Cranberries |
Antioxidant, Mitochondrial regeneartion |
|
Embelin |
C17H26O |
|
Berries of the Embelia ribes plant |
Mitochondrial biogenesis |
|
Epicatechin |
C15H14O6 |
|
Cocoa beans, Tea, Grapes |
Respiraion, Antioxidant, Mitophagy |
|
Epigallocatechin-3 -gallate |
C21H22O13 |
|
Tea and Cocoa beans |
Respiration, Mitochondrial biogenesis |
|
Ferulic acid |
C10H10O4 |
|
Rice, Oats, Wheat, Oranges, Apples |
Ionic balance, maintain mitochondrial integrity, Antioxidant |
|
Gastrodin |
C13H18O7 |
|
Gastordia elata |
Antioxidant, Respiration |
|
Geniposide |
C17H24O10 |
|
Gardenia jasminoids |
Ionic balance |
|
Genistein |
C15H10O5 |
|
Soybeans |
Respiration, Mitochondrial integrity |
|
Ginsenoside Rg5 |
C42H70O12 |
|
Gardenia Jasminoids, Hardy Rubber tree, Chinese Foxglove, Beverly Bells flower, Xuan Shen, and Figwort plant |
Mitochondrial integrity, Energy metabolism, Antioxidant |
|
Hesperidin |
C28H34O15 |
|
Oranges and Lemons |
Antioxidant, ATP production, Modulating membrane potential |
|
Honokiol |
C18H18O2 |
|
Magnolia tree |
Respiration, Enhance ATP production, Antioxidant, Mitochondrial regeneration |
|
Hydroxytyrosol |
C8H10O3 |
|
Olive leaves and oil |
Respiration, Antioxidant, Mitochondrial dynamics |
|
Hyperoside |
C21H20O12 |
|
Mountain Hawthorn Plant Weeping Forsythia Shrub, Holoparasite vine, Perennial Shrub/Flower Tree, Deadnettle, Sage, Rhododendron, and Aconitaceae, Garcinia |
Mitochondrial regeneration, Fission |
|
Icariin |
C33H40O15 |
|
Horny Goat Weed |
Improve mitochondrial dynamics and integrity |
|
Isoliquiritigenin |
C15H12O4 |
|
Roots of licorice family |
Antioxidant, Modulate AMP/ATP ratio |
|
Isorhamnetin |
C16H12O7 |
|
Red turnip, Goldenrod, Mustard Leaf, Almonds, Chives, Dill Weed, Fennel Leaves, and Ginkgo Biloba |
Antioxidant |
|
Isorhynchophylline |
C22H28N2O4 |
|
Gou-Teng and Gambier |
Mitochondrial degeneration |
|
Kaempferol |
C15H10O6 |
|
Spinach, Kale, Broccoli Sap and Berries |
Antioxidant, Mitophagy, Ca uptake |
|
Leonurine |
C14H21N3O5 |
|
Plant Herba Leonuri, Lion’s ear, and Lion’s tail |
Modulate mitochondrial activity |
|
Ligustrazine |
C8H12N2 |
|
Chuanxiong |
Membrane potential |
|
Liquiritigenin |
C15H12O4 |
|
Licorice, Jacaranda Obtusifolia Flower, Indian Kino Tree |
Mitochondrial integrity |
|
Luteolin |
C15H10O6 |
|
Clover blossoms, Rosemary, Oregano, Parsley, Basil, Mint, Celery, Broccoli, Artichoke, Oranges |
Mitochondrial permeability, Antioxidant, Mitophagy |
|
Lycopene |
C40H56 |
|
Watermelon, Tomatoes, Grapefruit, Papaya, Mangoes |
Antioxidant, Structural integrity |
|
Magnolol |
C18H18O2 |
|
Houpu magnolia and in M. grandiflora |
Inhibit respiration |
|
Malvidin |
C17H15O7+ |
|
Blueberries, Grapes, Black berries, Raspberries, Soybean |
Basal respiration, Antioxidant, Mitochindrial dynamics |
|
Naringenin |
C15H12O5 |
|
Tomatoes, Grapefruit, Citrus fruits |
Antioxidant, Mitophagy, Respiration |
|
Nicotine |
C10H14N2 |
|
Potatoes, Eggplants, Bell peppers and Tobacco leaves |
Leaky mitochondria |
|
Oleuropein |
C25H32O13 |
|
Olive Tree |
Antioxidant, Block protein processing |
|
Oligonol |
Mixture of polyphenols |
|
Lychee fruits, Green Tea Extract |
Mitochondrial biogenesis |
|
Orientin |
C21H20O11 |
|
Bamboo Leaves, Passion Flowers, Ocimum Sanctum, Acai Plum, Buckwheat Sprouts, Millets |
Antioxidant, Mitochondrial integrity |
|
Pinocembrin |
C15H12O4 |
|
Pinus Heartwood, Eucalyptus, Populus, Euphorbia, Honey, Damiana |
Mitochondrial dynamics |
|
Polydatin |
C20H22O8 |
|
Grape Skin |
Antioxidant, improve mitochondrial membrane potential and integrity, Ca influx, ATP production |
|
Procyanidin |
C30H26O13 |
|
Grape seed, Apples, Cocoa beans, bark of Pine tree |
Modulating mitochindrial protein profile, Antioxidant, Mitochondrial dynamics |
|
Protocatechuic acid |
C7H6O4 |
|
Plums, Gooseberries, Grapes, Nuts, and Almonds |
Antioxidant, Mitochondrial integrity |
|
Puerarin |
C21H20O9 |
|
Kudzu plant |
ATP production, Modulating mitochindrial fusion and fission, mitochondrial gene expression, antioxidant |
|
Quercetin |
C15H10O7 |
|
Berries, Cherries, Apples, Onions |
Respiration, ATP production, ETC capacity, Mitophagy, Antioxidant |
|
Resveratrol |
C14H12O3 |
|
Grape, Almonds, Blue berries, Crane berries, Plum |
Respiration, Fatty acid oxidation, Mitophagy, Antioxidant, Mitochondrial biogenesis |
|
Rhein |
C15H8O6 |
|
Rhubarb |
Modulating membrane potential, ATP production, Enchancing mitochindrial dynamics |
|
Rutin |
C27H30O16 |
|
Buckwheat, Japanese Pagoda Tree, Eucalyptus, Lime Tree Flowers, Elder Flowers, Hawthorn, Rue, Ginko, Apples |
Mitochondrial biogenesis, Antioxidant |
|
Salidroside |
C14H20O7 |
|
Rhodiola Plants |
Autophagy |
|
Salvianolic acid A/B |
C36H30O16 |
|
Red sage |
Restore mitochindrial menbrane potential and integrity, Antioxidant, Modulate mitochondrial dynamics |
|
Schisandrin |
C24H32O7 |
|
Magnolia Vine |
Ionic balance, antioxidant |
|
Scutellarin |
C21H18O12 |
|
Barbed Skullcap and Blue Skullcap |
Mitochondrial biogenesis, Reduce lipid peroxidation, Antioxidant |
|
Silibinin |
C25H22O10 |
|
Milk Thistle |
Improve mitochondrial dynamics, membrane potential |
|
Songorine |
C22H31NO3 |
|
Wolfsbane |
Mitochondrial biogenesis |
|
Stevioside |
C38H60O18 |
|
Candy leaf |
Ionic balance |
|
Sulforaphene |
C6H9NOS2 |
|
Cauliflower, Cabbage, Kale, and Bok choy |
Mitophagy |
|
Tanshinone IIA |
C19H18O3 |
|
Dashen |
Improve mitochondrial structure, Antioxidant |
|
Tomatidine |
C27H45NO2 |
|
Tomatoes |
Mitophagy |
|
Tormentic acid |
C30H48O5 |
|
Olives, Strawberries, Mangos, Rose Fruits, Apples, Mulberry, Quince |
Antioxidant, Mitochondrial integrity |
|
Toxicarioside |
C30H44011 |
|
Mitophagy |
|
|
Vanillin |
C8H8O3 |
|
Vanilla orchid |
Antioxidant |
|
Xanthohumol |
C21H22O5 |
|
Female Inflorescences of Humulus lupus |
Promotes fusion |
|
α-Mangostin |
C24H26O6 |
|
Mangosteen |
ATP synthesis, Membrane potential |
Plant Secondary Metabolites (PSM) Modulate the AMPK Pathway
Several PSMs have been identified to modulate the AMP-activated protein kinase (AMPK) pathway, a central regulator of cellular energy homeostasis. AMPK senses cellular energy levels by monitoring the AMP/ATP ratio and, when activated, promotes catabolic processes that generate ATP while suppressing energy-intensive anabolic pathways [27]. Through their interaction with the AMPK pathway, PSMs enhance mitochondrial function, support metabolic health, and provide protective effects against various diseases. Berberine and resveratrol has been shown to directly activate AMPK by influencing its upstream kinases, like liver kinase B1 (LKB1) and calcium/calmodulin-dependent protein kinase kinase 2 (CaMKK2). Berberine increases the AMP/ATP ratio, mimicking a low-energy state, which triggers LKB1-mediated AMPK activation [21, 28]. Resveratrol, meanwhile, activates AMPK via mechanisms involving increased intracellular calcium, stimulating CaMKK2 [28]. Curcumin and catechins indirectly activate AMPK by reducing oxidative stress and improving mitochondrial function. For instance, curcumin enhances mitochondrial efficiency and reduces reactive oxygen species (ROS) levels, creating a favorable environment for AMPK activation. Through AMPK activation, PSMs stimulate the transcriptional coactivator PGC-1α (peroxisome proliferator-activated receptor gamma coactivator 1-alpha) [29]. PGC-1α drives mitochondrial biogenesis by upregulating genes involved in mitochondrial replication and oxidative phosphorylation, thus improving energy production and metabolic flexibility. AMPK activation by PSMs improves lipid and glucose metabolism, reducing insulin resistance and enhancing fatty acid oxidation. For example, quercetin and epigallocatechin gallate (EGCG) promote AMPK-mediated suppression of acetyl-CoA carboxylase (ACC), enhancing fatty acid breakdown. PSMs also influence the AMPK pathway in concert with other signaling networks. For example, resveratrol activates sirtuin 1 (SIRT1), which synergizes with AMPK to enhance mitochondrial biogenesis and energy homeostasis [30]. Thus, PSMs modulate the AMPK pathway through direct and indirect mechanisms, promoting energy homeostasis, mitochondrial biogenesis, and metabolic health. These effects position PSMs as potential therapeutic agents for conditions such as obesity, diabetes, and age-related mitochondrial dysfunction.
Modulation of the Sirtuin Pathway by PSMs
PSMs modulate the sirtuin pathway, a critical regulator of cellular metabolism, stress resistance, and aging. Sirtuins are a family of NAD+ dependent deacetylases and ADP-ribosyl transferases that influence mitochondrial biogenesis, energy metabolism, and cellular survival [31]. By activating sirtuins, PSMs enhance mitochondrial function and protect against metabolic and age-related diseases. Resveratrol, quercetin, and curcumin are well-known activators of SIRT1, the most studied member of the sirtuin family. Resveratrol directly interacts with SIRT1, enhancing its deacetylase activity. SIRT1 activation deacetylates key transcriptional regulators like peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), promoting mitochondrial biogenesis, improving oxidative phosphorylation, and enhancing cellular energy efficiency [32, 33]. PSMs indirectly enhance sirtuin activity by increasing intracellular NAD+ levels, a critical cofactor for sirtuin function. For instance, compounds like polyphenols and alkaloids can stimulate NAD+ biosynthesis by activating enzymes in the NAD+ salvage pathway, thereby sustaining sirtuin-mediated mitochondrial and metabolic regulation [32, 34].
SIRT3, a mitochondrial sirtuin, regulates the activity of enzymes involved in oxidative phosphorylation, fatty acid oxidation, and antioxidant defense [31]. Resveratrol and EGCG enhance SIRT3 activity, leading to deacetylation of proteins like superoxide dismutase 2 (SOD2) and ATP synthase, which reduces reactive oxygen species (ROS) production and supports mitochondrial energy metabolism [35, 36]. Curcumin and catechins modulate sirtuins to reduce oxidative stress and inflammation. SIRT1 activation by these metabolites upregulates antioxidant enzymes and suppresses pro-inflammatory signaling pathways, protecting mitochondrial and cellular integrity under stress conditions [32, 37]. Many PSMs activate sirtuins in synergy with AMPK, another key metabolic regulator [32]. This crosstalk amplifies the beneficial effects on mitochondrial biogenesis, energy metabolism, and stress resistance.
PSMs modulate the sirtuin pathway by directly activating sirtuins, enhancing NAD+ availability, and promoting mitochondrial health. These effects highlight the therapeutic potential of PSMs in combating metabolic dysfunction, aging, and oxidative stress-related diseases.
PSMs target Nrf2 Pathway
Plant secondary metabolites (PSMs) modulate the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway, a key regulator of cellular defense mechanisms against oxidative stress and inflammation [38]. The Nrf2 pathway controls the expression of genes encoding antioxidant enzymes, detoxification proteins, and other cytoprotective molecules, making it crucial for maintaining mitochondrial and cellular health. Curcumin, resveratrol, and sulforaphane, activate Nrf2 by disrupting its interaction with Kelch-like ECH-associated protein 1 (Keap1) [39, 40, 41]. Under normal conditions, Keap1 binds Nrf2 and targets it for degradation. PSMs induce oxidative or electrophilic modifications in Keap1, releasing Nrf2 to translocate into the nucleus and initiate gene transcription. Activated Nrf2 enhances the expression of antioxidant enzymes such as superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), and heme oxygenase-1 (HO-1). Catechins and quercetin have been shown to boost these defenses, reducing reactive oxygen species (ROS) levels and protecting mitochondria from oxidative damage [42, 43]. PSMs modulate the Nrf2 pathway to promote mitochondrial health. Nrf2 activation improves mitochondrial function by reducing oxidative damage and supporting the expression of genes involved in mitochondrial quality control and biogenesis. For example, resveratrol enhances mitochondrial resilience through Nrf2-mediated antioxidant defenses [40]. Flavonoids and isothiocyanates stimulate Nrf2-dependent expression of phase II detoxification enzymes, such as glutathione-S-transferases (GSTs) and NAD(P)H quinone oxidoreductase 1 (NQO1) [44, 45]. These enzymes neutralize toxic intermediates, safeguarding mitochondrial integrity. By activating Nrf2, PSMs suppress pro-inflammatory signaling pathways, including NF-κB. This crosstalk reduces chronic inflammation, a driver of mitochondrial dysfunction and metabolic diseases. This pathway works alongside other protective mechanisms, such as AMPK and sirtuins, amplifying their impact on mitochondrial and cellular health. PSMs activate the Nrf2 pathway to enhance antioxidant defenses, support mitochondrial function, and reduce inflammation. These effects make PSMs valuable in preventing oxidative stress-related diseases and promoting metabolic and cellular resilience.
PSMs aid in maintenance of mitochondrial structure and function
The antioxidant effects of PSMs significantly modulate mitochondrial structure and function by mitigating oxidative stress and preserving mitochondrial integrity. These metabolites, including polyphenols, terpenoids, and alkaloids, act as potent scavengers of reactive oxygen species (ROS), which are critical regulators of mitochondrial dynamics, quality control, and bioenergetics [19, 20]. Mitochondria are a major site of ROS production, especially under stress. Excessive ROS can damage mitochondrial membranes, proteins, and DNA, leading to impaired structure and function. Polyphenols (e.g., flavonoids like quercetin and resveratrol), neutralize ROS, reducing oxidative damage to mitochondrial lipids, proteins, and mtDNA. Prevent mitochondrial membrane permeabilization, preserving mitochondrial integrity. Carotenoids (e.g., β-carotene), protect mitochondrial membranes by preventing lipid peroxidation [46]. Antioxidant PSMs stabilize mitochondrial membrane potential, a key factor for ATP production and ion homeostasis. Flavonoids (e.g., catechins), prevent the collapse of ΔΨm by reducing oxidative stress on membrane proteins and complexes of the electron transport chain (ETC). Phenolic acids (e.g., ferulic acid), strengthen mitochondrial membranes by reducing oxidative damage to cardiolipin, a lipid critical for ETC function [47].
ROS often suppress mitochondrial biogenesis by damaging signaling pathways involved in mitochondrial renewal. Antioxidant PSMs activate PGC-1α (peroxisome proliferator-activated receptor-gamma coactivator 1-alpha), a key regulator of mitochondrial biogenesis, through indirect modulation of oxidative stress. Stimulate pathways such as AMPK and SIRT1, which are sensitive to the oxidative state and promote mitochondrial turnover and growth [47]. Antioxidant PSMs enhance mitochondrial efficiency by protecting the electron transport chain (ETC) from oxidative damage. They prevent inhibition of ETC complexes caused by ROS-induced modifications and reduce ROS-mediated uncoupling of oxidative phosphorylation, ensuring efficient ATP production [46]. Excessive ROS can trigger the opening of the mitochondrial permeability transition pore (mPTP), leading to swelling and cell death. Terpenoids (e.g., saponins) inhibit ROS-mediated mPTP opening, preventing mitochondrial swelling and apoptosis. Alkaloids (e.g., berberine) protect mitochondria by stabilizing the mPTP under oxidative stress conditions [48]. ROS are key signals for mitophagy (selective degradation of damaged mitochondria). Excessive ROS, however, can overwhelm the system, leading to mitochondrial dysfunction. Antioxidant PSMs normalize ROS levels, allowing proper activation of mitophagy pathways (e.g., PINK1/Parkin) and promote the selective removal of damaged mitochondria, maintaining a healthy mitochondrial population. Under abiotic and biotic stress, antioxidant PSMs help maintain mitochondrial structure and function by enhancing stress signaling pathways, such as those mediated by salicylic acid and jasmonic acid and support mitochondrial resilience to fluctuating ROS levels, ensuring energy supply during stress [49, 50]. The antioxidant effects of plant secondary metabolites preserve mitochondrial structure and function by reducing ROS levels, stabilizing mitochondrial membranes, maintaining ΔΨm, and supporting biogenesis and dynamics. These actions ensure mitochondrial health, energy production, and cellular adaptation to environmental stress. The ability of PSMs to fine-tune oxidative balance highlights their importance in organismal resilience and stress tolerance.
PSMs regulate mitochondrial dynamics
PSMs regulate mitochondrial dynamics by influencing the processes of mitochondrial fusion, fission, and their interplay with quality control mechanisms. Mitochondrial dynamics is critical for maintaining mitochondrial function, ensuring cellular homeostasis, and responding to environmental stress (Figure 2). Mitochondrial Fusion involves merging mitochondrial membranes to maintain mitochondrial integrity, distribute mitochondrial DNA (mtDNA), and buffer against stress. Polyphenolic Compounds like resveratrol and quercetin promote mitochondrial fusion by enhancing the expression of fusion-related proteins such as MFN1/2 (Mitofusin 1 and 2) and OPA1 (Optic Atrophy 1) [51, 52]. Also, support oxidative balance, reducing damage to mitochondria, which facilitates effective fusion. Carotenoids, like β-carotene, stabilize mitochondrial membranes, indirectly favoring fusion over fission under normal or moderate stress conditions [46]. Mitochondrial Fission enables the segregation of damaged mitochondria for removal via mitophagy and contributes to mitochondrial biogenesis. Terpenoids and Phenolics increase activity of DRP1 (Dynamin-related protein 1), the primary mediator of mitochondrial fission and mitigate the excessive production of ROS, which triggers protective mitochondrial fission during stress [53]. Some alkaloids like nicotine or berberine influence mitochondrial fission to adapt to stress conditions by maintaining energy balance and ensuring rapid mitochondrial turnover [21, 54].
Certain PSMs regulate the dynamic equilibrium between fusion and fission, a critical factor for maintaining mitochondrial function. Flavonoids (e.g., catechins) help fine-tune the fusion-fission balance by modulating signaling pathways such as AMPK and mTOR, which indirectly affect mitochondrial dynamics [55]. Phenolic acids (e.g., gallic acid) protect mitochondria from oxidative stress, ensuring proper interplay between fusion and fission processes [56].
PSMs enhance mitochondrial membrane stability, which is vital for maintaining dynamics. Antioxidants like ascorbic acid (vitamin C, a metabolite derivative) preserve ΔΨm, indirectly supporting fusion and suppressing unnecessary fission [57]. Phenolics reduce lipid peroxidation in mitochondrial membranes, preventing structural disruptions. Mitochondrial fission often precedes mitophagy to isolate damaged parts of mitochondria. PSMs like curcumin and resveratrol activate mitophagy pathways (e.g., PINK1/Parkin), which are tightly linked to mitochondrial fission, ensuring that damaged mitochondria are removed efficiently [28, 29].
Under abiotic or biotic stress, PSMs act as ROS scavengers, limiting oxidative damage and favoring controlled mitochondrial dynamics. Thereby, Influence stress-responsive transcription factors that regulate genes associated with mitochondrial fusion and fission (e.g., through modulation of salicylic acid or jasmonic acid pathways) [49, 50]. Plant secondary metabolites regulate mitochondrial dynamics by maintaining the balance between fusion and fission, stabilizing mitochondrial membranes, and influencing signaling pathways involved in stress responses and mitochondrial quality control. These actions optimize mitochondrial function, enabling plants to adapt to environmental challenges and maintain energy homeostasis. The STRING 12.0 database was used to build the protein-protein interaction network with all the genes targeted by PSM’s and involved in maintenance of mitochondrial dynamics (Figure 2) [58]. The interaction value of “high confidence >0.7” was used for the analysis. The thickness of the network edges reflects the strength of the data. The proteins are listed in the Table 2 with their potential functions. The network indicates that PSM target proteins work in a concerted fashion to bring about the modulation in the mitochondrial structure and function. MAPK acts as a signaling protein in controlling the gene expression, cell division and stress responses.
Table 2: List of genes targeted by different PSMs and their potential function in maintenance of mitochondrial dynamics and function
|
GENE |
NAME |
FUNCTION |
|
BAK1 |
BCL2 Antagonist/Killer 1 |
BAK1 is a pro-apoptotic protein that promotes mitochondrial outer membrane permeabilization, leading to the release of apoptogenic factors like cytochrome c and the activation of caspases, thereby inducing apoptosis |
|
BAX |
BCL2 Associated X |
BAX is a pro-apoptotic protein that promotes cell death by increasing mitochondrial membrane permeability, leading to the release of cytochrome c and activation of caspases |
|
BCL2 |
B-cell lymphoma 2 |
BCL2 is a protein that inhibits apoptosis, promoting cell survival by preventing the release of apoptogenic factors from the mitochondria |
|
BCL2L1 |
Bcl-2-like protein 1 |
BCL2L1 is a protein that regulates apoptosis by acting as either an inhibitor or activator of cell death, depending on its isoform |
|
BMS1 |
BMS1 Ribosome Biogenesis Factor |
BMS1 is a protein that plays a crucial role in the assembly of the small ribosomal subunit by facilitating the processing and maturation of pre-ribosomal RNA |
|
CAMKK2 |
Calcium/Calmodulin-Dependent Protein Kinase 2 |
CAMKK2 is an enzyme that regulates various physiological processes, including energy homeostasis, glucose metabolism, and neuronal signaling, by phosphorylating downstream kinases like CaMK1, CaMK4, and AMPK. |
|
CASP3 |
Caspase 3 |
CASP3 is a crucial effector caspase that mediates the execution phase of apoptosis by cleaving various cellular proteins following activation by initiator caspases |
|
CASP9 |
Caspase 9 |
CASP9 is an initiator caspase that plays a crucial role in apoptosis by activating downstream effector caspases, leading to programmed cell death |
|
CYCS |
Cytochrome c, somatic |
CYCS is a small heme protein that plays a critical role in the mitochondrial electron transport chain and apoptosis by transferring electrons and triggering caspase activation |
|
DENR |
Density-Regulated Protein |
DENR is involved in the regulation of translation reinitiation and ribosome recycling, playing a crucial role in protein synthesis |
|
GABPA |
GA Binding Protein Transcription Factor Subunit Alpha |
GABPA is a transcription factor that regulates gene expression, mitochondrial function, and cellular differentiation by binding to specific DNA sequences |
|
KEAP1 |
Kelch-like ECH-associated protein 1 |
KEAP1 is a regulatory protein that controls the activity of the transcription factor Nrf2, playing a crucial role in the cellular response to oxidative stress by promoting the degradation of Nrf2 under normal conditions |
|
MAPK1 |
Mitogen-activated protein kinase 1 also Known as ERK2 |
MAPK1 (ERK2) is an enzyme that regulates cell proliferation, differentiation, transcription, and development by integrating multiple biochemical signals. |
|
MAPK8 |
Mitogen-Activated Protein Kinase 8 |
MAPK8 also known as JNK1, is an enzyme that regulates cellular processes such as proliferation, differentiation, transcription, and apoptosis by integrating multiple biochemical signals |
|
MFN1 |
Mitofusin 1 |
MFN1 is a mitochondrial outer membrane GTPase that mediates mitochondrial fusion, helping maintain mitochondrial network integrity and function |
|
MFN2 |
Mitofusin 2 |
MFN2 is a mitochondrial membrane protein that regulates mitochondrial fusion, morphology, and function, and also plays a role in endoplasmic reticulum-mitochondrial tethering |
|
MTOR |
Mechanistic Target of Rapamycin |
MTOR is a serine/threonine protein kinase that regulates cell growth, proliferation, motility, survival, protein synthesis, autophagy, and transcription by forming two distinct complexes, mTORC1 and mTORC2 |
|
NFKB1 |
Nuclear Factor Kappa B Subunit 1 |
NFKB1 is a transcription factor that regulates immune and inflammatory responses, cell growth, and apoptosis by controlling the expression of various genes |
|
OPA1 |
Optic Atrophy 1 |
OPA1 is a mitochondrial dynamin-like GTPase that regulates mitochondrial fusion, cristae structure, and energy production, playing a crucial role in maintaining mitochondrial integrity and function |
|
PINK1 |
PTEN-induced putative kinase 1 |
PINK1 is a mitochondrial serine/threonine-protein kinase that protects cells from stress-induced mitochondrial dysfunction by initiating mitophagy, the process of removing damaged mitochondria |
|
PPARGC1A |
Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-Alpha |
PPARGC1A is a transcriptional coactivator that regulates genes involved in energy metabolism, mitochondrial biogenesis, and muscle fiber type determination |
|
PRKAA1 |
Protein Kinase AMP-Activated Catalytic Subunit Alpha 1 |
PRKAA1 is a catalytic subunit of AMPK that acts as a cellular energy sensor, regulating metabolic pathways by phosphorylating key enzymes in response to changes in cellular energy status |
|
SARM1 |
Sterile Alpha and TIR Motif Containing 1 |
SARM1 is an enzyme that acts as a sensor of metabolic stress and an executioner of neuronal cell body and axon death by hydrolyzing NAD+ |
|
SIRT1 |
Sirtuin 1 |
SIRT1 is a NAD+-dependent deacetylase that regulates cellular stress responses, metabolism, and aging by deacetylating various transcription factors and proteins |
|
SIRT3 |
Sirtuin 3 |
SIRT3 is a mitochondrial enzyme that regulates cellular metabolism, oxidative stress, and energy homeostasis by deacetylating and activating various mitochondrial proteins |
|
SOD2 |
Superoxide Dismutase 2 (Mn) |
SOD2 is a mitochondrial enzyme that protects cells from oxidative stress by converting superoxide radicals into hydrogen peroxide and oxygen |
|
STK11 |
Serine/threonine-protein kinase 11 |
The STK11 gene, also known as LKB1, encodes a serine/threonine kinase that regulates cell polarity and functions as a tumor suppressor |
|
TAOK2 |
TAO Kinase 2 |
TAOK2 is a serine/threonine-protein kinase that regulates various cellular processes, including stress response, apoptosis, and cytoskeletal organization, by activating the MAPK14/p38 MAPK signaling pathway |
Role of PSMs in mitophagy
PSMs play a crucial role in regulating mitophagy, a selective form of autophagy that eliminates damaged or dysfunctional mitochondria. Mitophagy is vital for cellular homeostasis, stress responses, and adaptation to environmental changes. PSMs such as phenolics, terpenoids, and alkaloids influence mitophagy through various mechanisms. Compounds like flavonoids, phenolic acids, and tannins modulate signaling pathways such as AMPK (AMP-activated protein kinase) and mTOR (mechanistic target of rapamycin) [30, 59]. Activation of AMPK promotes mitophagy by upregulating autophagy-related genes (ATGs) and enhancing mitochondrial quality control. Carotenoids and saponins, can reduce oxidative stress by stabilizing mitochondrial membranes, indirectly promoting mitophagy [46, 60].
PSMs, especially those with strong antioxidant properties, reduce excessive reactive oxygen species (ROS) production. Since ROS act as a key signal for mitophagy, PSMs help fine-tune this response, preventing overactivation or suppression of mitophagy. For example, resveratrol (a stilbenoid) and curcumin (a polyphenol) directly lower oxidative stress and enhance mitochondrial turnover through PINK1/Parkin-mediated mitophagy [51, 29]. Some of the PSMs can regulate mitochondrial fusion and fission processes, which are prerequisites for mitophagy (Figure 2). They can enhance mitochondrial fission by modulating the expression or activity of proteins like DRP1 (dynamin-related protein 1). PSMs also interact with phytohormones such as jasmonic acid and salicylic acid, which are known to influence cellular autophagy [49, 50].
These signaling molecules may amplify mitophagy under stress conditions like pathogen attack or abiotic stress. During biotic or abiotic stress, PSMs contribute to the removal of damaged mitochondria through stress-responsive pathways. For instance, alkaloids like nicotine can activate stress-response genes that are linked to mitochondrial quality control mechanisms [54]. PSMs may regulate mitophagy indirectly by mediating inter-organelle communication (e.g., signaling between mitochondria and the ER). This helps coordinate energy metabolism and stress adaptation. Plant secondary metabolites regulate mitophagy by modulating oxidative stress, mitochondrial dynamics, and autophagy-related signaling pathways. This regulatory role not only aids in maintaining mitochondrial health but also enhances cell or organism’s resilience to environmental challenges. Understanding these mechanisms can provide insights into improving stress tolerance and metabolic efficiency in organisms.
PSMs with apoptotic activity
PSMs play a significant role in modulating mitochondrial apoptotic pathways by targeting key molecular processes involved in mitochondrial membrane integrity, reactive oxygen species (ROS) production, and pro-apoptotic signaling. Apoptosis is a regulated form of cell death often mediated by mitochondria, particularly through the intrinsic (mitochondrial) pathway (Figure 3). The mitochondrial outer membrane permeability (MOMP) is a critical step in apoptosis, controlled by Bcl-2 family proteins (anti-apoptotic like Bcl-2/Bcl-xL and pro-apoptotic like Bax/Bak). Polyphenol compounds like resveratrol and quercetin can modulate the balance between anti-apoptotic and pro-apoptotic proteins by upregulate Bcl-2/Bcl-xL to stabilize mitochondrial membranes and prevent apoptosis [61, 62]. Down-regulate Bax/Bak to inhibit the release of cytochrome c. Terpenoids (e.g., carotenoids and saponins) and protect mitochondrial membranes from stress-induced depolarization, reducing the likelihood of cytochrome c release [63, 64]. Cytochrome c release from mitochondria into the cytosol is a hallmark of apoptosis, as it activates the apoptosome and downstream caspases. Flavonoids reduce oxidative stress and stabilize mitochondrial membranes, directly inhibiting cytochrome c release [24].
Alkaloids (e.g., berberine) inhibit stress-induced mitochondrial swelling and permeability transition pore (mPTP) opening, preventing cytochrome c leakage [65]. Excessive ROS production from mitochondria can trigger apoptosis by damaging mitochondrial components and activating pro-apoptotic signaling. Antioxidant PSMs like Polyphenols (e.g., catechins, curcumin) scavenge ROS, preventing oxidative damage to mitochondrial DNA (mtDNA), proteins, and lipids [56]. Lower ROS levels to suppress activation of stress-responsive apoptotic pathways (e.g., JNK and p38 MAPK). Phenolic acids (e.g., ferulic acid) maintain redox balance and protect mitochondrial function under stress [66]. Loss of ΔΨm is an early event in mitochondrial apoptosis, leading to the activation of apoptotic pathways. Flavonoids and terpenoids stabilize ΔΨm by inhibiting oxidative damage to membrane components. Supporting the activity of the electron transport chain (ETC) and reducing ETC-derived ROS [22, 24].
PSMs modulate apoptotic signaling pathways by targeting key upstream regulators. Flavonoids activate the AMPK pathway, enhancing mitochondrial resilience and suppressing apoptosis [67]. Meanwhile, compounds like curcumin and resveratrol inhibit the pro-inflammatory NF-κB pathway, reducing stress-induced apoptosis and promoting cellular survival under stress conditions [68]. Caspases are crucial enzymes in the process of apoptosis, with caspase-9 serving as an initiator activated upon apoptosome formation and caspase-3 acting as an executioner that cleaves cellular components to complete cell death. Polyphenols inhibit apoptosis by preventing the release of cytochrome c and the assembly of the apoptosome, thereby blocking the activation of caspase-9 and caspase-3 [56]. Similarly, alkaloids suppress caspase activity either directly or indirectly by modulating upstream factors such as reactive oxygen species (ROS) and Bcl-2 family proteins, ultimately interfering with the apoptotic pathway [65]. Under moderate stress, certain PSMs precondition mitochondria by inducing mild, protective levels of reactive oxygen species (ROS), which enhance mitochondrial defense mechanisms. Phytohormones like salicylic acid and jasmonates regulate mitochondrial signaling, fine-tuning stress responses and delaying apoptosis under stress conditions [49, 50]. Additionally, isoprenoids support mitochondrial resilience by promoting biogenesis and repair, thereby reducing the likelihood of apoptotic signaling during prolonged stress. The mitochondrial permeability transition pore (mPTP) plays a critical role in regulating mitochondrial apoptosis. Plant secondary metabolites (PSMs) inhibit mPTP opening, preserving mitochondrial membrane potential (ΔΨm) and preventing the release of apoptogenic factors such as cytochrome c [20]. Compounds like curcumin and resveratrol stabilize the mPTP under oxidative stress, protecting mitochondria from apoptotic signaling.
PSMs exhibit dual roles in apoptosis depending on the context (Figure 3). In cancer cells, PSMs enhance apoptosis by selectively targeting dysfunctional mitochondria, inducing ROS-mediated cell death in tumor cells while sparing normal cells. Conversely, in stress-tolerant cells, such as plants under abiotic stress, PSMs prevent excessive apoptosis, promoting resilience and survival under adverse conditions. PSMs modulate mitochondrial apoptotic pathways by balancing oxidative stress, stabilizing mitochondrial membranes, and regulating pro- and anti-apoptotic proteins and signaling cascades. These effects contribute to the protective or pro-apoptotic roles of PSMs, depending on the physiological context, making them valuable in stress resilience, disease prevention, and therapeutic applications.
CONSTRAINTS IN USING PLANT SECONDARY METABOLITES AS MITO-NUTRACEUTICALS
Secondary metabolites in plants are not consistently present because their production is often influenced by environmental conditions, developmental stages, and stress factors [69]. These compounds are not essential for basic metabolic functions but play crucial roles in plant defense, pollinator attraction, and stress adaptation. For example, alkaloids may accumulate in response to herbivore attacks, while phenolics are produced under UV stress [70]. Additionally, secondary metabolite levels vary between species, tissues, and even individual plants within the same species [71, 72].
Plant secondary metabolites also interact with other foods in diverse ways, influencing their bioavailability, activity, and effects. These interactions involve enhancement of bioavailability, inhibition of nutrient absorption, synergistic effects, and co-metabolism, highlighting the complexity of their roles in a mixed diet [73, 74]. Certain compounds improve the absorption of secondary metabolites. For instance, flavonoids like quercetin are better absorbed when consumed with fat-rich foods, as their solubility increases in the presence of lipids. Similarly, vitamin C enhances the stability and absorption of polyphenols, boosting their antioxidant properties [75]. Some secondary metabolites act as anti-nutrients by forming complexes with dietary proteins, carbohydrates, or minerals, reducing their bioavailability. Tannins bind to iron and proteins, lowering digestibility, while oxalates reduce calcium absorption, potentially leading to deficiencies or kidney stone formation [76]. Saponins interfere with fat absorption by forming complexes with lipids, and alkaloids can inhibit digestive enzymes, impairing nutrient utilization [77]. A comprehensive view of constraints in exploiting the PSMs as mito-nutraceuticals is summarized in Figure 4.
Secondary metabolites often work synergistically with other foods to enhance health benefits. For example, the combination of polyphenols from tea and anthocyanins from berries amplifies antioxidant activity [78]. Similarly, curcumin (from turmeric) and piperine (from black pepper) work together, with piperine enhancing curcumin’s bioavailability and anti-inflammatory properties [79]. Dietary fiber from whole grains promotes the fermentation of plant polyphenols in the gut, boosting beneficial microbial metabolites. Secondary metabolites also interact with gut microbiota and enzymes during digestion, influencing their effects [80]. Polyphenols from tea or fruits are metabolized by gut microbes into bioactive compounds, which are enhanced by prebiotic-rich foods like fiber. Lipid co-metabolism occurs when fat-rich foods improve the solubility and absorption of fat-soluble metabolites like carotenoids. Conversely, tannins and phytates may reduce nutrient availability by forming insoluble complexes, though foods like vitamin C-rich citrus can counteract these effects [81, 82]. While secondary metabolites may reduce nutrient bioavailability in some cases, they often exhibit significant health benefits, such as antioxidant, anti-inflammatory, and therapeutic properties. Their interactions with other foods underscore the importance of a novel methods for their targeted delivery to maximize their positive effects while minimizing potential drawbacks.
FUTURE DIRECTIONS
To overcome the constraints discussed above, one of the possible ways by which plant secondary metabolites can be exploited maximum for nutrient and nutraceutical activity is by developing plant secondary metabolite-loaded nanoparticles. Such formulation can offer several advantages, particularly in the fields of food science and medicine. They include:
- Enhanced Bioavailability: Many plant secondary metabolites, such as flavonoids, alkaloids, and terpenoids, have low water solubility and poor bioavailability. Nanoparticles improve their solubility, absorption, and uptake in biological systems, ensuring higher therapeutic efficiency [83].
- Improved Stability: Secondary metabolites are prone to degradation by environmental factors such as heat, light, and pH changes. Encapsulation within nanoparticles protects these compounds, maintaining their activity and prolonging shelf life [83].
- Controlled Release: Nanoparticles allow for sustained or controlled release of secondary metabolites, ensuring prolonged therapeutic or biological effects. This reduces the need for frequent dosing, improving and patient compliance [84].
- Targeted Delivery: Nanoparticles can be engineered to deliver plant metabolites to specific cells, tissues, or organs, minimizing off-target effects and maximizing efficacy. It protects the molecules from enzymatic and microbial bio-transformations in the digestive system and enables them to be delivered intact. This is particularly beneficial for cancer therapy, where targeted delivery can reduce toxicity to healthy cells [84].
- Multifunctionality: Nanoparticles can carry multiple secondary metabolites or combine them with other therapeutic agents, creating synergistic effects. For example, co-delivery of antioxidants and anti-inflammatory agents enhances overall efficacy [85].
- Reduced Toxicity: By targeting specific sites and controlling release, nanoparticles minimize the systemic exposure of secondary metabolites, reducing potential side effects or toxicity [85].
- Versatile Applications: Plant secondary metabolite-loaded nanoparticles have diverse applications.
- Biocompatibility and Sustainability: Plant-derived metabolites are natural and generally biocompatible, and the use of nanoparticles can be aligned with eco-friendly approaches, reducing reliance on synthetic chemicals.
- Overcoming Drug Resistance: In medicine, nanoparticles can enhance the effectiveness of plant metabolites against drug-resistant pathogens or cancer cells by improving intracellular delivery and bioactivity [86].
Plant secondary metabolite-loaded nanoparticles combine the therapeutic potential of natural compounds with the advanced delivery capabilities of nanotechnology, offering a promising approach for food and nutraceutical applications. In summary, plant secondary metabolites modulate mitochondrial structure and function through pathways that enhance energy production, promote biogenesis, regulate antioxidant defenses, and maintain mitochondrial quality control. These molecular mechanisms position PSMs as potential therapeutic agents for improving mitochondrial health and combating diseases linked to mitochondrial dysfunction. Improving methods for targeted delivery can revolutionize mitochondrial therapeutics and can greatly impact human health.
Funding: Authors acknowledge fund support from the National Science Foundation HBCU-UP RIA Program (Award #2400398)
Institutional Review Board Statement: NA
Informed Consent Statement: NA
Data Availability Statement: NA
Acknowledgements: Authors wish to thank Dr. John Williams, Chair, Department of Natural Sciences, Albany State University for providing required infrastructure to carry out this research.
Conflict of Interest: The authors declare no conflicts of interest.
References
- Casanova A, Wevers A, Navarro-Ledesma S, Pruimboom L. Mitochondria: It is all about energy. Front Physiol 14 (2023): 1114231.
- Liu H, Wang S, Wang J, et al. Energy metabolism in health and diseases. Signal Transduct Target Ther 10 (2025): 69.
- Chen TH, Lin SH, Lee MY, Wang HC, Tsai KF, et al. Mitochondrial alterations and signatures in hepatocellular carcinoma. Cancer Metastasis Rev 44 (2025): 34.
- Liu Y, Wang H, Zhang S, et al. The role of mitochondrial biogenesis, mitochondrial dynamics and mitophagy in gastrointestinal tumors. Cancer Cell Int 25 (2025): 46.
- Marino Y, Inferrera F, Genovese T, Cuzzocrea S, Fusco R, et al. Mitochondrial dynamics: Molecular mechanism and implications in endometriosis. Biochimie. Published online January (2025).
- Tábara LC, Segawa M, Prudent J. Molecular mechanisms of mitochondrial dynamics. Nat Rev Mol Cell Biol 26 (2025): 123-146.
- Yang P, Shuai W, Wang X, et al. Mitophagy in Neurodegenerative Diseases: Mechanisms of Action and the Advances of Drug Discovery. J Med Chem. Published online February (2025).
- Wang Q, Yuan Y, Liu J, Li C, Jiang X. The role of mitochondria in aging, cell death, and tumor immunity. Front Immunol 15 (2024): 1520072.
- Monaghan RM. The fundamental role of mitochondria-endoplasmic reticulum contacts in ageing and declining healthspan. Open Biol 15 (2025): 240287.
- Kamarulzaman NT, Makpol S. The link between Mitochondria and Sarcopenia. J Physiol Biochem. Published online February (2025).
- Murillo Carrasco AG, Chammas R, Furuya TK. Mitochondrial DNA alterations in precision oncology: Emerging roles in diagnostics and therapeutics. Clinics (Sao Paulo) 80 (2025): 100570.
- Kyriazis ID, Vassi E, Alvanou M, et al. The impact of diet upon mitochondrial physiology (Review). Int J Mol Med 50 (2022): 135.
- Putti R, Sica R, Migliaccio V, Lionetti L. Diet impact on mitochondrial bioenergetics and dynamics. Front Physiol 6 (2015): 109.
- Chen D, Li X, Zhang L, Zhu M, Gao L. A high-fat diet impairs mitochondrial biogenesis, mitochondrial dynamics, and the respiratory chain complex in rat myocardial tissues. J Cell Biochem 119 (2018): 9602.
- Langley MR, Yoon H, Kim HN, et al. High fat diet consumption results in mitochondrial dysfunction, oxidative stress, and oligodendrocyte loss in the central nervous system. Biochim Biophys Acta Mol Basis Dis 1866 (2020): 165630.
- Abad-Jiménez Z, López-Domènech S, Pelechá M, et al. Calorie restriction modulates mitochondrial dynamics and autophagy in leukocytes of patients with obesity. Free Radic Biol Med 225 (2024): 677-686.
- Simonenko SY, Bogdanova DA, Kuldyushev NA. Emerging Roles of Vitamin B12 in Aging and Inflammation. Int J Mol Sci 25 (2024): 5044.
- Fiorani M, Guidarelli A, Cantoni O. Mitochondrial reactive oxygen species: the effects of mitochondrial ascorbic acid vs untargeted and mitochondria-targeted antioxidants. Int J Radiat Biol 97 (2021): 1055-1062.
- Liang Z, Currais A, Soriano-Castell D, Schubert D, Maher P. Natural products targeting mitochondria: emerging therapeutics for age-associated neurological disorders. Pharmacol Ther 221 (2021): 107749.
- Anchimowicz J, Zielonka P, Jakiela S. Plant Secondary Metabolites as Modulators of Mitochondrial Health: An Overview of Their Anti-Oxidant, Anti-Apoptotic, and Mitophagic Mechanisms. Int J Mol Sci 26 (2025): 380.
- Fang X, Wu H, Wei J, Miao R, Zhang Y, et al. Research progress on the pharmacological effects of berberine targeting mitochondria. Front Endocrinol (Lausanne) 13 (2022): 982145.
- Zhang P, Liu H, Yu Y, Peng S, Zeng A, Song L. Terpenoids mediated cell apoptotsis in cervical cancer: Mechanisms, advances and prospects. Fitoterapia 180 (2025): 106323.
- Ademowo OS, Oyebode O, Edward R, Conway ME, et al. Effects of carotenoids on mitochondrial dysfunction. Biochem Soc Trans 52 (2024): 65-74.
- Kumar S, Chhabra V, Shenoy S, et al. Role of Flavonoids in Modulation of Mitochondria Dynamics during Oxidative Stress. Mini Rev Med Chem 24 (2024): 908-919.
- Koh YC, Ho CT, Pan MH. The Role of Mitochondria in Phytochemically Mediated Disease Amelioration. J Agric Food Chem 71 (2023): 6775-6788.
- Oliveira MR, Nabavi SF, Daglia M, Rastrelli L, Nabavi SM. Epigallocatechin gallate and mitochondria-A story of life and death. Pharmacol Res 104 (2016): 70-85.
- Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol 19 (2018): 121-135.
- de Oliveira MR, Nabavi SF, Manayi A, Daglia M, Hajheydari Z, et al. Resveratrol and the mitochondria: From triggering the intrinsic apoptotic pathway to inducing mitochondrial biogenesis, a mechanistic view. Biochim Biophys Acta 1860 (2016): 727-745.
- Sathyabhama M, Priya Dharshini LC, Karthikeyan A, Kalaiselvi S, Min T. The Credible Role of Curcumin in Oxidative Stress-Mediated Mitochondrial Dysfunction in Mammals. Biomolecules 12 (2022): 1405.
- Grahame Hardie D. Regulation of AMP-activated protein kinase by natural and synthetic activators. Acta Pharm Sin B 6 (2016): 1-19.
- Kupis W, Palyga J, Tomal E, Niewiadomska E. The role of sirtuins in cellular homeostasis. J Physiol Biochem 72 (2016): 371-380.
- Rahnasto-Rilla M, Tyni J, Huovinen M, et al. Natural polyphenols as sirtuin 6 modulators. Sci Rep 8 (2018): 4163.
- Heger V, Tyni J, Hunyadi A, Horáková L, Lahtela-Kakkonen M, et al. Quercetin based derivatives as sirtuin inhibitors. Biomed Pharmacother 111 (2019): 1326-1333.
- Rahnasto-Rilla M, Järvenpää J, Huovinen M, et al. Effects of galloflavin and ellagic acid on sirtuin 6 and its anti-tumorigenic activities. Biomed Pharmacother 131 (2020): 110701.
- Ungurianu A, Zanfirescu A, Margina D. Sirtuins, resveratrol and the intertwining cellular pathways connecting them. Ageing Res Rev 88 (2023): 101936.
- Payne A, Nahashon S, Taka E, Adinew GM, Soliman KFA. Epigallocatechin-3-Gallate (EGCG): New Therapeutic Perspectives for Neuroprotection, Aging, and Neuroinflammation for the Modern Age. Biomolecules 12 (2022): 371.
- Zendedel E, Butler AE, Atkin SL, Sahebkar A. Impact of curcumin on sirtuins: A review. J Cell Biochem 119 (2018): 10291-10300.
- Dewanjee S, Bhattacharya H, Bhattacharyya C, et al. Nrf2/Keap1/ARE regulation by plant secondary metabolites: a new horizon in brain tumor management. Cell Commun Signal 22 (2024): 497.
- Ashrafizadeh M, Ahmadi Z, Mohammadinejad R, Farkhondeh T, Samarghandian S. Curcumin Activates the Nrf2 Pathway and Induces Cellular Protection Against Oxidative Injury. Curr Mol Med 20 (2020): 116-133.
- Farkhondeh T, Folgado SL, Pourbagher-Shahri AM, Ashrafizadeh M, Samarghandian S. The therapeutic effect of resveratrol: Focusing on the Nrf2 signaling pathway. Biomed Pharmacother 127 (2020): 110234.
- Houghton CA, Fassett RG, Coombes JS. Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician's Expectation Be Matched by the Reality?. Oxid Med Cell Longev (2016): 7857186.
- Sheng Y, Sun Y, Tang Y, et al. Catechins: Protective mechanism of antioxidant stress in atherosclerosis. Front Pharmacol 14 (2023): 1144878.
- Zamanian MY, Soltani A, Khodarahmi Z, et al. Targeting Nrf2 signaling pathway by quercetin in the prevention and treatment of neurological disorders: An overview and update on new developments. Fundam Clin Pharmacol 37 (2023): 1050-1064.
- Mendonca P, Soliman KFA. Flavonoids Activation of the Transcription Factor Nrf2 as a Hypothesis Approach for the Prevention and Modulation of SARS-CoV-2 Infection Severity. Antioxidants (Basel) 9 (2020): 659.
- Hoch CC, Shoykhet M, Weiser T, et al. Isothiocyanates in medicine: A comprehensive review on phenylethyl-, allyl-, and benzyl-isothiocyanates. Pharmacol Res 201 (2024): 107107.
- Ademowo OS, Oyebode O, Edward R, Conway ME, et al. Effects of carotenoids on mitochondrial dysfunction. Biochem Soc Trans 52 (2024): 65-74.
- Fakhri S, Abdian S, Zarneshan SN, Akkol EK, Farzaei MH, et al. Targeting Mitochondria by Plant Secondary Metabolites: A Promising Strategy in Combating Parkinson's Disease. Int J Mol Sci 22 (2021): 12570.
- Guo J, Huang M, Hou S, et al. Therapeutic Potential of Terpenoids in Cancer Treatment: Targeting Mitochondrial Pathways. Cancer Rep (Hoboken) 7 (2024): e70006.
- Poór P. Effects of Salicylic Acid on the Metabolism of Mitochondrial Reactive Oxygen Species in Plants. Biomolecules 10 (2020): 341.
- Li J, Yu G, Wang X, Guo C, Wang Y, Wang X. Jasmonic acid plays an important role in mediating retrograde signaling under mitochondrial translational stress to balance plant growth and defense. Plant Commun 6 (2025): 101133.
- Zhou J, Yang Z, Shen R, et al. Resveratrol Improves Mitochondrial Biogenesis Function and Activates PGC-1α Pathway in a Preclinical Model of Early Brain Injury Following Subarachnoid Hemorrhage. Front Mol Biosci 8 (2021): 620683.
- Ho CL, Kao NJ, Lin CI, Cross TL, Lin SH. Quercetin Increases Mitochondrial Biogenesis and Reduces Free Radicals in Neuronal SH-SY5Y Cells. Nutrients 14 (2022): 3310.
- Chen Q, Ruan D, Shi J, Du D, Bian C. The multifaceted roles of natural products in mitochondrial dysfunction. Front Pharmacol 14 (2023): 1093038.
- Borkar NA, Thompson MA, Bartman CM, Sathish V, et al. Nicotine affects mitochondrial structure and function in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 325 (2023): L803-L818.
- Chen B, Zhang W, Lin C, Zhang L. A Comprehensive Review on Beneficial Effects of Catechins on Secondary Mitochondrial Diseases. Int J Mol Sci 23 (2022): 11569.
- Chodari L, Dilsiz Aytemir M, Vahedi P, et al. Targeting Mitochondrial Biogenesis with Polyphenol Compounds. Oxid Med Cell Longev (2021): 4946711.
- Aumailley L, Bourassa S, Gotti C, Droit A, Lebel M. Vitamin C modulates the levels of several proteins of the mitochondrial complex III and its activity in the mouse liver. Redox Biol 57 (2022): 102491.
- Szklarczyk D, Kirsch R, Koutrouli M, Nastou K, Mehryary F, et al. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic acids research 51 (2023): D638–D646.
- Wu Q, Lv Q, Liu X, et al. Natural compounds from botanical drugs targeting mTOR signaling pathway as promising therapeutics for atherosclerosis: A review. Front Pharmacol 14 (2023): 1083875.
- Qiu WQ, Yu L, He CL, et al. Two 18-norspirostane steroidal saponins as novel mitophagy enhancers improve Alzheimer's disease. Clin Transl Med 13 (2023): e1390.
- Brockmueller A, Buhrmann C, Shayan P, Shakibaei M. Resveratrol induces apoptosis by modulating the reciprocal crosstalk between p53 and Sirt-1 in the CRC tumor microenvironment. Front Immunol 14 (2023): 1225530.
- Nguyen LT, Lee YH, Sharma AR, et al. Quercetin induces apoptosis and cell cycle arrest in triple-negative breast cancer cells through modulation of Foxo3a activity. Korean J Physiol Pharmacol 21 (2017): 205-213.
- Koklesova L, Liskova A, Samec M, et al. Carotenoids in Cancer Apoptosis-The Road from Bench to Bedside and Back. Cancers (Basel) 12 (2020): 2425.
- Zhu X, Jiang H, Li J, Xu J, Fei Z. Anticancer Effects of Paris Saponins by Apoptosis and PI3K/AKT Pathway in Gefitinib-Resistant Non-Small Cell Lung Cancer. Med Sci Monit 22 (2016): 1435-1441.
- Chen D, Ma Y, Guo Z, et al. Two Natural Alkaloids Synergistically Induce Apoptosis in Breast Cancer Cells by Inhibiting STAT3 Activation. Molecules 25 (2020): 216.
- Kampa M, Alexaki VI, Notas G, et al. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: potential mechanisms of action. Breast Cancer Res 6 (2004): R63-R74.
- Hardie DG. AMPK: a target for drugs and natural products with effects on both diabetes and cancer. Diabetes 62 (2013): 2164-2172.
- Alharbi KS, Afzal O, Almalki WH, et al. Nuclear factor-kappa B (NF-κB) inhibition as a therapeutic target for plant nutraceuticals in mitigating inflammatory lung diseases. Chem Biol Interact 354 (2022): 109842.
- Reshi ZA, Ahmad W, Lukatkin AS, Javed SB. From Nature to Lab: A Review of Secondary Metabolite Biosynthetic Pathways, Environmental Influences, and In Vitro Approaches. Metabolites 13 (2023): 895.
- Guerriero G, Berni R, Muñoz-Sanchez JA, et al. Production of Plant Secondary Metabolites: Examples, Tips and Suggestions for Biotechnologists. Genes (Basel) 9 (2018): 309.
- Ghasemzadeh A, Jaafar HZ, Ashkani S, et al. Variation in secondary metabolite production as well as antioxidant and antibacterial activities of Zingiber zerumbet (L.) at different stages of growth. BMC Complement Altern Med 16 (2016): 104.
- Karimi A, Krähmer A, Herwig N, Schulz H, Hadian J, et al. Variation of Secondary Metabolite Profile of Zataria multiflora Populations Linked to Geographic, Climatic, and Edaphic Factors. Front Plant Sci 11 (2020): 969.
- Zyzelewicz D, Oracz J. Bioavailability and Bioactivity of Plant Antioxidants. Antioxidants (Basel) 11 (2022): 2336.
- Cosme P, Rodríguez AB, Espino J, Garrido M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants (Basel) 9 (2020): 1263.
- Di Lorenzo C, Colombo F, Biella S, Stockley C, Restani P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 13 (2021): 273.
- Kim EY, Ham SK, Bradke D, Ma Q, Han O. Ascorbic acid offsets the inhibitory effect of bioactive dietary polyphenolic compounds on transepithelial iron transport in Caco-2 intestinal cells. J Nutr 141 (2011): 828-834.
- Marrelli M, Conforti F, Araniti F, Statti GA. Effects of Saponins on Lipid Metabolism: A Review of Potential Health Benefits in the Treatment of Obesity. Molecules 21 (2016): 1404.
- Diep T, Pook C, Yoo M. Phenolic and Anthocyanin Compounds and Antioxidant Activity of Tamarillo (Solanum betaceum). Antioxidants (Basel) 9 (2020): 169.
- Heidari H, Bagherniya M, Majeed M, Sathyapalan T, et al. Curcumin-piperine co-supplementation and human health: A comprehensive review of preclinical and clinical studies. Phytother Res 37 (2023): 1462-1487.
- Wang X, Qi Y, Zheng H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants (Basel) 11 (2022): 1212.
- Petroski W, Minich DM. Is There Such a Thing as "Anti-Nutrients"? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients 12 (2020): 2929.
- Salim R, Nehvi IB, Mir RA, Tyagi A, Ali S, et al. A review on anti-nutritional factors: unraveling the natural gateways to human health. Front Nutr 10 (2023): 1215873.
- Zhuo Y, Zhao YG, Zhang Y. Enhancing Drug Solubility, Bioavailability, and Targeted Therapeutic Applications through Magnetic Nanoparticles. Molecules 29 (2024): 4854.
- Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology 16 (2018): 71.
- Al Bostami RD, Abuwatfa WH, Husseini GA. Recent Advances in Nanoparticle-Based Co-Delivery Systems for Cancer Therapy. Nanomaterials (Basel) 12 (2022): 2672.
- Ortíz R, Quiñonero F, García-Pinel B, et al. Nanomedicine to Overcome Multidrug Resistance Mechanisms in Colon and Pancreatic Cancer: Recent Progress. Cancers (Basel) 13 (2021): 2058.


















































































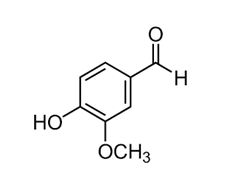






 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 