A Proprietary Dual Polyphenolic Ingredient for Endurance, Muscle Performance, and Muscle Protection in Healthy Subjects: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Clinical Study
Shankaranarayanan Jeyakodi*,1, Arunkanth Krishnakumar1, Mohan Muttanahally Eraiah2, Jestin V. Thomas3, Lincy Benzy Joshua3
1Research and Development, Zeus Hygia Lifesciences Pvt Ltd, Hyderabad, India
2BGS Global Institute of Medical Sciences, Bangalore, India
3Leads Clinical Research and Bio Services Pvt. Ltd., Bengaluru, India
*Corresponding author: Shankaranarayanan Jeyakodi, Research and Development, Zeus Hygia Lifesciences Pvt Ltd, Hyderabad, India.
Received: 11 October 2024; Accepted: 18 October 2024; Published: 29 October 2024
Article Information
Citation: Shankaranarayanan Jeyakodi, Arunkanth Krishnakumar, Mohan Muttanahally Eraiah, Jestin V. Thomas, Lincy Benzy Joshua. A Proprietary Dual Polyphenolic Ingredient for Endurance, Muscle Performance, and Muscle Protection in Healthy Subjects: A Randomized, Double-Blind, Placebo- Controlled, Parallel-Group Clinical Study. Fortune Journal of Health Sciences.7 (2024): 602-614.
View / Download Pdf Share at FacebookAbstract
Background and objectives: The potential of herbal substances can be harnessed to improve endurance, muscle performance, and muscle protection in addition to physical activity and diet. The present study aimed to evaluate the efficacy of Gremin®, a proprietary dual polyphenolic ingredient (PDPI) in enhancing endurance, muscle performance, and muscle protection in healthy adults compared to placebo.
Methods: Sixty healthy adults took PDPI and a placebo for 14 days at a once-daily dose of 500 mg. Endurance measured by physical performance tests, VO2 max, and the six-minute walk test, isometric muscle strength assessed with a handheld dynamometer, and fatigue evaluated using the Multidimensional Fatigue Inventory (MFI) questionnaire were considered the primary objectives. Secondary objectives included muscle recovery (based on the levels of lactate dehydrogenase and C-reactive protein), muscle mass and lean body mass measured using dual-energy X-ray absorptiometry (DEXA) scans, and the safety assessments (blood tests) including adverse events.
Results: PDPI showed significantly enhanced endurance evidenced by the results of the 6-minute walk test, improved muscle strength as seen from the results of the isometric muscle strength study, and reduced fatigue as assessed using the MFI questionnaire compared to the placebo at the end of the study. DEXA scan results showed PDPI significantly increased the lean body mass and fat-free mass compared to its baseline. Throughout the study, PDPI was well-tolerated without any clinically notable adverse events or abnormal blood test results.
Conclusion: The present clinical evidence highlights the effectiveness of Gremin® in significantly enhancing endurance, improving muscle performance, and providing robust muscle protection, making it a potential supplement for athletes and active individuals looking to elevate their physical performance and recovery. However, long-term clinical study in healthy subjects may be warranted to confirm the long-term safety and effectiveness of Gremin®.
Keywords
<p>Gremin<sup>®</sup>, endurance, muscle performance, muscle protection, safety</p>
Article Details
Introduction
Endurance, muscle performance, and muscle protection are closely interconnected. Endurance is the capacity to endure hardship or adversity, particularly over an extended period, and can be physical and mental. For instance, athletes train to enhance their physical endurance for long-distance events, while mental endurance enables individuals to persevere through difficult situations without giving up. Muscle performance refers to muscular strength, which is the ability of a muscle to generate maximum force during a specific task or exercise. Endurance supports muscle performance by enabling athletes to ensure their muscles can continue to perform optimally even as they tire [1, 2]. Muscle protection ensures the muscles are not overly strained during long bouts of endurance activity or intense muscle performance tasks. It helps prevent injuries caused by overloading the muscles, thus sustaining an athlete's ability to train and compete consistently [3]. Together, these three elements enable athletes and active individuals to perform at their best, recover effectively, and reduce the risk of injury, contributing to long-term success and health in their respective activities [4]. Androgenic anabolic steroids are a category of substances that are used to enhance endurance, muscle performance, and muscle protection. These substances can pose significant health risks, including cardiovascular problems, hormonal imbalances, liver and kidney damage, and psychological effects like addiction, mood disorders, and distorted body image. These substances can also lead to ethical and legal issues, such as being banned from sports, creating unfair advantages, and resulting in legal consequences. Long-term use may reduce the body's natural ability to perform and recover, potentially causing chronic health conditions. Additionally, they can give users a false sense of security, leading to overtraining and masking underlying health or fitness issues that require proper attention [5].
Physical activity [6] and nutrition [7] are the most effective and widely used approaches to prevent chronic diseases. The use of herbal actives in addition to physical activity and nutrition can be a safer approach to enhance endurance, muscle performance, and muscle protection in healthy individuals. Various herbal substances are continually explored for their potential benefits in improving endurance, muscle performance, and muscle protection. Curcumin, the active compound found in turmeric, has been widely studied for its anti-inflammatory, antioxidant, and muscle recovery properties [8, 9], which suggest it may have the potential to enhance endurance, muscle performance, and muscle protection. Chlorogenic acid, a polyphenol found in various plants, particularly in coffee beans could be useful for enhancing endurance, muscle performance, and muscle protection because of its ability to reduce oxidative stress [10] and inflammation [10], which are common consequences of prolonged physical activity, such as endurance exercise. Chlorogenic acid has been shown to influence glucose metabolism and fat utilization [11], which could potentially improve endurance by allowing for more efficient energy production during exercise.
The proprietary dual polyphenolic ingredient (PDPI) is the first ingredient in the market where the synergistic benefits of natural polyphenols namely the green coffee bean extract rich in chlorogenic acid and curcumin from Curcuma longa, infused into a food ingredient carrier to explore its potential health benefits. At the preclinical level, PDPI showed muscle recovery effects as observed from the reduced levels of lactate dehydrogenase and creatinine kinase in mice with barium chloride-induced skeletal muscle injury [12]. In a placebo-controlled clinical study involving 16 healthy subjects undergoing acute injury eccentric exercise protocol, PDPI safely and significantly reduced pain and showed health benefits related to muscle recovery, muscle protection, and endurance [13]. PDPI was found to be safe with an LD50 of >5000 mg/kg body weight of female Wistar rats. The study was conducted as per the OECD guideline 423. Given the potential benefits of curcumin and chlorogenic acid on endurance, muscle performance, and muscle protection, and considering the encouraging safety and efficacy results of the previously reported preclinical and clinical studies of PDPI, the present clinical study was conducted to evaluate the effect of PDPI in healthy adults for endurance as the primary objective and muscle health, muscle recovery, and safety as the secondary objectives.
Materials and Methods
Study design
The study was designed as a randomized, double-blind, placebo-controlled, parallel-group clinical study. Healthy moderately active subjects aged 30-60 years (both inclusive) were included in the study as per the inclusion and exclusion criteria. The study was carried out at the BGS Global Institute of Medical Sciences, Bengaluru, Karnataka, India after having received written informed consent from all the subjects. The study was initiated after approval (Reference number: BGSGIMSIEC/APP/DEC/06) from the Institutional Ethics Committee of the BGS Global Institute of Medical Sciences, Bengaluru, Karnataka, India, and registered with Clinical Trials Registry - India on February 5, 2024, with the registration number CTRI/2024/02/062298. The study was conducted in accordance with ICH-GCP (International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use –Good Clinical Practice) guidelines, ethical principles that have their origin in the Declaration of Helsinki, and with the local regulations. A total of three visits were planned in the study. During Visit 1 (Day -7 to 0), all the enrolled subjects underwent screening procedures during which they received instructions on the study assessments, the exercise protocol, food intake to be followed, and about maintaining a subject diary. Additionally, subjects were advised to avoid strenuous or unaccustomed exercise until the end of the study. Subjects who passed the eligibility criteria were randomized in a ratio of 1:1 to receive one capsule of test product or placebo, 30 minutes after meals, once a day for 14 days. Baseline assessments for the primary and secondary outcomes were carried out on Visit 1 for the randomized subjects. The assessments were repeated on Visit 2 (Day 7±2) and Visit 3 (Day 14±2), which was the end of the intervention period.
Participants
Participants involved in the study were screened and selected based on the inclusion and exclusion criteria. Subjects who were healthy males and females of age 30 to 60 years (both inclusive), moderately physically active subjects (regular aerobic exercise for at least 2-3 hours per week) with body mass index (BMI) ≤ 30 kg/m2, no known musculoskeletal pathology, non-smokers, female subjects of childbearing age who agreed to use approved birth control methods during the study and had a negative urine pregnancy test at screening, willing to adhere to their routine diet, physical activity, and general lifestyle throughout the study and who were willing and able to give informed consent and comply with the study procedures were considered for inclusion in the study. Subjects not meeting the inclusion criteria and those currently on or taken in the prior month any anti-inflammatory / analgesic / antioxidant drugs, those with symptoms of viral infection, including COVID-19, known HIV or hepatitis B positive or any other immunocompromised state, those with history of abnormal liver or renal function tests, laboratory findings indicating an active inflammatory or infectious process, or any known disease within the last three months, those with history of uncontrolled or severe medical conditions, such as diabetes, liver disease, or hypertension, cardiac or neurological disorders, autoimmune diseases, including rheumatoid arthritis, lupus, or type 1 diabetes, gastrointestinal tract bleeding / peptic ulcer disease, psychiatric disorders / current alcoholism, or drug abuse, smoking, abuse / alcohol addiction, eating disorders, such as bulimia or binge eating, endocrine abnormalities, including stable thyroid disease, any major surgery, including cardiovascular surgery or surgery involving the esophagus, stomach, duodenum, or bariatric surgery, those with open wounds, those with injury involving the skeletal system or deep soft tissue injury and on steroids or ergogenic users in the last three months, allergic to any of the natural constituents of the investigational product, pregnant and lactating women, those currently participating or having participated in another clinical trial during the last three months prior to the beginning of this study and any additional condition(s) that, in the investigator's opinion, could interfere with the subject's intervention, assessment, or adherence to the protocol, and therefore warrant exclusion from the study or could prevent the subject from completing the study were excluded from the study.
Interventions, randomization and blinding
PDPI, the test substance evaluated in this study is a proprietary dual polyphenolic ingredient (Gremin®) formulation containing green coffee bean extract and curcumin from Curcuma longa. PDPI is the first ingredient in the market where synergistic benefits of natural polyphenols, green coffee bean extract (chlorogenic acid), and curcumin, infused into a food ingredient carrier to explore its potential health benefits. The test product was formulated as opaque colored capsules. Each capsule contained 500 mg of PDPI (Gremin®). Similar-looking placebo capsules were prepared using microcrystalline cellulose. Both the test product and placebo capsules were manufactured in a GMP-certified manufacturing facility and were provided by Zeus Hygia Life Sciences, India. All the randomized subjects were instructed to take the given capsules once a day after meals for 14 days and they were instructed to record the date, time, and amount taken for each time in their subject diary. Randomization was performed using block randomization. Each randomized subject received a xx-digit randomization number. Randomized subjects who terminated their study participation for any reason, regardless of whether the investigational product was taken or not, retained their randomization number. Subjects were randomly assigned to the two groups in a ratio of 1:1. The study was conducted as a double-blind study as both the investigator and subjects were not aware of the intervention.
Outcomes
The primary objectives of the study were to evaluate the effects of PDPI on endurance, isometric muscle strength and fatigue compared to placebo. Therefore, mean changes in endurance, isometric muscle strength, and fatigue between the two intervention groups from baseline to end of the intervention period were considered as the primary outcomes. The secondary objectives were to assess the impact of PDPI on muscle recovery, muscle mass and lean body mass, and safety of PDPI compared to placebo. Therefore, mean changes in muscle recovery, muscle mass, and lean body mass between the two supplement groups from baseline to the end of the intervention period were considered as the secondary outcomes. Endurance was assessed by physical performance tests, the 6-minute walk test (6MWT), and maximum oxygen uptake (VO2 max). The physical performance tests were conducted by asking the subjects to perform squats and push-ups in one set until failure [13]. The 6MWT was developed by the American Thoracic Society and it was officially introduced in 2002, coming along with a comprehensive guideline. The 6MWT is a sub-maximal exercise test used to assess aerobic capacity and endurance. The distance covered over a time of 6 minutes is used as the outcome by which to compare changes in performance capacity. The test was initially designed to help in the assessment of patients with cardiopulmonary issues. Gradually, it was introduced in numerous other conditions. It evaluates the functional capacity of the individual and it provides valuable information regarding all the systems during physical activity, including pulmonary and cardiovascular systems, blood circulation, neuromuscular units, body metabolism, and peripheral circulation [14, 15]. The VO2 max is an important indicator of physical efficiency (especially aerobic efficiency). It displays how much oxygen your body can use during maximal intensity exercise and is thought to be one of the accurate measures of aerobic or cardiovascular fitness. It measures the maximum amount of oxygen that the body can use in one minute per kilogram of body weight (mL/kg/min) [16, 17]. The VO2 max was calculated by using the resting heart rate and maximal heart rate.
Isometric muscle strength was measured using a handheld dynamometer. A handheld dynamometer is an appropriate and convenient method to assess muscle strength in a clinical setting due to its strong reliability and validity [18, 19]. It is used to measure the maximum strength within the forearm muscles, making it particularly useful for sports that involve activities such as throwing, lifting, and catching. Fatigue was evaluated by the Multidimensional Fatigue Inventory (MFI) questionnaire. MFI is a 20-item self-report instrument designed to measure various dimensions of fatigue, including general fatigue, physical fatigue, mental fatigue, reduced motivation, and reduced activity each measured by a subscale. The scale has been validated in a variety of participant populations. MFI scoring was done by respondents using a scale ranging from 1 to 7 to indicate how aptly certain statements regarding fatigue represent their experiences. Several positively phrased items are reverse-scored. Higher total scores correspond with more acute levels of fatigue [20]. All of these assessments for the primary outcomes were carried out at the baseline, on Day 7, and at the end of the study (Day 14). Muscle recovery was found by measuring the levels of lactate dehydrogenase (LDH) and C-reactive protein (CRP) in the blood. The muscle mass and lean body mass were assessed by dual-energy X-ray absorptiometry (DEXA) scan. Assessments for muscle recovery, muscle mass, lean body mass were carried out at the baseline and the end of the study. Safety assessments were performed at the baseline and the end of the study by the physical examination, noting the incidence of abnormal vital signs (blood pressure, heart rate, and temperature) and clinically significant changes in laboratory parameters such as serum aspartate aminotransferase (AST), serum alanine aminotransferase (ALT), complete blood count (CBC), and serum creatinine. Safety was also assessed by monitoring the incidence of adverse events (AE) / serious adverse events (SAEs) reported during the study.
Statistical methods
A sample size of 60 subjects (30 per group) was deemed sufficient to detect a clinically significant difference between groups with 80% power and a 5% level of significance, assuming a standard deviation of 1.49 at the end of treatment and an attrition rate of 15%. Recorded data were analyzed using appropriate statistical tools within groups and between the intervention groups. Mean differences within and between groups were assessed inferentially at each data collection point using t-tests for all outcome measures. MFI scores were classified into domains / scales. Scores were summarized by intervention groups and visits and assessed by using a t-test with a 5% level of significance. All continuous study assessments were summarized by intervention and time point using descriptive statistics (n, mean). All categorical study assessments were summarized by intervention and time points using frequency counts and percentages. Hypothesis testing, unless otherwise indicated, was two-sided and performed at the 0.05 significance level. The analysis was performed on the safety population.
Results
Participants’ flow
The study was initiated in February 2024 and completed in March 2024. A total of 73 subjects participated in the screening process. Thirteen subjects failed the screening and the remaining 60 eligible subjects were randomized into two groups in a ratio of 1:1 with 30 subjects in the test product group and 30 subjects in the placebo group. Three subjects from the placebo group and one subject from the test product group did not turn up for Day 7 visit. Additionally, one subject from the placebo group did not return for Day 14 visit. Overall, 55 subjects (29 subjects in the test group and 26 subjects in the placebo group) completed the study with more than 97% compliance to the intervention, and therefore data from all the completed subjects were analyzed. The flow of participants in the study is shown in Figure 1.
Baseline demographic characteristics
The study included male and female subjects of Indian origin with 19 (65.52%) males and 10 (34.48%) females in the test product group and 18 (69.23%) males and 8 (30.77%) females in the placebo group. The baseline demographic characteristics of the test group and the placebo group are presented in Table 1. Results of the baseline demographic characteristics namely gender, age, weight, height, and BMI were comparable between the study groups, and no significant (p>0.05) difference was found between the groups.
Table 1: Baseline demographic characteristics
|
Study parameters |
PDPI group (n = 29) |
Placebo group (n = 26) |
|
Males (n, %) |
19 (65.52%) |
18 (69.23%) |
|
Females (n, %) |
10 (34.48%) |
8 (30.77%) |
|
Age (years)a |
36.83 ± 4.41 |
37.00 ± 4.45 |
|
Height (cm)a |
165.03 ± 10.24 |
163.73 ± 8.40 |
|
Weight (kg)a |
68.11 ± 10.88 |
66.37 ± 9.50 |
|
BMI (kg/m2)a |
24.93 ± 2.86 |
24.72 ± 2.40 |
aEach value represents mean ± SD; BMI – Body mass index
Effect of PDPI on endurance
Physical performance tests, the 6-minute walk test (6MWT), and maximum oxygen uptake (VO2 max) assessments were carried out to understand the effect of the interventions on endurance.
Physical performance
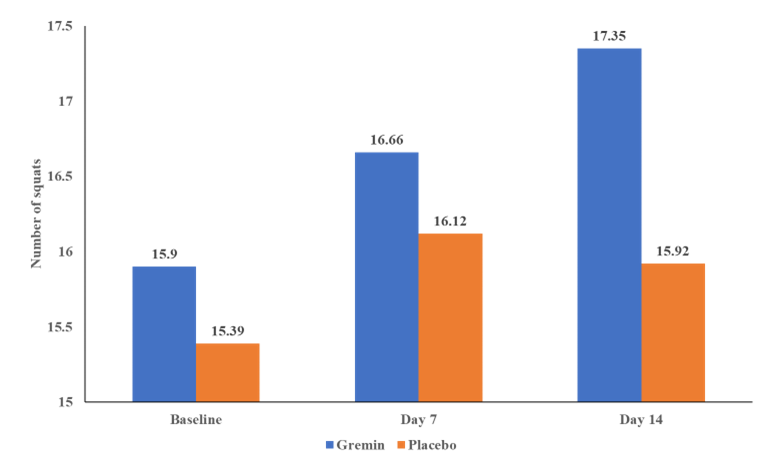
The physical performance was evaluated based on the number of squats and push-ups performed by subjects in one set until failure. The mean (±SD) number of squats performed by subjects in each group is presented in Figure 2.
The number of squats performed in the test product group at the baseline was 15.90 ± 8.84, which was increased to 16.66 ± 10.15 on Day 7 and then further increased to 17.35 ± 8.44 on Day 14. A similar increasing trend was seen in the number of squats in the placebo group on Day 7 (16.12 ± 6.48) compared to that of the baseline (15.39 ± 6.36) but not on Day 14 (15.92 ± 5.74) from that of Day 7. In terms of percentage, test product group showed 9.12% improvement compared to the 3.44% improvement in the placebo at the end of the study, however, the differences observed in the number of squats at all time points from the baseline within each group and between the groups were not statistically significant (p>0.05). The number of push-ups in the test product at the baseline was 9.62 ± 7.04, which was increased to 10.69 ± 7.83 on Day 7 and then further increased to 11.69 ± 9.04. The increase in the number of push-ups seen in the placebo group on Day 7 (9.81 ± 5.21) from that of the baseline (9.35 ± 4.90) was minimal and it decreased on Day 14 (9.58 ± 5.54) from that of Day 7. In terms of percentage, test showed 21.52% improvement compared to the 2.46% improvement in the placebo at the end of the study, however, the differences observed in the number of push-ups at all time points from the baseline within each group and between the groups were not statistically significant (p>0.05). The results of the push-ups performed by subjects in each group are presented in Figure 3.
The 6-minute walk test
Results of the 6MWT performed in terms of the distance covered in meters by subjects in each group are presented in Figure 4. In the 6MWT, the test group showed significantly (p<0.05) higher distance covered on Day 14 (573.79 ± 35.4), which was 2.65% higher compared to its baseline (558.97 ± 33.74). Whereas in the placebo group, the distance covered on Day 14 (565.39 ± 43.84 or 0.14% improvement) was not significantly (p>0.05) different from its baseline (564.62 ± 44.47). The mean change from baseline in the distance covered on Day 14 in the test group (14.83 ± 33.23) was significantly (p<0.05) different from that of the placebo group (0.77 ± 3.92) indicating the effectiveness of PDPI in the 6MWT over the placebo group. A similar trend was observed in the results of the speed (m/minute) assessed in the 6MWT, where the test group showed significantly (p<0.05) higher speed on Day 14 (95.63 ± 5.90), which was 2.65% higher compared to its baseline (93.16 ± 5.62) but the same was not significantly (p>0.05) different in the placebo on Day 14 (94.23 ± 7.31, 0.14% improvement) compared to its baseline (94.10 ± 7.41) (Figure 5). The mean ± SD change in the speed on Day 14 in the test group (2.47 ± 5.54) was significantly (p<0.05) different from that of the placebo group (0.13 ± 0.66) indicating the effectiveness of PDPI on speed in the 6MWT over the placebo group.
The VO2 max
Results of the VO2 max of the subjects in the test and placebo groups are given in Figure 6. The VO2 max improved by 3.75% in the PDPI group compared to 1.5% improvement in the placebo group. However, there was no significant (p>0.05) difference in the levels of VO2 max within the test group and placebo group as well as between the test and placebo groups at all time points.
Effect on isometric muscle strength
The test group showed a significant increase (p<0.05) in the isometric muscle strength of the dominant hand by 10.59% on Day 14 (29.65 ± 7.21) from the baseline (26.81 ± 7.54). Whereas, in the placebo group there was no significant (p>0.05) difference in the isometric muscle strength of the dominant hand on Day 14 (26.49 ± 6.86) from the baseline (25.17 ± 6.83), which was up by 5.24% from the baseline. However, the difference observed between the test and the placebo group at any time point was not significant (p>0.05) (Figure 7). The test group showed a significant increase in the isometric muscle strength of the non-dominant hand by 17.05% on Day 7 (22.38 ± 6.33, p<0.05) and by 35.56% Day 14 (25.92 ± 8.87, p<0.05) from the baseline (19.12 ± 7.00). Whereas, in the placebo group there was no significant (p>0.05) difference in the isometric muscle strength of the non-dominant hand on Day 7 (20.99 ± 5.65), which was up by 8.93% and Day 14 (21.07 ± 3.66), which was up by 9.34% from the baseline (19.27 ± 6.69). The difference observed between the test and the placebo group on Day 14 was statistically significant (p<0.05) (Figure 8).
Effect on fatigue
Results of the MFI questionnaire indicated that the test product significantly (p<0.05) decreased the scores of reduced motivation (by 22.94%), mental fatigue (45.80%), and the total MFI (by 18.82%) compared to placebo (reduced motivation, down by 15.05%, mental fatigue, down by 11.08%, and the total MFI, down by 1.94%) on Day 14. Additionally, the test product showed a significant reduction from its baseline in the scores of reduced motivation and mental fatigue on Day 7 and Day 14 and in the scores of total MFI on Day 14. The reduction in the reduced motivation by the test product on Day 14 compared to placebo may not be considered meaningful as the placebo had significantly (p<0.05) higher scores of reduced motivation at the baseline compared to the baseline level in the test product group. The test product group and placebo group did not show any significant effects on general fatigue, physical fatigue, and reduced activity within the group and between the groups at any of the time point (Table 2).
Table 2: Results of the MFI questionnaire
|
Parameters |
PDPI (n = 29) |
Placebo (n = 26) |
||||
|
Baseline |
Day 7 |
Day 14 |
Baseline |
Day 7 |
Day 14 |
|
|
General fatigue |
9.07 ± 3.52 |
9.41 ± 2.93 |
8.72 ± 2.12 |
8.77 ± 2.46 |
8.35 ± 2.93 |
9.04 ± 2.11 |
|
Physical fatigue |
7.07 ± 3.40 |
6.93 ± 2.96 |
6.45 ± 3.05 |
7.00 ± 3.06 |
7.81 ± 3.02 |
7.62 ± 2.74 |
|
Reduced activity |
8.24 ± 3.06 |
8.62 ± 3.23 |
7.52 ± 2.43 |
7.85 ± 3.11 |
8.69 ± 3.25 |
8.73 ± 2.99 |
|
Reduced motivation |
10.07 ± 2.07# |
9.14 ± 1.94* |
7.76 ± 2.55*# |
11.23 ± 2.12 |
9.58 ± 2.86* |
9.54 ± 2.90* |
|
Mental fatigue |
9.17 ± 3.17 |
6.76 ± 3.14* |
4.97 ± 1.59*# |
8.39 ± 1.92 |
7.62 ± 3.01 |
7.46 ± 2.96 |
|
Total MFI |
43.62 ± 7.18 |
40.86 ± 9.18 |
35.41 ± 7.60*# |
43.23 ± 6.03 |
42.04 ± 7.50 |
42.39 ± 7.54 |
Each value represents mean ± SD. *p<0.05 – significantly different compared to the respective baseline; #p<0.05 - significantly different compared to the placebo; MFI – Multidimensional Fatigue Inventory questionnaire.
Effect on muscle recovery
Levels of the LDH and CRP of subjects in the test product group and placebo group are presented in Table 3. There was a significant (p<0.05) reduction in the LDH levels of the subjects in the test product group (8.42%) on Day 14 (183.17 ± 30.12 U/L) from its baseline (200.00 ± 34.60 U/L) while the reduction in the LDH levels of the placebo group (3.63%) on Day 14 (187.08 ± 36.00 U/L) from its baseline (194.12 ± 38.40 U/L) was not significant indicating muscle health benefit of PDPI. However, there was no significant (p>0.05) difference in the levels of LDH between the two groups. Within the group and between the group analysis did not show any significant (p>0.05) difference in the levels of CRP of subjects in the test product group and placebo group, though there was a 29.25% reduction in the test product group and 11.39% worsening in the placebo group.
Table 3: Results of the muscle recovery parameters
|
Parameters |
PDPI group (n = 29) |
Placebo group (n = 26) |
||
|
Baseline |
Day 14 |
Baseline |
Day 14 |
|
|
LDH (U/L) |
200.00 ± 34.60 |
183.17 ± 30.12* |
194.12 ± 38.40 |
187.08 ± 36.00 |
|
CRP (mg/L) |
2.53 ± 3.61 |
1.79 ± 1.77 |
1.58 ± 1.35 |
1.76 ± 2.09 |
Each value represents mean ± SD. *p<0.05 – significantly different compared to the respective baseline; CRP – C-reactive protein, LDH – Lactate dehydrogenase
Muscle mass and lean body mass assessed by DEXA scan
In the DEXA scan, the test product group showed a significant (p<0.05) increase in the lean body mass (38480.50 ± 9505.31 g) and fat-free mass (41043.67 ± 10117.12 g) at the end of the study (Day 14), which was 1.38% and 2.13% improvement respectively from the baseline lean body mass (37958.17 ± 9550.90 g) and fat-free mass (40188.17 ± 9963.09 g) but in the placebo group, there was no significant change in the lean body mass (38057.17 ± 6048.08 g) and fat-free mass (40273.58 ± 6364.45 g) at the end of the study (Day 14), which was 0.12% and 0.21% improvement respectively from the baseline lean body mass (38011.58 ± 6791.19 g) and fat-free mass (40188.50 ± 7114.33 g). There were no significant differences seen within each group or between the two groups in the tissue mass, fat mass, and bone mineral content (BMC). The DEXA scan results are shown in Table 4.
Table 4: Results of the DEXA scan
|
Parameters |
PDPI group (n = 12) |
Placebo group (n = 12) |
||
|
Baseline |
Day 14 |
Baseline |
Day 14 |
|
|
Tissue (g) |
60804.75 ± 12436.77 |
61228.17 ± 12615.81 |
60142.17 ± 8928.89 |
60024.25 ± 8744.11 |
|
Fat (g) |
22846.58 ± 4616.11 |
22747.58 ± 4529.55 |
22130.83 ± 5484.99 |
21967.08 ± 5437.09 |
|
Lean (g) |
37958.17 ± 9550.90 |
38480.50 ± 9505.31* |
38011.58 ± 6791.19 |
38057.17 ± 6048.08 |
|
BMC (g) |
2230.08 ± 432.19 |
2229.92 ± 451.45 |
2177.08 ± 383.11 |
2216.33 ± 371.92 |
|
Fat-free mass (g) |
40188.17 ± 9963.09 |
41043.67 ± 10117.12* |
40188.50 ± 7114.33 |
40273.58 ± 6364.45 |
Each value represents mean ± SD; *p<0.05 – significantly different compared to the respective baseline; BMC – Bone mineral content
Safety Assessments
Vital signs, clinical chemistry and hematology parameters
The vital signs namely systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), respiratory rate (RR), temperature, and blood oxygen level (SpO2) were within the normal levels in all the subjects in both the groups throughout the study. No clinically significant difference in the vital signs was found between the groups at the end of the study. Similarly, there were no clinically significant changes observed in the clinical chemistry and hematology parameters of both groups (Table 5).
Table 5: Results of the vital signs, clinical chemistry and hematology parameters
|
Parameters |
PDPI group (n = 29) |
Placebo group (n = 26) |
||
|
Baseline |
Day 14 |
Baseline |
Day 14 |
|
|
SBP (mmHg) |
119.83 ± 7.91 |
122.03 ± 8.23 |
119.73 ± 9.54 |
120.65 ± 8.55 |
|
DBP (mmHg) |
77.28 ± 9.96 |
76.62 ± 10.41 |
77.42 ± 9.68 |
75.69 ± 8.71 |
|
HR (bpm) |
82.03 ± 11.06 |
83.55 ± 9.34 |
85.58 ± 9.10 |
82.23 ± 7.90 |
|
RR (b/m) |
16.90 ± 0.82 |
16.9 ± 0.86 |
17.42 ± 0.81 |
17.00 ± 0.75 |
|
Temperature (°F) |
97.91 ± 0.52 |
97.56 ± 0.42 |
97.79 ± 0.47 |
97.54 ± 0.66 |
|
SpO2 (%) |
97.41 ± 0.63 |
97.03 ± 1.02 |
97.00 ± 1.36 |
97.23 ± 1.31 |
|
Hemoglobin (g/dL) |
13.29 ± 2.09 |
13.16 ± 2.08 |
13.12 ± 1.37 |
13.30 ± 1.51 |
|
RBC (million cells/mm3) |
4.95 ± 0.65 |
4.92 ± 0.67 |
4.86 ± 0.59 |
4.92 ± 0.63 |
|
PCV (%) |
41.27 ± 5.97 |
41.12 ± 5.81 |
40.79 ± 3.97 |
41.45 ± 4.57 |
|
MCV (fL) |
83.70 ± 8.24 |
83.93 ± 8.47 |
84.61 ± 8.73 |
84.97 ± 8.84 |
|
MCH (pg) |
26.97 ± 3.19 |
26.83 3.29 |
27.24 3.40 |
27.30 3.32 |
|
MCHC (g/dL) |
32.16 ± 0.96 |
31.82 0.99 |
32.12 0.94 |
31.74 1.95 |
|
Total WBC (cells/mm3) |
6899.66 ± 1896.24 |
7333.10 ± 2436.95 |
6893.08 ± 1787.38 |
6934.23 ± 1560.77 |
|
Neutrophils (%) |
59.98 ± 8.46 |
61.35 ± 9.64 |
57.64 ± 7.14 |
57.94 ± 6.63 |
|
Lymphocytes (%) |
29.74 ± 6.20 |
28.43 ± 7.13 |
31.67 ± 6.11 |
30.50 ± 8.28 |
|
Eosinophils (%) |
3.81 ± 4.44 |
3.77 ± 4.22 |
3.90 ± 2.49 |
3.68 ± 2.10 |
|
Monocytes (%) |
5.99 ± 1.79 |
5.70 ± 1.66 |
6.19 ± 1.34 |
6.22 ± 1.47 |
|
Basophils (%) |
0.49 ± 0.31 |
0.58 ± 0.33 |
0.61 ± 0.33 |
0.66 ± 0.30 |
|
Platelets (lakhs/cm2) |
3.02 ± 0.77 |
3.01 ± 0.83 |
2.81 ± 0.80 |
2.93 ± 0.74 |
|
AST (U/L) |
26.07 ± 11.37 |
27.07 ± 13.64 |
24.39 ± 11.95 |
26.81 ± 13.83 |
|
ALT (U/L) |
29.10 ± 23.06 |
31.14 ± 25.33 |
23.12 ± 13.02 |
24.69 ± 12.79 |
|
Serum creatinine (mg/dL) |
0.80 ± 0.14 |
0.85 ± 0.16 |
0.84 ± 0.22 |
0.87 ± 0.25 |
Each value represents mean ± SD; ALT – Alanine transaminase, AST – Aspartate transaminase, DBP – Diastolic blood pressure, HR – Heart rate, MCH - mean corpuscular hemoglobin, MCHC - mean corpuscular hemoglobin concentration, MCV – Mean corpuscular volume, PCV – Packed cell volume, RBC – Red blood cells, RR – Respiratory rate, SBP – Systolic blood pressure, SpO2 – Blood oxygen level, WBC – White blood cells.
Adverse events
A total of five adverse events (AEs) were reported during the study with two (headache -1 and viral fever - 1) in the test product group and three (cold – 3) in the placebo group. All the AEs were mild in severity and were considered by the investigator as not related to the intervention. The outcomes of all the reported AEs were noted as resolved before the end of the study. None of the subjects reported any serious adverse event (SAE) or were withdrawn from the study due to an AE or an SAE.
Discussion
Physical activity, especially sports, places significant stress on tendons and the muscle-tendon junction, heightening the risk of tendon injuries. The main cause of tendon degeneration appears to be mechanical overload combined with insufficient recovery, resulting in the failure of traction and tension within the tendon’s collagen fibers. Repeated muscle contractions lead to ongoing microtrauma in tendons, and the slow turnover of collagen further impedes proper recovery [21]. Sports injuries like fractures, dislocations, and ligament or tendon ruptures are often the result of high-energy trauma. In cases of ligament and tendon ruptures, elevated levels of creatine kinase and lactate dehydrogenase are commonly observed [22]. In the present study, PDPI at a once-daily dose of 500 mg for 14 days enhanced endurance as observed from the results of the 6MWT, improved muscle strength as evident from the results of the isometric muscle strength study, and reduced fatigue as assessed using the MFI questionnaire. These primary objective endpoints observed with test product were significantly better than placebo indicating the potential endurance enhancing benefits of PDPI. The results observed in this study are broadly in line with the results of the clinical study of PDPI in healthy moderately active subjects undergoing acute injury exercise protocol [13]. However, we did not find any significant effects between the test product and placebo group on the number of push-ups, squats, VO2 max, levels of lactate dehydrogenase and CRP, isokinetic muscle strength of the dominant hand, general fatigue, physical fatigue and reduced motivation in the MFI questionnaire in this clinical study possibly because of the lack of any stress exercise protocol and the involvement of healthy moderately active subjects. It is to be noted that, PDPI at preclinical level significantly reduced levels of lactate dehydrogenase and creatinine kinase in mice with barium chloride-induced skeletal muscle injury [12]. In the DEXA scan, the test product group showed significant increase in the lean body mass and fat-free mass compared to its baseline. However, there was no significant difference between the test product and the placebo in the DEXA scan results. The lack of significant difference between the groups could be due to the shorter duration of the supplementation.
Results of the current study in a way support the muscle strength-enhancing effects of chlorogenic acid studied at the preclinical level. Chlorogenic acid was reported to have improved muscle strength by regulating mitochondrial function and cellular energy metabolism in a rat model of resistance training [23]. Chlorogenic acid on account of its antioxidant activity [10] may improve muscle recovery and endurance and because of its anti-inflammatory activity [10] could help in quicker recovery post-exercise. Chlorogenic acid could provide more efficient energy use during physical activities by improving glucose and lipid metabolism [11]. The beneficial effects of curcumin on muscle injury were confirmed in a meta-analysis that analyzed 14 clinical study publications. The meta-analysis showed that curcumin significantly mitigated skeletal muscle damage, with notable improvements in creatine kinase levels, muscle soreness, IL-6 levels, and range of motion. It also highlighted that the effect of curcumin was more pronounced for untrained individuals or those less exposed to muscle-damaging exercise [24]. PDPI exhibited muscle recovery effects as observed from the reduced levels of creatinine kinase from Day 3 till the end of the study (Day 10) in a study involving healthy moderately active adults following the acute injury eccentric exercise protocol [13]. In the same study, pain as assessed by the visual analogue scale and LDH levels were also found reduced and total iron levels were improved upon supplementation with PDPI. Results of reported clinical and preclinical studies of PDPI [12, 13], chlorogenic acid [10, 11, 23], and curcumin [24] along with the results of the current study on PDPI supports the endurance, muscle performance, and muscle protective properties of PDPI when administered at a once-daily dose of 500 mg for 14 days. PDPI was found to be safe and well tolerated as evidenced by the clinically non-significant results of the clinical biochemistry and hematology parameters, vital signs, and the reported adverse events in the current clinical study. The safety of PDPI was also demonstrated in the previously reported clinical study [13]. Earlier, in an acute oral toxicity study conducted as per OECD guideline 423, PDPI was found safe with LD50 of >5000 mg/kg body weight of female Wistar rats. In summary, at the end of this clinical study, PDPI increased the number of squats by 9.12% compared to the 3.44% increase seen in the placebo, increased the number of push-ups by 21.52% compared to the 2.46% increase seen in the placebo, increased the distance covered and speed by 2.65% in the 6MWT compared to the 0.14% increase seen distance covered and speed in the placebo, increased the VO2 max by 3.75% compared to the 1.5% increase seen in the placebo, increased the isometric muscle strength of the dominant hand by 10.54% compared to the 5.24% increase seen in the placebo, increased the isometric muscle strength of the non-dominant hand by 35.56% compared to the 9.34% increase seen in the placebo, increased the lean body mass by 1.38% compared to the 0.12% increase seen in the placebo, increased the fat-free mass by 2.13% compared to the 0.21% increase seen in the placebo, decreased the total MFI by 18.82% compared to the 1.94% decrease in the placebo, reduced the LDH levels by 8.42% compared to the 3.63% reduction seen in the placebo, and reduced the CRP by 29.25% compared to the 11.39% worsening seen in the placebo. Further clinical study of PDPI is warranted to assess its long-term efficacy and safety in healthy subjects.
Conclusion
Overall, Gremin® significantly enhanced endurance, improved muscle strength, lean body mass, fat-free mass and reduced fatigue in healthy subjects at a once-daily dose of 500 mg for 14 days and was found safe without any untoward adverse events. The present clinical evidence highlights the effectiveness of Gremin® in significantly enhancing endurance, improving muscle performance, and providing robust muscle protection, making it a potential supplement for athletes and active individuals looking to elevate their physical performance and recovery. However, long-term clinical study in healthy subjects may be warranted to confirm the long-term safety and effectiveness of Gremin®.
Acknowledgements
The authors acknowledge Zeus Hygia Lifesciences Pvt Ltd, India for supplying the investigational products used in this study.
Funding
This research project was funded by Zeus Hygia Lifesciences Pvt Ltd., India.
Data availability statement
The datasets generated and / or analyzed during the current study are available from the authors on reasonable request.
Conflict of interests
Authors Shankaranarayanan Jeyakodi and Arunkanth Krishnakumar are employees of Zeus Hygia Lifesciences Pvt Ltd., India. Other authors declare that they have no conflict of interests.
References
- Powers SK, Howley ET, Quindry J. Exercise Physiology: Theory and Application to Fitness and Performance (12th ed.). McGraw-Hill 2024.
- Joyner MJ, Coyle Endurance exercise performance: the physiology of champions. The Journal of Physiology 586 (2008): 35-44.
- Hyldahl RD, Hubal MJ. Lengthening our perspective: morphological, cellular, and molecular responses to eccentric exercise. Muscle & Nerve (49) 2014: 155-70.
- Frontera WR. Bigard The benefits of strength training in the elderly. Science & Sports 17 (2002): 109-116.
- Pope HG Jr, Wood RI, Rogol A, Nyberg F, Bowers L, Bhasin S. Adverse health consequences of performance-enhancing drugs: an Endocrine Society scientific statement. Endocrine reviews 35 (2014): 341-75.
- Rhodes RE, Janssen I, Bredin SSD, Warburton DER, Bauman A. Physical activity: Health impact, prevalence, correlates and interventions. Psychology & health 32 (2017): 942-975.
- Roberts CK, Barnard Effects of exercise and diet on chronic disease. Journal of applied physiology 98 (2005): 3-30.
- Nanavati K, Rutherfurd-Markwick K, Lee SJ, Bishop NC, Ali A. Effect of curcumin supplementation on exercise-induced muscle damage: a narrative review. European Journal of Nutrition 61 (2022): 3835-55.
- Fernández-Lázaro D, Mielgo-Ayuso J, Seco Calvo J, Córdova Martínez A, Caballero García A, Fernandez-Lazaro CI. Modulation of exercise-induced muscle damage, inflammation, and oxidative markers by curcumin supplementation in a physically active population: A systematic review. Nutrients 12 (2020): 501.
- Yun N, Kang JW, Lee SM. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: molecular evidence of its antioxidant and anti-inflammatory properties. The Journal of nutritional biochemistry 23 (2012): 1249-55.
- Zhang LT, Chang CQ, Liu Y, Chen ZM. Effect of chlorogenic acid on disordered glucose and lipid metabolism in db/db mice and its mechanism. Zhongguo yi xue ke xue yuan xue bao 33 (2011): 281-6.
- Jeyakodi S, Krishnakumar A, Chellappan DK, Subbanna R, Bansal S. Beneficial effect of Gremin, a proprietary dual antioxidant formulation on skeletal muscle injury – In vivo. Journal of Nutraceuticals and Food Science 6 (2021): 34.
- Jeyakodi S, Krishnakumar A, Bansal K, Patil R, Bhosale N, Ranade A. Effect of dual polyphenolic ingredient on muscle injury and performance: A prospective, double-blind, placebo-controlled, randomized, interventional study in healthy volunteers. Journal of Food Technology and Nutrition Sciences 4 (2022): 1-9.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. American Journal of Respiratory and critical care medicine 166 (2002): 111-7.
- Bohannon RW, Bubela DJ, Wang YC, Magasi SS, Gershon RC. Six-Minute Walk Test Vs. Three-Minute Step Test for Measuring Functional Endurance. Journal of strength and conditioning research 29 (2015): 3240-3244.
- Uth N, Sørensen H, Overgaard K, Pedersen PK. Estimation of VO2 max from the ratio between HRmax and HRrest--the Heart Rate Ratio Method. European Journal of applied physiology 91 (2004): 111-5.
- Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. Journal of the American College of Cardiology 37 (2001): 153-6.
- Le-Ngoc L, Janssen J. Validity and reliability of a hand-held dynamometer for dynamic muscle strength assessment. In: Kim C-T, editor. Rehabilitation Medicine. In Tech. 2012.
- Beaudart C, Rolland Y, Cruz-Jentoft AJ, Bauer JM, Sieber C, Cooper C, et al. Assessment of muscle function and physical performance in daily clinical practice: A position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Calcified Tissue International 105 (2019): 1-14.
- Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. Journal of psychosomatic research 39 (1995): 315-25.
- Córdova A, Drobnic F, Noriega-González D, Caballero-García A, Roche E, Alvarez-Mon M. Is curcumine useful in the treatment and prevention of the tendinopathy and myotendinous junction Injury? A Scoping Review. Nutrients 15 (2023): 384.
- Miyamoto T, Oguma Y, Sato Y, Kobayashi T, Ito E, Tani M, et al. Elevated creatine kinase and lactic acid dehydrogenase and decreased osteocalcin and uncarboxylated osteocalcin are associated with bone stress injuries in young female athletes. Scientific Reports 8 (2018): 18019.
- Ommati MM, Farshad O, Mousavi K, Khalili M, Jamshidzadeh A, Heidar R. Chlorogenic acid supplementation improves skeletal muscle mitochondrial function in a rat model of resistance training. Biologia 75 (2020): 1221-30.
- Liu X, Lin L, Hu G. Meta-analysis of the effect of curcumin supplementation on skeletal muscle damage status. PLoS One 19 (2024): e0299135.









 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 