Reduction in Cardiac STAT3 Phosphorylation at Site Ser-727 Subsequent to mTOR Overactivation Exacerbated Myocardial Ischemia Reperfusion Injury in Type 1 Diabetic Rats
Weiyi Xia†,1, Xiang Xie†,2, Chunyan Wang3, Liangqing Zhang1, Yin Cai1,4, Zhidong Ge2, Sheng Wang5, Zhengyuan Xia*,1,6,7, Danyong Liu*,1,3
1Department of Anesthesiology, Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong, China
2Department of Anesthesiology, The Second Affiliated Hospital and Yuying Children's Hospital, Wenzhou Medical University, Wenzhou, China
3Department of Anesthesiology, Affiliated Shenzhen Maternity and Child Healthcare Hospital, Southern Medical University, Shenzhen, Guangdong, China
4Department of Health Technology and Informatics, the Hong Kong Polytechnic University, Hong Kong, China
5Department of Anesthesiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
6State Key Laboratory of Pharmaceutical Biotechnology, University of Hong Kong, Hong Kong SAR, China
7Department of Anesthesiology and Perioperative Medicine, Zhengzhou Central Hospital Affiliated to Zhengzhou University, Zhengzhou, Henan, China.
†These authors equally contributed to this study
*Corresponding author: Zhengyuan Xia, Department of Anesthesiology, Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong, China. E-mail: zyxia@hku.hk
Danyong Liu, Department of Anesthesiology, Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong, China. E-mail: danyong_Liu@163.com
Received: 06 June 2025; Accepted: 16 June 2025; Published: 21 June 2025
Article Information
Citation: Weiyi Xia, Xiang Xie, Chunyan Wang, Liangqing Zhang, Yin Cai, Zhidong Ge, Sheng Wang, Zhengyuan Xia, Danyong Liu. Reduction in Cardiac STAT3 Phosphorylation at Site Ser-727 Subsequent to mTOR Overactivation Exacerbated Myocardial Ischemia Reperfusion Injury in Type 1 Diabetic Rats. Fortune Journal of Health Sciences. 8 (2025): 607-617.
View / Download Pdf Share at FacebookAbstract
Reduced levels of myocardial STAT3 activity in diabetic hearts may contribute to the increased susceptibility to ischemia-reperfusion injury (I/RI). The protein mammalian target of rapamycin (mTOR) can regulate metabolism and cell processes and plays major roles in the dynamics of I/RI. However, the role of mTOR in the regulation of myocardial STAT3 and thereby its impact on myocardial I/RI in diabetes at relatively late stages of the disease is unknown. Type Ι diabetes was induced by Streptozotocin in Sprague-Dawley rats. Myocardial I/RI was achieved with coronary occlusion for 30 minutes followed by reperfusion for 2 hours in the absence or presence of the mTOR inhibitor rapamycin. In vitro cardiomyocyte hypoxia/re-oxygenation (H/R) was established in H9C2 cells. In type Ι diabetic rats, myocardial levels of troponin-I (Tn- I), lipid peroxidation products 15-F2t-Isoprostane (15-F2t-Iso) and malondialdehyde (MDA), and the protein expression of mTOR were all significantly increased?while SOD activity and the level of phosphorylated STAT3 (p-STAT3-Ser727) were both significantly decreased compared to non-diabetic rats. Myocardial I/RI significantly increased the infarct size and further increased the mTOR activation and decreased p- STAT3-Ser727 compared to diabetic rats without I/RI. The selective mTOR inhibitor rapamycin reversed these changes and conferred cardioprotective effect. In H9C2 cells, high glucose (HG) significantly increased lactic dehydrogenase (LDH) release, apoptosis cells, reactive oxygen species (ROS) production and the activation of mTOR, but decreased p-STAT3-Ser727. H/R further increased cellular injury, while mTOR gene knock-down significantly reduced H/R injury. It is concluded that myocardial mTOR was enhanced in type I diabetes which is attributable to the increased myocardial susceptibility to I/RI. Enhancing cardiac p-STAT3-Ser727 may represent a mechanism by which mTOR inhibition attenuated myocardial I/RI in type I diabetes.
Keywords
<p>Diabetes; myocardial ischemia reperfusion injury; mTOR; STAT3; ROS; Apoptosis</p>
Article Details
Introduction
Myocardial ischemia reperfusion injury (I/RI) is a leading cause of death especially for subjects with diabetes, but its underlying mechanisms are largely unknown. Myocardial infarction is irreversible and is the characteristic consequence of sustained myocardial ischemia with or without reperfusion. Increasing duration of ischemia causes progressive irreversible injury and thus, reducing infarct size is the first goal for I/RI protection. The pathophysiology of I/RI mainly includes autophagy, necrosis, apoptosis, necroptosis, pyroptosis and ferroptosis(1,2). A great number of signaling molecules participate in I/RI process, for example, autacoids, such as adenosine and bradykinin, lipid molecules that all have closed relationship with protein STAT3, a key protein in the two cardiac pro-survival RISK and SAFE pathways. These two signaling pathways correlate with each other and play central roles in I/RI and ischemia preconditioning(3,4). The mammalian target of rapamycin (mTOR) protein is a 289 KD serine/threonine kinase, which can regulate cell growth and survival(5), mTOR also plays important roles and functions as nutrient energy sensor facilitating the regulation of autophagy(6). With respect to the heart, mTOR is a necessary component for the physiological regulation of functions related to cardiac structure and cardiac metabolism(7). mTOR also facilitates the maintenance of normal microvascular barrier functions and endothelial permeability. Previous research has indicated that the PI3K/Akt/mTOR signaling pathway is mediated by insulin(6).The mTOR-dependent angiogenesis signaling pathway, and the mTOR activation- signaling pathway are major signaling pathways related to cardioprotection(7). Das et al. reported that application of the mTOR inhibitor rapamycin (0.25 mg/kg, i.p.) prior to ischemia consequently reduced I/RI in mice by inducing the activation of the janus kinase 2 (JAK2) Signal Transducer and Activator of Transcription signaling (STAT3) (8). These findings demonstrated that mTOR was a possible regulator of STAT3. STAT3 has also been found to play central roles in maintaining cardiomyocyte function, modulating conditions in the cardiac microenvironment, and is known to communicate with cardiac fibroblasts (9,10). STAT3 participated in myocardial preconditioning, and the phosphorylation of STAT3 at sites of tyrosine705 and serine727 have been shown to confer cardioprotection. In Heusch et al studies, they demonstrated the STAT3 phosphorylation at tyrosine705 was significantly greater at 120 minutes reperfusion after treatment with remote ischemic preconditioning (RIPC) in pigs, and activation of STAT3 was necessary for ischemic postconditioning in pigs, while RIPC in human seems to rely more on STAT5 than on STAT3 possibly because the underlying signaling be different between species (11,12). Our researches have also indicated that STAT3 played crucial roles in both ischemic preconditioning and postconditioning cardioprotection in human and in rodents (13,14). Furthermore, expression of STAT3 was found to have been reduced in the myocardium of subjects with diabetes, and this may constitute the main reason why diabetes afflicted subjects were more likely to experience myocardial I/RI and were less responsive to protective treatments including pre- and postconditioning (14,15). However, whether or not mTOR functions as an upstream effector of STAT3 in myocardial I/RI in type I diabetes is unclear. Therefore, we sought to use streptozotocin (STZ) - induced type I diabetic rats and high glucose exposed H9C2 cells to facilitate investigations of the roles of mTOR-STAT3 in myocardial I/RI in type I diabetes. Our main goal was to provide evidence facilitating the identification of novel treatment targets for myocardial I/RI in diabetes.
Methods
In Vivo Studies
Animals and diabetes model
6-8-week-old specific pathogen free (SPF) male Sprague–Dawley rats (260 ± 10 g) received standard care and were given free access to food and water. The animals were maintained at a constant air temperature of 23 °C, a constant humidity of 50 %, and a 12 hour (h) light and 12 h dark cycle. The experimental procedures were carried out in accordance with the US National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and were approved by the Ethical Committee on Animal Research of the Guangdong Medical University. Diabetes in rats were induced by injection of streptozotocin (STZ; 60 mg/kg, STZ was dissolved in 0.1 M citrate buffer and pH = 4.5; Sigma, St. Louis, MO) into the tail vein and following the protocol as described in more detail previously and rats with fasting blood glucose at 16.7 mmol/L or higher 72 h after STZ injection were recognized as diabetic (16,17).
Experimental procedure
The rats were randomly assigned into following groups: (1) Ctrl:Control (2) D8w: Rats at 8-weeks of diabetes; (3) D8w+I/R: D8w rats with ischemia/reperfusion (I/R); (4) D8w+I/R+Rapamycin: D8w rats treated with Rapamycin (Sigma-Aldrich, St.
Louis, MO) before being subjected to I/R. At 4 weeks of diabetes, diabetic rats started to receive 1 mg/kg/day of rapamycin via orogastric gavage for a duration of 4 weeks before the diabetic rats (D8w) were subjected to I/R study(16).
The rats were anesthetized by intraperitoneal injection of 3% sodium pentobarbital (50mg/kg) and placed on a temperature-controlled heating pad to expose the left anterior descending (LAD) between the third and fourth ribs. We established I/R using 5–0 suture passed through the LAD arteries to form a slipknot for 30 minutes (min), and reperfusion was established by releasing the knot to restore blood flow, and the duration of reperfusion was 2 hours (h) as described before(18,19). TTC (1%,2,3,5-triphenyltetrazolium chloride) based staining was used to facilitate the determinations of myocardial infarction as described(20). Upon the completion of reperfusion, normal regions of left ventricles (LV) were defined by way of ligating the left anterior descending and infusing 5 % Evans Blue (Sigma, St Louis, MO) through the right jugular vein. We then applied an overdose of pentobarbital through injection to euthanize rats. We subsequently dissected and froze the hearts for 15 min at -20 °C, and then sliced the hearts into five 1-1.5 mm sections. Slices were submerged in 1% TTC (pH 7.4) for 20 min at room temperature to facilitate delineation of infarcted areas. Images were captured through the use of an Axisoplus Image-Capturing System (Zeiss). We subsequently analyzed the images by use of Axiovision Rel. 4.5 Image- Analyzing software.
Assessment of DNA fragmentation through terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
The levels of fragmentation of DNA were assessed using terminal deoxynucleotidyl transferase nick end labeling (TUNEL) assay kits provided by Roche Applied Science (Indianapolis, IN, USA). In brief, the cells were fixed by using 4 % polyoxymethylene on the slides. Then, the samples were washed thrice with PBS for 5 min each time, followed by 2 min of permeabilization using 0.1 % (v/v) Triton X-100. Samples were further permeabilized by using proteinase K (30 mg/ml, 30 min, 37° C). We then washed the samples in PBS following the manufacturer’s protocols with respect to detection of apoptotic cells. DNase I was used to facilitate induction of DNA strand breaking and as positive control, whereby we omitted TdT from the mixture and reaction for our negatively oriented control. Positive identifications of TUNEL-based staining were observed and determined under fluorescence microscopy (TE2000U, Nikon, Tokyo, Japan) and by using a B-2A filter (excitation filter of 450- 490 nm, 505 nm dichroic mirror, bandpass filter of 520 nm).
Dihydroethidium (DHE) staining
DHE staining was used for reactive oxygen species (ROS) detection following manufacturer’s protocols. Red fluorescence was emitted when DHE was oxidized by superoxide, and it was detected by fluorescence microscopy (Olympus, Tokyo, Japan) as described (21).
mTOR siRNA gene silencing studies in H9C2 cells.
We cultured H9C2 cardiomyocytes at 37 °C in a constant atmosphere of 5 % CO2 and Dulbecco's modified Eagle's medium (DMEM) (Gibco Laboratories) containing 10% Fetal bovine serum (FBS) (Gibco Laboratories). In in vitro experiments, we aimed to confirm the protective effect of mTOR gene silencing. In order to reduce the interference of other factors, we chose mTOR siRNA. We used commercially supplied mTOR siRNA (Santa Cruz Biotechnology, Texas, USA) to knock down mTOR expression following the manufacturer’s protocols. We seeded and assigned 2*105 H9C2 cells each into 4 treatment groups composed as follows: (1) normal levels of glucose (NG): 5.5 mM; (2) high glucose (HG): 30 mM glucose; (3) HG + H/R; (4) HG +H/R + siRNA mTOR. With respect to control siRNA or mTOR siRNA treatment groups, we incubated H9C2cells in either NG or HG in DMEM for 48 hours(h) prior to subjecting the H9C2 cells to H/R. 6 hours of hypoxic conditions were accomplished by equilibration of a humidified plexiglass chamber which contained myocytes and a constant atmosphere of 5%CO2 and 95%N2. Hypoxic conditions were confirmed by measuring O2 concentrations in chambers and the hypoxia was set at oxygen levels fell to ≤ 0.1%. Post-hypoxia, we transferred cells to a CO2 incubator to facilitate re-oxygenation for 12 hours and then we collected cells and mediums for further analyses.
Measurement of Lactate dehydrogenase (LDH) activity
LDH is a major indicator of myocardial I/RI injury. Thus, we examined levels of LDH in H9C2 culture mediums by LDH Cytotoxicity Assay Kits (Roche, USA) followed the manufacturer’s protocols as described (22).
Western blotting assays
In our animal-based experiments, we homogenized rats’ LV tissues in 1× lysis buffer acquired from Cell Signaling Technology (Beverly, MA) and centrifuged samples at 13, 200 g for 30 min, while H9C2 cells in ice-cold 1× lysis buffer were centrifuged for 10 min. The supernatant was collected as total protein. We then used Bradford protein assays to determinate the protein concentrations. Then, we sampled equal amounts of protein homogenates, resolved by the SDS-PAGE (7.5-12.5%). Next, we transferred the samples to polyvinylidene nitrocellulose membranes and completed processing as described previously(23, 24). Antibodies for cleaved- caspase3, mTOR, phosphorylation-mTOR (p-mTOR), p-STAT3-Ser727, Total- STAT3, GAPDH antibodies, and secondary antibody were all purchased from Cell Signaling Technology (Beverly, MA). The protein bands were detected through the standard protocols for the ECL method. Densitometry for protein band assessments was completed in Image J Software (National Institutes of Health, USA). We reported data for normalized protein band density in arbitrary units.
Statistical analyses
Analyses were performed in a blinded manner. Data are presented as mean ± the standard error of the mean (± S.E.M), and were analyzed with one-way analysis of variance (ANOVA) and Tukey’s post hoc tests to facilitate determinations of statistical significance between different treatment groups (GraphPad Prism Software, Version 8.0). Differences were considered as statistically significant at p< 0.05.
Results
mTOR inhibition with Rapamycin attenuated myocardial I/RI in diabetes
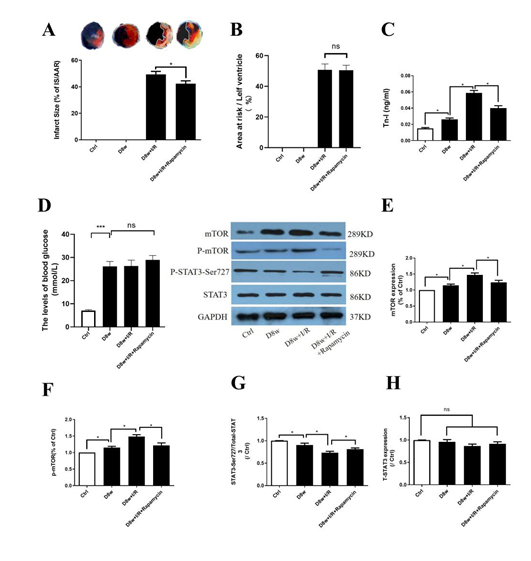
As Fig. 1A and B indicates, mTOR inhibitor rapamycin significantly reduced post- ischemic myocardial infarction size (IS) (Fig. 1A, B; D8w +I/R+rapamycin vs. D8w+I/R) and significantly reduced the release of Tn-I (Fig.1C; D8w+I/R+rapamycin vs. D8w+I/R). The blood glucose levels in rats at 8 weeks of diabetes were 27.8 ± 2.4 mM and 28.3 ± 2.6 mM respectively in the D8w and D8w+I/R groups at baseline, which were significantly higher than that in the non-diabetic group (7.5 ± 0.7 mM, p<0.05,Fig. 1D). 4 weeks of treatment with rapamycin at 1 mg/kg /day in diabetic rats did not have significant impact on blood glucose (28.8 ± 2.3 mM in D8w+I/R+rapamycin at baseline, p>0.05 vs. D8w or D8w+I/R, Fig. 1C). The levels of cardiac mTOR protein and phosphorylated mTOR (p-mTOR) increased significantly in D8w rats as compared non-diabetic control groups (Figs.1E and 1F). In contrast, the levels of phosphorylation of STAT3 at Ser727 (p-STAT3-Ser727) were significantly decreased (Fig.1G), while total STAT3 did not significantly differ between D8w rats and non-diabetic control(Figs.1H), and I/R further decreased p- STAT3-Ser727 (all p< 0.05; D8w vs. Ctrl, or D8w+I/R vs. D8w, Figs.1F and 1G). Treatment with mTOR inhibitor rapamycin reduced the expression of mTOR and enhanced activation of p-STAT3-Ser727 during I/RI.
Figure 1: The effects of mTOR inhibitor Rapamycin on post-ischemic myocardial injury. A: infarct size (IS); B: area at risk; C: serum Tn-I release; D: the levels of blood glucose; E: mTOR protein expression; F: phosphorylated mTOR (p-mTOR) protein expression; G: protein expression of p-STAT3(Ser727); H: STAT3 protein expression. Ischemia reperfusion (I/R) was achieved by 30 minutes ischemia followed by 2 hours reperfusion in diabetic rats. Data are expressed as mean ± S.E.M (n = 6 per group), *p<0.05.
mTOR inhibition with Rapamycin decreased post-ischemic myocardial ROS production
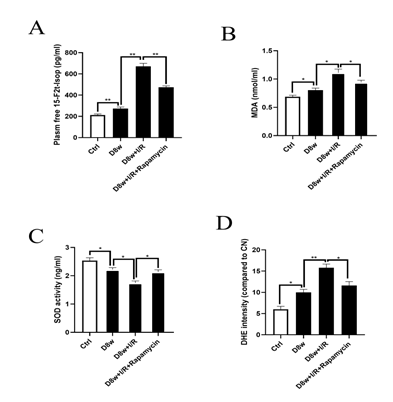
As Figs. 2A, 2B and 2D indicated, the myocardial lipid peroxidation products 15-F2t-Iso and MDA were significantly increased that was concomitant with increased production of ROS evidenced as increased Dihydroethdium (DHE) staining in diabetic rats, while the SOD level was significantly decreased (Fig. 2C) as compared to control(all p<0.05; D8w vs. Ctrl), and these changes were further exacerbated by I/R (p<0.05, D8w+I/R vs. D8w). Treatment with rapamycin reduced the post-ischemia myocardial ROS level induced by I/R and attenuated the increases in 15-F2t-Iso and MDA (all p<0.05; D8w+I/R+rapamycin vs. D8w+I/R).
Figure 2: mTOR inhibition with Rapamycin decreased post-ischemic myocardial ROS production. A: 15-F2t-Isoprostane(15-F2t-Iso) release; B: MDA release; C: SOD activity; D: DHE staining. Ischemia reperfusion (I/R) was achieved by 30 minutes ischemia followed by 2 hours reperfusion in diabetic rats. Data are expressed as mean ± S.E.M (n =6 per group), *p<0.05.
Effects of mTOR gene knockdown on hypoxia re-oxygenation(H/R) injury in H9C2 cells
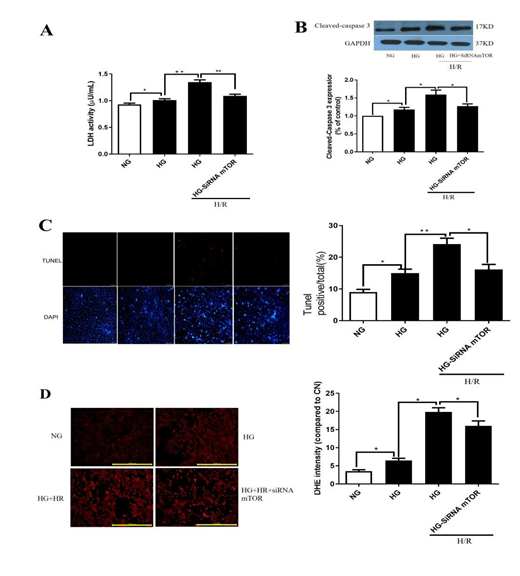
Results exemplified in Fig.3A indicated that LDH release increased significantly under high glucose (HG) in comparison to normal glucose (p< 0.05, HG vs.NG). Moreover, H/R further increased the levels of LDH release and exacerbated cell injury (p<0.05, HG+H/R vs. HG). mTOR gene knockdown via siRNA significantly suppressed H/R induced increases of LDH release and post-hypoxic cell injury under HG (all p<0.05, HG+H/R+siRNA mTOR vs. HG+H/R). As shown in Figs. 3B and 3C, the expression of protein cleaved-caspase3 and the percentage of apoptotic cells were significantly increased after HG treatment (p< 0.05; HG vs.NG,). And, H/R further increased the levels of expression of cleaved-caspase3 and apoptotic cell death (p< 0.05; HG+H/R vs. HG). Application of mTOR siRNA decreased the levels of expression of cleaved-caspase3 and apoptotic cell death (Figs. 3B and 3C; all p< 0.05; HG+H/R+siRNA mTOR vs. HG+H/R). As exemplified in Fig. 3D, DHE staining indicated that HG induced a significant increase of ROS (p< 0.05; HG vs.NG), which was further significantly increased by H/R. mTOR gene silence with siRNA prevented H/R induced further increase of ROS under HG conditions (all p< 0.05; HG+H/R vs. HG; HG+H/R+siRNA mTOR vs. HG+H/R). The changes in the patterns of apoptotic cell death largely mirrored the changes in the levels of ROS production assessed by DHE staining (Fig. 3D).
Figure 3: Effects of mTOR gene knockdown on hypoxia re-oxygenation(H/R) injury in H9C2 cells. A: LDH releasing; B: protein cleaved-caspase3 expression; C: TUNEL staining; D: DHE staining. In HG group, H9C2 cells were subjected to 30 mM high glucose for 48 hours, and then all cells subjected to 6 hours hypoxia and 12 hours reperfusion. Data are expressed as mean ± S.E.M of two independent experiments each performed in triplicate. (n=6 per group) *p< 0.05.
Effects of mTOR gene knockdown on mTOR and STAT3 protein expressions
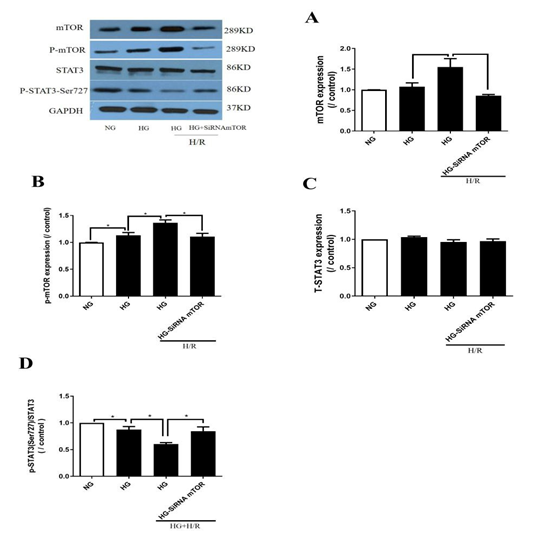
As shown in Figs. 4A and 4B, the protein level of mTOR increased moderately while the p-mTOR proteins increased significantly under HG compared to NG (p< 0.05; HG vs. NG) and H/R further exacerbated these increases (p< 0.05; HG+H/R vs. HG). After knockdown of mTOR, mTOR and p-mTOR were both significantly decreased (p< 0.05; HG+H/R+siRNA mTOR vs. HG+H/R, Figs. 4A, 4B). Fig. 4C demonstrated that total STAT3 did not significantly affected by HG, H/R, nor by mTOR gene silencing. The results showed that p-STAT3-Ser727 decreased significantly under HG as compared to NG (p< 0.05; HG vs.NG, Fig. 4D), and H/R further decreased p-STAT3-Ser727 in HG condition (p< 0.05; NG+H/R vs. NG; HG+H/R vs. HG). In contrast, mTOR gene silencing restored the expression of p- STAT3 -Ser727 that was otherwise reduced by H/R (p< 0.05; HG+H/R+siRNA mTOR vs. HG+H/R, Fig. 4D).
Figure 4: Effects of mTOR gene knockdown on mTOR and STAT3 protein expressions. A: protein mTOR expression; B: protein p-mTOR expression; C: protein STAT3 expression; D: protein p-STAT3(Ser727) expression. In HG group, H9C2 cells were subjected to 30 mM high glucose for 48 hours, and then subjected to 6 hours hypoxia and 12 hours of reoxygenation. Data are expressed as mean ± S.E.M of two independent experiments each performed in triplicate.( n=6 per group.) *P<0.05.
Discussion
The findings from our study indicated that hyperglycemia induced significant activation of cardiac mTOR and induced reductions in p-STAT3-Ser727 activation. Further, we showed that inhibition of mTOR could reduce myocardial I/R or H/R injury under HG condition through enhancement of p-STAT3-Ser727 activation. Our current study characterized the dynamics of mTOR-p-STAT3-Ser727 with respect to myocardial I/RI in hearts impacted by diabetes, this offered additional insights regarding the mechanism of diabetic myocardial I/RI. Infarct size is the major endpoint of myocardial I/R, and it could be affected by various kinds of factors such as gender, age as well as complications such as hypertension, hyperlipidaemia and especially diabetes (25). In animal studies, although studies showed some controversial results but it can be confirmed that infarct size is significantly affected by the type of animal models and the duration or severity of diabetes. In clinical study, the outcome of acute myocardial infarction in diabetic patients was obviously worse than that in non-diabetic patients, and cardioprotective efficacy of interventions like preconditioning was not good in diabetes as compared to non-diabetes. The degree of preserved neural pathways, and inherent cardioprotective phenotype in different patient cohorts may affect the efficiency to cardioprotective treatments. Further studies are needed to unlock the mechanism of diabetes on the severity of myocardial ischemic insults and to find the treatment target. mTOR is a type of serine/threonine kinase which functions mainly as a nutrient and energy sensor, and plays essential roles in protein synthesis and autophagy, cell growth and survival (26,27). AMPK/mTOR has been identified as a major signaling pathway in the mTOR family and regulates autophagy. However, there is research which showed that AMPK did not directly regulate mTOR, but instead mTOR could be regulated by Akt (28).
In the current study, we found that the levels of mTOR and p-mTOR were both increased, and other research indicated that mTOR participates in the regulation of autophagy and can be regulated by other upstream effectors, including oxidative stress (29). It has been reported that mTOR modulated autophagy and protected cardiomyocytes from oxidative stress-induced toxicity (30,31), and vice versa, oxidative stress may provoke mTOR activation and subsequently impair autophagy (32). Research revealed that the chronic rapamycin treatment improves cardiac function in T2D mice through attenuation of oxidative stress and alteration of antioxidants and contractile as well as glucose metabolic protein expression (33). mTOR has been shown to be essential in preventing apoptosis during ischemic postconditioning mediated cardiac protection in rats (31). In our study, application of mTOR inhibitor rapamycin in in vivo study or mTOR gene knockdown in H9C2 cells both decreased the myocardial I/RI or H/R injury, manifested by significantly decreased ROS level that was concomitant with reduced expression of protein cleaved-caspase3 and significant reduction in apoptotic cell death. Continuous activation of mTOR was detrimental to vascular systems (34), and thus reducing the activation of mTOR could reduce vasculopathy and improve microcirculatory coronary flows following cardiac transplantation (35,36). Based upon these findings, mTOR not only plays a key role as a regulator of autophagy and apoptosis but also plays an important role with respect to myocardial I/RI through its effect upon other types of signaling (37).
In our study, we found that reduced activation of mTOR with rapamycin consequently decreased post-ischemic ROS levels and oxidative damage manifested as reductions in 15-F2t-Iso and MDA. Our finding that rapamycin confirms cardioprotection against myocardial I/RI in type I diabetes is in line with findings of a recent study in which application of rapamycin reduced myocardial I/RI in conscious rabbits (38). Our current study further explored the mechanism whereby rapamycin confers cardioprotection in type I diabetes. Collectively, findings gained from our current study support the hypothesis that inhibition of mTOR in diabetes may confer myocardial protective effects through reducing oxidative stress and subsequently enhance cardiac p-STAT3, although the underlying mechanisms and dynamics merit additional rigorous investigations. In deed, we have shown previously that antioxidant treatment with N-Acetylcysteine enhanced cardiac p-STAT3 and reduced post-ischemic myocardial injury in type 1 diabetic rats (39). Study has shown that after prolonged use of rapamycin, it can inhibit mTOR C2 receptors, thereby weakening insulin sensitivity and changing blood sugar levels. Because it was used in small doses in our study, there was a slight change in blood glucose but there was no statistical difference, and this finding is in keeping with a previous study (40). The mechanism whereby rapamycin inhibits mTOR expression may be through the formation of complex with FKBP-12 protein, which then binds to the FRB region of mTOR and inhibits the activation of mTOR, thereby inhibiting the function of downstream related factors (41).
Signal transduction and activation of transcription 3 (STAT3) is the major member of signaling pathway of survival activating factor enhancement pathway (SAFE) that has significant roles in myocardial I/RI especially in diabetes (13). The expression of STAT3 decreased in the heart of diabetes which may be the reason why diabetic hearts are prone to myocardial I/RI (26). STAT3 was essential for ischemic preconditioning (IPC), ischemic postconditioning (IPostC) and remote IPC (RIPC). However, as Heusch et al reported, in separate studies involving IPC, IPostC and RIPC studies, the STAT3 phosphorylation at-Tyr705 (p-STAT3-Tyr705) were all significantly increased by IPC, IPostC or RIPC. However, after having been analyzed the phosphorylation of STAT3 and other signaling proteins in left ventricular biopsies of IPC, IPostC and RIPC in one approach, they only observed that the STAT3 phosphorylation at-Tyr705 at early reperfusion was significantly increased along with infarct size reduction by IPC, but only trend wise by IPostC and RIPC. These studies demonstrated that STAT3 phosphorylation at-Tyr705 is necessary in conditioning cardioprotection but it may have its own specific time course in myocardial conditioning (11,12). Janus kinase 2(JAK2) is one of the upstream effectors of STAT3 production, and as reported STAT3 Tyr705 was directly regulated by JAK2 whereas STAT3 Ser727 was regulated by ERK/MAPK (27). Some researchers have reported that STAT3 was also affected by protein kinase C (PKC) (15,42), while other findings have provided evidence that STAT3 has an effect on autophagy by way of serving as the downstream effector of mTOR (43). In this study, we found that mTOR inhibition or gene knockdown could subsequently restore I/R or H/R induced reduction in p- STAT3 Ser727 and attenuate myocardial I/RI. Recent research appears to support our findings in that treatments with rapamycin increased the levels of expression of p- STAT3-Tyr705(44). Inhibition of mammalian target of rapamycin protects against reperfusion injury in diabetic heart through STAT3 signaling (45). Thus, our findings suggested that mTOR is an important regulator in myocardial I/RI. However, if mTOR regulates autophagy through STAT3, the dynamics and mechanism underlying this process need further research. Autophagy can have protective effects during ischemia while contrastingly plays detrimental roles during reperfusion, thus, the relationship between STAT3 and autophagy deserves more research (46,47). In our another study exploring the effects of RIPC, we demonstrated that STAT3 gene knockdown cancelled simulated-RIPC mediated cardioprotective effect, and that RIPC conferred its cardioprotection against myocardial I/RI mainly through PKC- STAT3(Ser727) (14). Further study is needed to define the roles and interplays in between mTOR and STAT3 and their impact on autophagy as well as on in the context of myocardial I/RI in diabetes.
The limitation of this study is due to the fact that the autophagic response can exhibit different patterns in type 1 and type 2 diabetic cardiomyopathy. T1DM mice with low insulin levels exhibit controversial autophagy in STZ-induced T1DM metabolic cardiomyopathy compared to T2DM mice (48). We used only a type 1 diabetes model, while in clinical settings most diabetic patients were type II diabetes. In type II diabetic mice with myocardial I/R, chronic application of Rapatar, a novel nanoformulated micellar of rapamycin, has been shown to attenuate myocardial I/RI that was associated with increased STAT3 activation (49). In our current study, we extended the study to explore the role of mTOR in type I diabetes in the context of myocardial I/RI. Compared to type II diabetes, the major pathological change of type I diabetes is the insulin deficiency and high glucose was the primary element. The type II diabetes was a polygenetic disease, it can be divided into two kinds, with or without obesity, and several genes can predispose individuals to developing the disease, so its mechanism is more diverse. Therefore, on the basis of our initial findings regarding the mechanism of myocardial I/RI in type I diabetes, future study shall be extended to study the impacts and mechanisms of type II diabetes on myocardial I/RI.
Conclusion
Our study demonstrated that increased activation of mTOR in the heart of type I diabetic rats causes subsequent reduction in p-STAT3-Ser727 and exacerbated myocardial I/RI. Further, we found that knockdown of mTOR can increase the expression p-STAT3-Ser727 and reduce cardiomyocyte H/R injury. The mechanistic insights gained in the current study may help facilitate the development of targeted therapies against myocardial I/RI in diabetes.
Declarations of Conflict of interest
No conflicts of interest are declared by the authors.
Funding
This study was supported by the funding from the National Natural Science Foundation of China (82270306).
Statement
A preprint has previously been published (50). The original contributions presented in this study are included in the article, further inquiries can be directed to the corresponding authors.
Author contributions
DL, WX and ZX conceived the work. WX, CW, LZ and YC performed the experiments. DL, ZG and SW participated in data analysis and interpretation. WX and XX drafted the manuscript. ZX and DL revised and approved the manuscript. All authors read and approved the final manuscript.
Acknowledgment
The authors acknowledge for Mr. Peng Xing (Shenzhen Ivy-Valued Biotechnology Co. Ltd) for technical assistance during the experiments and acknowledge Vanscholar Editors Ltd, Canada for English language editing service.
References
- Heusch G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nature reviews Cardiology 17 (2020): 773-89.
- He J, Liu D, Zhao L, et al. Myocardial ischemia/reperfusion injury: Mechanisms of injury and implications for management (Review). Experimental and therapeutic medicine 23 (2022): 430.
- Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circulation research 116 (2015): 674- 99.
- Cheng X, Li H, Yan Z, et al. Ischemic limb preconditioning-induced anti-arrhythmic effect in reperfusion-induced myocardial injury: is it mediated by the RISK or SAFE pathway? Pflugers Archiv: European journal of physiology 474 (2022): 979-91.
- Oka SI, Hirata T, Suzuki W, et al. Thioredoxin-1 maintains mechanistic target of rapamycin (mTOR) function during oxidative stress in cardiomyocytes. The Journal of biological chemistry 292 (2017): 18988-9000.
- Wang S, Wang C, Yan F, et al. N-Acetylcysteine Attenuates Diabetic Myocardial Ischemia Reperfusion Injury through Inhibiting Excessive Autophagy. Mediators of inflammation (2017): 9257291.
- Yao H, Han X, Han X. The cardioprotection of the insulin-mediated PI3K/Akt/mTOR signaling pathway. American journal of cardiovascular drugs: drugs, devices, and other interventions 14 (2014): 433-42.
- Das A, Salloum FN, Durrant D, et al. Rapamycin protects against myocardial ischemia-reperfusion injury through JAK2-STAT3 signaling pathway. Journal of molecular and cellular cardiology 53 (2012): 858-69.
- O'Sullivan KE, Breen EP, Gallagher HC, et al. Understanding STAT3 signaling in ardiac ischemia. Basic research in cardiology 111 (2016): 27.
- Heusch G, Musiolik J, Gedik N, et al. Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circulation research 109 (2011): 1302-8.
- Lieder HR, Kleinbongard P, Skyschally A, et al. Vago- Splenic Axis in Signal Transduction of Remote Ischemic Preconditioning in Pigs and Rats. Circulation research 123 (2018): 1152-63.
- Kleinbongard P, Amanakis G, Skyschally A, et al. Reflection of Cardioprotection by Remote Ischemic Perconditioning in Attenuated ST-Segment Elevation During Ongoing Coronary Occlusion in Pigs: Evidence for Cardioprotection From Ischemic Injury. Circulation research 122 (2018): 1102-8.
- Wu Q, Wang T, et al. Cardiac protective effects of remote ischaemic preconditioning in children undergoing tetralogy of fallot repair surgery: a randomized controlled trial. European heart journal 39 (2018): 1028-37.
- Li H, Yao W, Liu Z, et al. Hyperglycemia Abrogates Ischemic Postconditioning Cardioprotection by Impairing AdipoR1/Caveolin-3/STAT3 Signaling in Diabetic Rats. Diabetes 65 (2016): 942-55.
- Wang C, Li H, et al. Repeated Non-Invasive Limb Ischemic Preconditioning Confers Cardio protection Through PKC-ԑ/STAT3 Signaling in Diabetic Rats. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology 45 (2018): 2107-21.
- Lei S, Li H, Xu J, et al. Hyperglycemia-induced protein kinase C β2 activation induces diastolic cardiac dysfunction in diabetic rats by impairing caveolin-3 expression and Akt/eNOS signaling. Diabetes 62 (2013): 2318-28.
- Zeng F, Luo J, Han H, et al. Allopurinol ameliorates liver injury in type 1 diabetic rats through activating Nrf2. International journal of immunopathology and pharmacology 35 (2021): 20587384211031417.
- Chu M, Planas O, Company A, et al. Unravelling the mechanism of cobalt-catalysed remote C-H nitration of 8-aminoquinolinamides and expansion of substrate scope towards 1-naphthylpicolinamide. Chemical science 11 (2020): 534- 42.
- Lindsey ML, Bolli R, Canty JM, et al. Guidelines for experimental models of myocardial ischemia and infarction. American journal of physiology Heart and circulatory physiology 314 (2018): H812-h38.
- He Y, Cai Y, Sun T, et al. MicroRNA-503 Exacerbates Myocardial Ischemia/Reperfusion Injury via Inhibiting PI3K/Akt- and STAT3-Dependent Prosurvival Signaling Pathways. Oxidative medicine and cellular longevity (2022): 3449739.
- Luo J, Yan D, Li S, et al. Allopurinol reduces oxidative stress and activates Nrf2/p62 to attenuate diabetic cardiomyopathy in rats. Journal of cellular and molecular medicine 24 (2020): 1760-73.
- Han RH, Huang HM, Han H, et al. Propofol postconditioning ameliorates hypoxia/reoxygenation induced H9c2 cell apoptosis and autophagy via upregulating forkhead transcription factors under hyperglycemia. Military Medical Research 8 (2021): 58.
- Cai Y, Liu H, Song E, et al. Deficiency of telomere-associated repressor activator protein 1 precipitates cardiac aging in mice via p53/PPARα signaling. Theranostics 11 (2021): 4710-27.
- Tan F, Cao Y, Zheng L, et al. Diabetes exacerbated sepsis-induced intestinal injury by promoting M1 macrophage polarization via miR-3061/Snail1 signaling. Frontiers in immunology 13 (2022): 922614.
- Kleinbongard P, Bøtker HE, Ovize M, et al. Co-morbidities and co- medications as confounders of cardioprotection-Does it matter in the clinical setting? British journal of pharmacology 177 (2020): 5252-69.
- Kim DH, Sarbassov DD, Ali SM, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110 (2002): 163-75.
- Ma J, Meng Y, Kwiatkowski DJ, et al. Mammalian target of rapamycin regulates murine and human cell differentiation through STAT3/p63/Jagged/Notch cascade. The Journal of clinical investigation 120 (2010): 103-14.
- Mourouzis I, Giagourta I, Galanopoulos G, et al. Thyroid hormone improves the mechanical performance of the post-infarcted diabetic myocardium: a response associated with up-regulation of Akt/mTOR and AMPK activation. Metabolism: clinical and experimental 62 (2013): 1387-93.
- Zhao D, Yang J, Yang L. Insights for Oxidative Stress and mTOR Signaling in Myocardial Ischemia/Reperfusion Injury under Diabetes. Oxidative medicine and cellular longevity (2017): 6437467.
- Chong ZZ, Shang YC, Maiese K. Cardiovascular disease and mTOR signaling. Trends in cardiovascular medicine 21 (2011): 151-5.
- Wagner C, Tillack D, Simonis G, et al. Ischemic post-conditioning reduces infarct size of the in vivo rat heart: role of PI3-K, mTOR, GSK-3beta, and apoptosis. Molecular and cellular biochemistry 339 (2010): 135-47.
- Fourcade S, Ferrer I, Pujol A. Oxidative stress, mitochondrial and proteostasis malfunction in adrenoleukodystrophy: A paradigm for axonal degeneration. Free radical biology & medicine 88 (2015): 18-29.
- Das A, Durrant D, Koka S, et al. Mammalian target of rapamycin (mTOR) inhibition with rapamycin improves cardiac function in type 2 diabetic mice: potential role of attenuated oxidative stress and altered contractile protein expression. The Journal of biological chemistry 289 (2014): 4145-60.
- Popescu NI, Lupu C, Lupu F. Extracellular protein disulfide isomerase regulates coagulation on endothelial cells through modulation of phosphatidylserine exposure. Blood 116 (2010): 993- 1001.
- Mancini D, Pinney S, Burkhoff D, et al. Use of rapamycin slows progression of cardiac transplantation vasculopathy. Circulation 108 (2003): 48- 53.
- Sinha SS, Pham MX, Vagelos RH, et al. Effect of rapamycin therapy on coronary artery physiology early after cardiac transplantation. American heart journal 155 (2008): 889.e1-6.
- Chen M, Zhao N, Shi W, et al. Glucose-dependent insulinotropic polypeptide/glucagon-like peptide 1 receptor agonist tirzepatide promotes branched chain amino acid catabolism to prevent myocardial infarction in non-diabetic mice. Cardiovascular research 121 (2025): 454-67.
- Samidurai A, Ockaili R, Cain C, et al. Preclinical model of type 1 diabetes and myocardial ischemia/reperfusion injury in conscious rabbits- demonstration of cardioprotection with rapamycin. STAR protocols 2 (2021): 100772.
- Lin J, Wang T, Li Y, et al. N-Acetylcysteine Restores Sevoflurane Postconditioning Cardioprotection against Myocardial Ischemia-Reperfusion Injury in Diabetic Rats. Journal of diabetes research (2016): 9213034.
- Lamming DW, Ye L, Sabatini DM, et al. Rapalogs and mTOR inhibitors as anti-aging therapeutics. The Journal of clinical investigation 123 (2013): 980-9.
- Yang H, Rudge DG, Koos JD, et al. mTOR kinase structure, mechanism and regulation. Nature 497 (2013): 217 23.
- Xuan YT, Guo Y, Zhu Y, et al. Role of the protein kinase C-epsilon-Raf-1-MEK-1/2-p44/42 MAPK signaling cascade in the activation of signal transducers and activators of transcription 1 and 3 and induction of cyclooxygenase-2 after ischemic preconditioning. Circulation 112 (2005): 1971-8.
- Yokoyama T, Kondo Y, Kondo S. Roles of mTOR and STAT3 in autophagy induced by telomere 3' overhang-specific DNA oligonucleotides. Autophagy 3 (2007): 496- 8.
- Samidurai A, Roh SK, Prakash M, et al. STAT3-miR- 17/20 signalling axis plays a critical role in attenuating myocardial infarction following rapamycin treatment in diabetic mice. Cardiovascular research 116 (2020): 2103- 15.
- Das A, Salloum FN, Filippone SM, et al. Inhibition of mammalian target of rapamycin protects against reperfusion injury in diabetic heart through STAT3 signaling. Basic research in cardiology 110 (2015): 31.
- Ma X, Liu H, Foyil SR, et al. Autophagy is impaired in cardiac ischemia-reperfusion injury. Autophagy 8 (2012): 1394-6.
- Ma X, Liu H, Foyil SR, et al. Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation 125 (2012): 3170-81.
- Zhou R, Zhang Z, Li X, et al. Autophagy in High-Fat Diet and Streptozotocin-Induced Metabolic Cardiomyopathy: Mechanisms and Therapeutic Implications. Int J Mol Sci 26 (2025).
- Samidurai A, Salloum FN, Durrant D, et al. Chronic treatment with novel nanoformulated micelles of rapamycin, Rapatar, protects diabetic heart against ischaemia/reperfusion injury. British journal of pharmacology 174 (2017): 4771- 84.
- Xie X, Zhao Z, Liu D, et al. Inhibiting mTOR enhanced Cardiac STAT3 Phosphorylation at Site Ser 727 and Attenuated Myocardial Ischemia Reperfusion Injury in Diabetic Rats.


 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 