Adenosine Mitigates Low-Dose Zidovudine-Induced Rat Liver Damage by Modulating Redox State and Pro-Inflammatory Cytokine Production
Martha Lucinda Contreras-Zentella1, Armando Butanda-Ochoa1, Lourdes Sánchez-Sevilla1, Rodolfo Paredes-Díaz2, and Rolando Hernández-Muñoz*, 1
1Department of Cellular Biology and Development, Institute of Cellular Physiology, National Autonomous University of Mexico (UNAM). Mexico City 04510.
2Imaging Unit, Institute of Cellular Physiology, National Autonomous University of Mexico (UNAM). University Ave. # 3000, Col. Copilco-Universidad, Coyoacán 04510 Mexico City, MEXICO
*Corresponding author: Rolando Hernández-Muñoz, Department of Cellular Biology and Development, Institute of Cellular Physiology, National Autonomous University of Mexico (UNAM). Mexico City 04510.
Received: 07 December 2024; Accepted: 12 December 2024; Published: 30 December 2024
Article Information
Citation: Martha Lucinda Contreras-Zentella, Armando Butanda-Ochoa, Lourdes Sánchez-Sevilla, Rodolfo Paredes-Díaz, and Rolando Hernández-Muñoz. Adenosine Mitigates Low-Dose Zidovudine-Induced Rat Liver Damage by Modulating Redox State and Pro-Inflammatory Cytokine Production. Fortune Journal of Health Sciences. 7 (2024): 753-766
View / Download Pdf Share at FacebookAbstract
Zidovudine (AZT) has significantly reduced mortality and morbidity among AIDS patients, but has also been linked to adverse events, including hepatotoxicity, which leads to liver steatosis. In contrast, adenosine (ADO) has shown effectiveness as a hepatoprotective agent against both acute and chronic liver damage. This study aimed to examine the adverse effects of chronic low-dose AZT administration and potential mitigation of these effects through co-administration with ADO in rats. We evaluated tissue inflammation and the liver redox state during the development of AZT-induced fatty liver. Wistar rats treated with AZT and adenosine were assessed for various parameters, including serum enzyme markers, histological changes, and serum levels of insulin, glucagon, pro-inflammatory cytokines, and redox state markers. Chronic oral AZT administration resulted in elevated serum liver enzyme activity and slight liver steatosis characterized by micro-vesicular fat distribution. These effects were accompanied by reduced insulin and glucagon levels, increased serum pro-inflammatory cytokine levels, and disruptions of cellular redox states. The co-administration of ADO reduced the harmful effects of AZT, reduced the production of most inflammatory molecules, increased serum insulin and glucagon levels, and restored the liver cellular redox state. In conclusion, our findings indicate a connection between blood insulin and glucagon levels and cellular redox state, altered by chronic low-dose AZT administration, which is likely linked to mitochondrial integrity and metabolism. Notably, these effects were partially alleviated by co-administration of ADO.
Keywords
<p>Liver redox state; Fatty liver; Inflammation; Insulin; Glucagon; Lactic acidosis; Ketosis</p>
Article Details
Inroduction
Highly active antiretroviral therapy (HAART) has greatly reduced mortality and morbidity in patients with AIDS [1]. Zidovudine (3′-Azido-3′-deoxythymidine or AZT), a nucleoside-analog reverse transcriptase inhibitor (NRTI), remains a key treatment for many HAART recipients in developing countries [2]. However, it is important to note that all HIV drugs used in HAART are linked to varying degrees of adverse events [3], often affecting organ systems, such as the liver, kidneys, and heart [4]. The antiretroviral activity of AZT relies on the formation of AZT 5′-triphosphate [5], which leads to DNA damage, activation of DNA repair mechanisms, and cell cycle arrest [6]. The entry of AZT into the mitochondria has been linked to negative effects on mitochondrial function, especially when it integrates into mitochondrial DNA (mtDNA) [7]. This interference disrupts mitochondrial bioenergetics and raises H2O2 production, thereby affecting respiratory complex-I function [8]. Increased H2O2 levels result in higher production of reactive oxygen species (ROS) and peroxynitrite, leading to single-strand DNA breaks, lipid peroxidation, protein oxidation/nitration, and mtDNA damage [9].
Prolonged exposure to antiretroviral drugs is associated with various long-term side effects such as micro-vesicular steatosis, steatohepatitis, and organ failure [10]. Long-term HAART use has also been linked to lactic acidosis, which is considered a sign of mitochondrial respiratory chain dysfunction [11]. Importantly, mitochondrial changes caused by AZT contribute to the connection between HIV, long-term HAART use, and conditions such as dyslipidemia and diabetes mellitus (DM) [12], which are both components of metabolic syndrome. Experimentally, the effects of AZT are concentration-dependent (ranging from 25 to 100 mg • kg-1) and depend on the exposure duration (ranging from 1 to 12 weeks of treatment). Zidovudine administration can cause lipodystrophy and disrupt lipid and glucose metabolism, leading to the release of pro-inflammatory chemokines and mediators that increase the risk of DM and liver steatosis [13]. Recent research has shown that a clinical dose of AZT (considered very low for rats) reduces liver DNA synthesis and mitosis in rats undergoing 70% partial hepatectomy (PH) because AZT interferes with liver regeneration by altering PH-triggered oxidative events involved in the proliferation process [14].
Previous research in our laboratory has shown that i.p. adenosine (ADO) administration (100 to 200 mg ● kg-1) acts as a hepatoprotective agent following acute ethanol intoxication [15], effectively preventing fat accumulation in the rat liver. Additionally, ADO significantly delayed the onset of fatty liver disease, hepatic necrosis, early oxidative stress, and lipid peroxidation caused by carbon tetrachloride administration. Notably, adenosine administration markedly reversed experimental cirrhosis and restored the liver function. These effects are associated with increased collagenolytic activity, reduced oxidative stress related to cirrhosis, and recovery of the proliferative capacity [16]. To explore further, we conducted prolonged oral administration of AZT at a dose slightly higher than typical for humans, but much lower than the safe dosing for rats (15 mg ● kg-1), for a treatment period of 1 to 8 weeks. In this study, we evaluated liver histology, inflammation markers, hepatic cell redox status, and changes in serum levels of pancreatic hormones. We also investigated the potential of co-administration of ADO to counteract the possible adverse effects of AZT.
Materials and Methods
Reagents and diagnostic kits
Zidovudine (AZT) and adenosine (ADO) were obtained from Biosynth (Carbosynth Ltd., China). Enzymes, coenzymes, and other reagents were purchased from Sigma-Aldrich Chemical Co. (Mexico). Clinical metabolites were analyzed using diagnostic kits from Spinreact (Barcelona, Spain).
Animal treatments
Male Wistar rats (n = 64) with an initial weight of 190 - 210 g (2 months old), were individually housed and divided into four experimental groups of four animals each. They had free access to laboratory chow and water in a controlled environment with a 12:12-hour light/dark cycle. Water consumption was carefully monitored to determine the appropriate volume for dissolving AZT and ADO in the drinking water. The study groups were as follows: A) control group (n = 16) receiving only water, B) control group receiving ADO (n = 16) at a dose of 30 mg • kg-1/day, C) AZT group (n = 16) receiving 15 mg • kg-1/day of AZT, and D) combined treatment group (n = 16) receiving 15 mg • kg-1/day of AZT and 30 mg • kg-1/day of ADO (n = 4 per experimental group and time). The treatment period lasted from one to eight weeks, with continuous intake of nucleosides. After this period, the rats were fasted overnight, anesthetized with isoflurane, and euthanized by decapitation, always conducted between 9:00 and 10:00 a.m. to minimize circadian variations in the intermediary metabolism. Whole blood was collected from the neck after decapitation, and serum was obtained by centrifugation. The liver and spleen were weighed, and organ-to-body weight ratios were calculated. Liver samples were collected for histological analysis, and the remaining liver tissue was stored at -20 °C. All experimental protocols were approved by the Biomedical-Ethics protocol of National Autonomous University of Mexico (UNAM), following the Federal Regulations for Animal Care and Experimentation (Ministry of Agriculture, SAGARPA, NOM-062-ZOO-1999). All methods are reported in accordance with the ARRIVE guidelines.
Determination of “clinical” serum parameters and “marker” enzyme activities
We quantified serum levels of glucose, triglycerides (TG), cholesterol, albumin, total bilirubin, direct bilirubin, urea, and creatinine using diagnostic kits. Additionally, we measured the activities of γ-glutamyl transferase (γ-GT), amylase, and lipase using diagnostic kits. For other enzyme activities, including lactate dehydrogenase (LDH), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and ornithine carbamoyl transferase (OCT), we applied previously established methods [17]. Serum arginase activity was quantified following the procedure described by Colombo and Konarska [18].
Measurement of Insulin, Glucagon, and Interleukin levels
Serum insulin and glucagon levels were quantified using ELISA kits from RayBiotech (USA), whereas serum concentrations of Interleukin-1β (IL-1β), Interleukin-4 (IL-4), Interleukin-6 (IL-6), Interleukin-10 (IL-10), and tumor necrosis factor-alpha (TNF-α) were measured using ELISA kits from Sigma-Aldrich Chemical Co. (Mexico).
Histological Assessment
Liver tissue samples were preserved in 20% formaldehyde solution (pH 7.0 with PBS), embedded in paraffin, and sectioned into 5 µm slices. These sections were stained with hematoxylin and eosin (H&E), following standard protocols. For histological analysis, at least four biological replicates were used for each treatment, and five sections were systematically captured from each sample using a zig-zag approach [19].
Estimation of NAD/NADH redox potential via pyruvate/lactate and acetoacetate/β-hydroxybutyrate (β-OH Butyrate) redox pairs
Buffered acid extracts from serum and liver samples were neutralized with 4 M K2CO3, and metabolites, including lactate, pyruvate, acetoacetate, and β-OH butyrate, were enzymatically determined as previously described [20].
Calculations and Statistics
Cytoplasmic and mitochondrial NAD/NADH ratios were calculated using the formula: NAD/NADH = [oxidized substrate] / [reduced substrate] • 1/Keq, considering the equilibrium constants for lactate and β-hydroxybutyrate dehydrogenases [20,21]. Linear correlation analyses were conducted using the Pearson’s equation. Results are presented as the mean ± SE, and statistical significance was evaluated using one-way analysis of variance (ANOVA). Comparisons between two groups were analyzed with Student’s t-test using GraphPad Prism 9.5.1 software. Statistical significance was set at p < 0.05.
Results
Morphometric analysis and “clinical” parameters measured in sera from rats treated with AZT and ADO
Neither zidovudine (AZT) nor ADO treatments significantly affected body weight gain, liver weight, or the behavior of the rats during the experimental procedure (data not shown). Administration of ADO alone significantly reduced serum glucose levels at 4 and 8 weeks compared with the controls (Table 1). AZT administration caused significant hypoglycemia at 2 and 8 weeks of treatment, which was corrected by the combined AZT and ADO treatment. When administered separately, ADO and AZT decreased serum TG levels compared with the controls, starting at week 2 (Table 1). Co-administration of AZT and ADO did not alter the effects induced by AZT or ADO alone. In contrast, serum cholesterol levels were significantly reduced only with concurrent administration of nucleosides at 2 and 8 weeks compared to controls (Table 1). Serum albumin and bilirubin levels were assessed as indicators of liver function in rats treated with AZT and ADO, respectively. For albumin, no changes were detected in most cases, except for AZT+ADO at four weeks, where a significant decrease was observed (Table 1). For total and direct bilirubin, a significant increase in total bilirubin was noted after two weeks of AZT administration, which promptly returned to control values (Table 1). Changes in total bilirubin appeared to be mainly linked to its indirect form, as serum direct bilirubin levels were only elevated at the beginning of the experiment with combined treatment (Table 1). When evaluating nitrogen metabolites (urea and creatinine) as indicators of kidney function, no significant changes were observed in animals treated with AZT and/or ADO (data not shown). Therefore, these results suggest that liver and kidney functions were not significantly affected by the experimental treatments.
Table 1: Clinical Parameters determined in sera from rats treated with zidovudine (AZT) and adenosine (ADO)
|
Parameter |
Time |
Controls |
Control ADO |
AZT |
AZT + ADO |
|
Glucose |
1 week |
88 ± 5 |
84 ± 7 |
80 ± 12 |
123 ± 10 |
|
2 weeks |
100 ± 6 |
90 ± 7 |
67 ± 6* |
124 ± 12 |
|
|
4 weeks |
107 ± 8 |
82 ± 6* |
101 ± 4 |
128 ± 11# |
|
|
8 weeks |
118 ± 10 |
90 ± 5* |
67 ± 3* |
123 ± 10# |
|
|
TG |
1 week |
57 ± 5 |
50 ± 8 |
50 ± 6 |
51 ± 5 |
|
2 weeks |
66 ± 6 |
38 ± 6* |
40 ± 7* |
40 ± 4* |
|
|
4 weeks |
50 ± 4 |
31 ± 3* |
34 ± 3* |
45 ± 7 |
|
|
8 weeks |
61 ± 7 |
35 ± 4* |
35 ± 6% |
33 ± 5* |
|
|
Cholesterol |
1 week |
58 ± 6 |
58 ± 9 |
47 ± 6 |
53 ± 5 |
|
2 weeks |
63 ± 12 |
47 ± 3 |
54 ± 4 |
42 ± 4* |
|
|
4 weeks |
60 ± 11 |
48 ± 3 |
48 ± 3 |
47 ± 7 |
|
|
8 weeks |
54 ± 5 |
49 ± 4 |
41 ± 6 |
35 ± 5* |
|
|
Albumin |
1 week |
3.1 ± 0.1 |
2.7 ± 0.2 |
2.7 ± 0.1* |
2.7 ± 0.2 |
|
2 weeks |
3.1 ± 0.2 |
2.7 ± 0.3 |
2.6 ± 0.1 |
2.7 ± 0.2 |
|
|
4 weeks |
3.7 ± 0.3 |
2.9 ± 0.4 |
2.9 ± 0.2 |
2.6 ± 0.1* |
|
|
8 weeks |
2.8 ± 0.3 |
2.8 ± 0.3 |
2.7 ± 0.3 |
3.3 ± 0.3 |
|
|
Bilirubin (T) |
1 week |
0.37 ± 0.11 |
0.38 ± 0.06 |
0.22 ± 0.04 |
0.22 ± 0.04 |
|
2 weeks |
0.19 ± 0.03 |
0.14 ± 0.02 |
0.66 ± 0.18* |
0.22 ± 0.04 |
|
|
4 weeks |
0.44 ± 0.08 |
0.20 ± 0.03* |
0.23 ± 0.04 |
0.31 ± 0.05 |
|
|
8 weeks |
0.29 ± 0.04 |
0.29 ± 0.05 |
0.14 ± 0.03* |
0.44 ± 0.07 |
|
|
Bilirubin (D) |
1 week |
0.05 ± 0.01 |
0.06 ± 0.01 |
0.04 ± 0.01 |
0.14 ± 0.03& |
|
2 weeks |
0.09 ± 0.02 |
0.02 ± 0.005* |
0.07 ± 0.01 |
0.07 ± 0.02 |
|
|
4 weeks |
0.10 ± 0.02 |
0.08 ± 0.03 |
0.05 ± 0.02 |
0.04 ± 0.01* |
|
|
8 weeks |
0.08 ± 0.02 |
0.05 ± 0.01 |
0.04 ± 0.01 |
0.03 ± 0.01 |
The “clinical Parameters” are the mean ± SE of 4 Independent observations per experimental group and time point. All the metabolites are expressed as mg dL-1 except the albumin which is in g . dL-1. Statistical significance: *p< 0.01 against controls, #p<0.01 vs. the AZT group, and &p<0.01 compared to controls and AZT alone groups
Effects of AZT and ADO on Serum Enzyme Markers for Liver Integrity
When evaluating enzyme activities as markers of liver integrity, including transaminases, LDH, and γ-GT, we found that AZT administration significantly increased serum ALT and AST activities after 8 weeks, and this effect was completely mitigated by concurrent ADO treatment (Figures 1A and B). An early increase in LDH activity was observed following AZT administration and persisted throughout the experiment; co-administration of ADO normalized LDH serum activity (Figure 1C). In contrast, AZT administration reduced γ-GT activity compared with that in control group, and this effect was unchanged by ADO administration (Figure 1D). Notably, the combined administration of AZT and ADO resulted in the lowest serum activities for all these enzymes (Figures 1A to D). Serum OTC activity increased after 4 weeks of AZT treatment, but the combined treatment led to progressive and greater enhancement of this enzyme activity (Figure 1E). Conversely, ADO alone or in combination with AZT induced a significant reduction in serum arginase activity, whereas the AZT-alone group showed a pattern of arginase activity similar to that of LDH (Figure 1F). We also evaluated serum enzyme activities as markers of pancreatic integrity, specifically amylase (Figure 1G) and lipase activities. Only lipase activity showed a significant increase at 8 weeks following AZT treatment, which was effectively mitigated by the co-administration of ADO (Figure 1H).
Figure 1: The impact of AZT and ADO on serum enzyme markers indicatives of liver and pancreas integrity. The data presented show the mean ± SEM for four individual samples in each experimental group, detailing serum levels of alanine aminotransferase (ALT; panel A), aspartate aminotransferase (AST; panel B), lactate dehydrogenase (LDH; panel C), γ-glutamyl transferase (γ-GT; panel D), ornithine transcarbamylase (OCT; panel E), and arginase (panel F), all measured in IU/L. The pancreatic enzymes amylase (panel G) and lipase (panel H) are also reported in IU/L. Control values are indicated by horizontal gray bars. Symbols and colors representing the different experimental groups are explained at the top of panel A. Statistical significance is denoted as follows: *p < 0.01 compared to the control group (horizontal gray bars); #p < 0.01 compared to the AZT-alone group; and &p < 0.01 compared to both the control and AZT-alone groups.
Effects of AZT and ADO on Serum Insulin and Glucagon Levels
We aimed to determine whether fluctuations in insulin and/or glucagon levels contribute to the metabolic effects induced by nucleosides in fasted animals. Oral administration of ADO alone initially increased insulin levels after two weeks, which then returned to normal levels. While AZT treatment alone did not lead to significant changes, combined treatment with ADO caused a substantial and sustained increase in insulin levels compared to both the control and AZT groups, which disappeared after 8 weeks of treatment (Figure 2A). Similarly, treatment with ADO alone or in combination with AZT increased serum glucagon concentrations (Figure 2B), maintaining the insulin/glucagon ratio in animals receiving the combined treatment. However, AZT administration increased this ratio at the latest experimental time point (Figure 2C).
Figure 2: Effects of AZT and ADO on serum activities of marker enzymes for liver and pancreas integrity. The data presented illustrate the mean ± SEM from four individual observations for each experimental group for insulin (panel A) and glucagon (panel B), with values expressed in picomoles per milliliter (pmoles • ml-1). Panel C shows the insulin to glucagon ratio. Control group values are represented by horizontal gray bars. Experimental groups are identified by specific symbols and colors, as indicated at the top of panel A. Statistical significance is marked as follows: *p < 0.01 compared to the control group (horizontal gray bars); #p < 0.01 relative to the group treated with AZT alone; and &p < 0.01 compared to both the control group and the AZT-alone group.
Histological evaluation of liver structure, serum levels of inflammatory cytokines, and liver TNF-α levels
Liver tissue slices were subjected to histological examination to assess the presence of various features: 1) fatty change, 2) cell ballooning, 3) inflammatory infiltrates (PMN and macrophage cells), 4) hepatocellular disorganization, and 5) interstitial fibrosis. Figure 3 shows micrographs of liver tissue specimens observed with a 40X objective. No histological changes were observed in the control group. In contrast, animals treated with AZT alone showed a gradual increase in micro-vesicles (likely fat droplets), becoming more evident from 2 to 4 weeks and peaking at 8 weeks (indicated by yellow arrows; Figure 3A). Co-administration with ADO also led to progressive but less pronounced accumulation of these micro-vesicles, which was significantly reduced by the end of the experiment (Figure 3A). Although we did not find histological evidence of liver steatosis (Fig. 3) accompanying the release of liver enzymes (Fig. 1) as harmful effects of AZT, changes in the enzymatically quantified liver TG levels were observed. Figure 3B shows that liver TG levels in controls (8.4 ± 0.7 mg • g-1) significantly increased after 4 - 8 weeks of AZT administration, peaking at 8 weeks and doubling compared to controls. In contrast, animals receiving the combination treatment had significantly lower liver TG levels than those in the AZT-alone group (Figure 3B).
Figure 3: Histological survey of livers from rats treated with AZT and ADO. The micrographs provided represent the four experimental groups: A) control, B) ADO, C) AZT, and D) AZT + ADO. For histological analysis, each treatment included four biological samples, with five sections or fields from each sample examined under a 40X objective. These sections were photographed using a zig-zag approach for thorough coverage. The accompanying table (below) shows the liver triglyceride amounts determined chemically. Yellow arrowheads indicate lipid droplets within hepatic parenchyma.
Despite the lack of clear histological evidence of liver inflammation due to AZT administration, changes in the serum levels of specific cytokines were noted. Treatment with ADO alone or in combination with AZT significantly reduced the levels of the inflammatory cytokine IL-1β, while AZT alone markedly increased its serum levels after 8 weeks, which was completely mitigated by concurrent ADO administration (Figure 4A). A similar trend was observed for IL-6, a pro-inflammatory cytokine. ADO alone significantly reduced IL-6 levels 2 weeks post-treatment, whereas AZT alone led to a significant increase after 4 weeks. Co-administration with ADO effectively reduced the AZT-induced increase in IL-6 levels (Figure 4B). The cytokine IL-4, which is associated with anti-inflammatory responses, showed an intriguing pattern. Treatment with ADO or AZT alone increased serum IL-4 levels at 1 - 2 weeks, like the co-administration of the nucleosides (Figure 4C). Interestingly, at the 8-week mark, an inverse relationship in serum IL-4 levels was observed between the AZT-alone and co-administered groups (Figure 4C). IL-10, known for its anti-inflammatory and regulatory effects, was robustly induced by ADO, whereas AZT alone showed only a modest peak at 2 weeks (Figure 4D). The effects of nucleosides on liver TNF-α levels are shown in Figure 4E. ADO alone induced an increase in TNF-α starting at 2 weeks, which then declined but remained significant at the end of the experiment (Figure 4E). Similar to IL-6, AZT induced a strong peak in liver TNF-α at 4 weeks, which was significantly reduced by combined treatment with ADO. After 8 weeks of nucleoside treatment, only a slight but significant peak in liver TNF-α was observed with ADO alone (Figure 4E).
Figure 4: Effects of AZT and ADO on serum concentration of cytokines and of liver TNF-α involved in the inflammatory process. The results show the mean ± SEM from four individual observations for each experimental group, focusing on interleukin-1β (IL-1β, panel A), interleukin-6 (IL-6, panel B), interleukin-4 (IL-4, panel C), and interleukin-10 (IL-10, panel D). Additionally, liver levels of tumor necrosis factor (TNF-α, panel E) were determined. Control values are represented by horizontal gray bars. The symbols and colors identifying the experimental groups are detailed at the top of panel A. Statistical significance is indicated as described in Figure 1.
Effects of AZT and ADO on Serum, Liver NAD/NADH Redox State (Cytoplasmic and Mitochondrial)
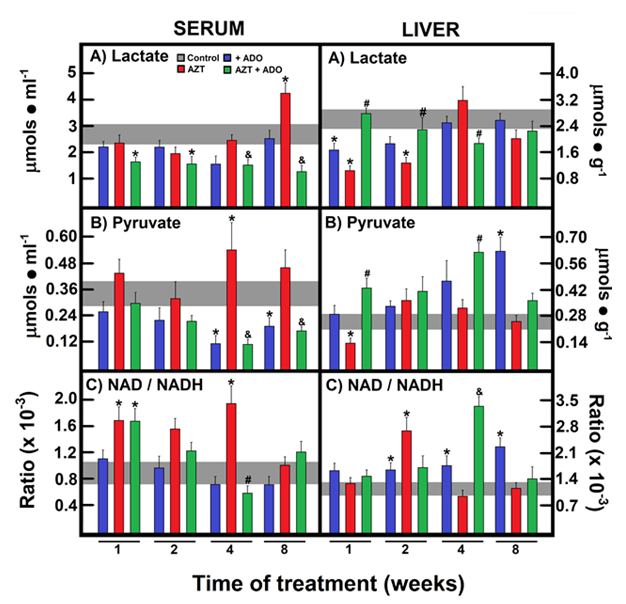
Our group recently uncovered a strong correlation between blood glucose variations, cellular redox state, and insulin/glucagon ratio [17-22]. Considering these observations, we quantified the concentrations of pyruvate/lactate and acetoacetate/β-OH-butyrate redox pairs to estimate the NAD/NADH redox state in both the cytoplasm and mitochondria and explored their potential reflection in serum levels in our experimental rat model. In the serum, lactate levels were significantly reduced in the group receiving the combined treatment of AZT and ADO at all experimental time points, compared to controls and/or AZT alone (Figure 5A). Conversely, AZT induced a significant increase in serum lactate levels at 8 weeks, which was an effect completely mitigated by co-administration with ADO (Figure 5A). ADO alone or in combination caused a decrease in serum pyruvate levels, whereas AZT alone led to a significant increase only after 4 weeks (Figure 5B). As a result, serum NAD/NADH ratios were markedly increased following AZT administration. When ADO was co-administered with AZT, this ratio also increased after 1 week of treatment but later declined, returning to the control range (Figure 5C). In contrast, AZT alone reduced liver lactate levels, whereas co-administration with ADO restored them, and ADO alone only lowered liver lactate levels during the first 2 weeks (Figure 5A). Liver pyruvate levels were increased by ADO alone or in combination with AZT, whereas AZT alone reduced liver pyruvate levels at the start of treatment (Figure 5B). ADO administration notably induced a more oxidized liver cytoplasmic redox state, with AZT showing a similar effect at two weeks, and the combined treatment resulted in a higher NAD/NADH ratio at four weeks (Figure 5C).
Figure 5: Effects of AZT and ADO on the cytoplasmic liver NAD/NADH redox state and their possible reflection in serum. The results present the mean ± SEM derived from four individual observations for each experimental group, examining serum and liver lactate levels (panel A), serum and liver pyruvate levels (panel B), and the serum and liver NAD/NADHcyto ratio (panel C). Control values are represented by horizontal gray bars. The symbols and colors identifying the experimental groups are specified at the top of panel A. Statistical significance is detailed as described in Figure 1.
For the acetoacetate/β-OH-butyrate redox pair, AZT administration significantly increased β-OH-butyrate levels, an effect that was normalized by co-administration with ADO (Figure 6A). AZT initially decreased acetoacetate levels, which were normalized following co-administration with ADO (Figure 6B). Consequently, AZT alone led to a lower NAD/NADH ratio at all-time points, an effect that was corrected in the AZT + ADO group, which had the highest NAD/NADH ratio at 1 week (Figure 6C). For the liver mitochondrial NAD/NADH redox state, AZT administration decreased serum β-OH-butyrate levels, which were corrected by combined treatment at 4 weeks, with no further changes thereafter (Figure 6A). Liver acetoacetate levels were significantly reduced by treatment with ADO alone, AZT alone, or their combination at early time points. However, the simultaneous administration of AZT and ADO induced a peak in acetoacetate levels at 4 weeks (Figure 6B). In animals treated with ADO or AZT alone, a reduced mitochondrial state was observed, especially in those receiving AZT; however, this was normalized by co-administration with ADO at later time points. ADO alone decreased the amount of ketone bodies in the serum (at 8 weeks) and in the liver (at 4 weeks) (Table 2). AZT treatment resulted in increased serum ketone body levels (ketosis) at 2 and 4 weeks, while the opposite trend was seen in the liver, suggesting an elevated release of these metabolites (Table 2). Co-administration with ADO prevented AZT-induced ketosis and tended to decrease liver ketone body levels, possibly due to an anti-lipogenic effect of ADO (Table 2).
Table 2: Serum and liver levels of total ketone bodies in rats treated with zidovudine (AZT) and adenosine (ADO)
|
Parameter |
Time |
Controls |
Control ADO |
AZT |
AZT + ADO |
|
Ketone Bodies (Serum) |
|||||
|
1 week |
0.49 ± 0.07 |
0.42 ± 0.08 |
0.36 ± 0.06 |
0.33 ± 0.08 |
|
|
2 weeks |
0.34 ± 0.05 |
0.56 ± 0.12 |
0.77 ± 0.16* |
0.35 ± 0.05** |
|
|
4 weeks |
0.31 ± 0.04 |
0.26 ± 0.05 |
0.49 ± 0.05* |
0.30 ± 0.04** |
|
|
8 weeks |
0.35 ± 0.05 |
0.16 ± 0.02* |
0.46 ± 0.04 |
0.33 ± 0.05 |
|
|
Ketone Bodies (Liver) |
|||||
|
1 week |
0.91 ± 0.14 |
0.66 ± 0.08 |
0.68 ± 0.11 |
0.79 ± 0.17 |
|
|
2 weeks |
1.03 ± 0.15 |
0.45 ± 0.06* |
0.52 ± 0.10* |
0.77 ± 0.11 |
|
|
4 weeks |
1.24 ± 0.20 |
0.69 ± 0.08* |
0.52 ± 0.06* |
0.30 ± 0.04*** |
|
|
8 weeks |
0.71 ± 0.15 |
0.56 ± 0.07 |
0.48 ± 0.04 |
0.60 ± 0.09 |
The total ketone bodies are the mean ± SE of 4 independent observations per experimental group and time point as the sum of β-OH-butyrate and acetoacetate and expressed as nmoles . ml-1 of serum or as nmoles . g-1 of liver. Statistical Significance: *p<0.01 against controls,**p<0.01 vs. the AZT group.
Correlations between serum levels of insulin, glucagon, cytokines, and NAD/NADH redox state
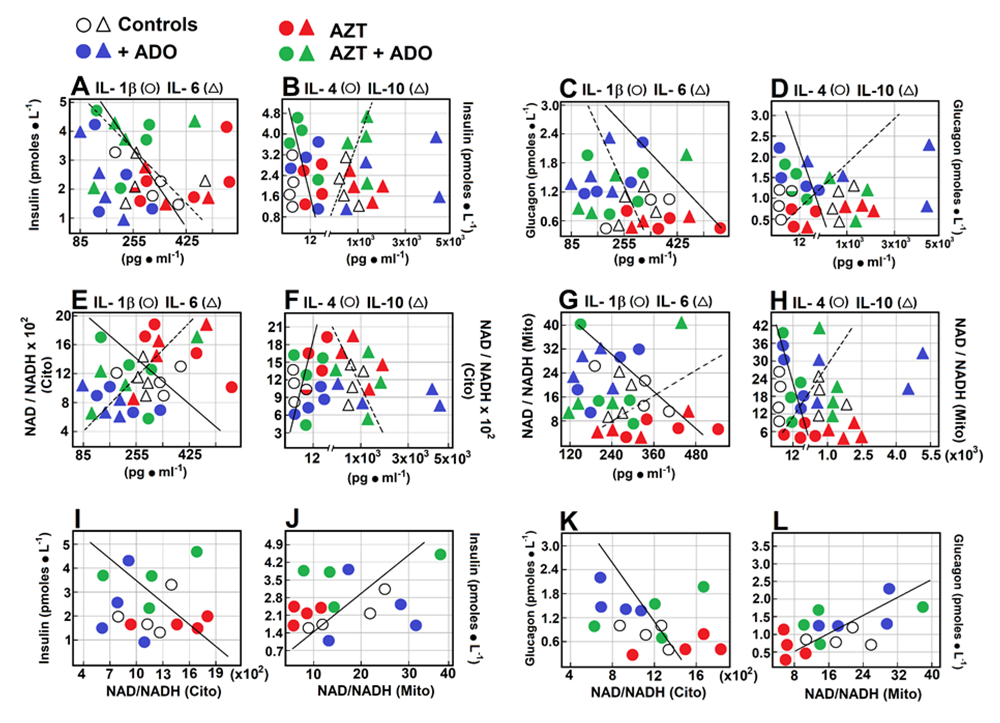
We examined the possible relationships between serum levels of pancreatic hormones, cytokines, and the NAD/NADH redox state. Insulin levels were inversely correlated with fluctuations in IL-1β (r = -0.469; Figure 7A), and no significant correlation was found with IL-6. Insulin levels did not affect IL-4 (Figure 7B) or IL-10 (Figure 7B) levels. When correlating glucagon levels, we observed a similar pattern to that of insulin but with higher correlation coefficients (Figures 7C and D). Glucagon levels were inversely correlated with IL-1β (r = -0.517, Figure 7C), had no effect on IL-6 levels, and showed a moderate inverse correlation with IL-4 (r = -0.357, Figure 7D), while demonstrating a direct correlation with changes in IL-10 levels (r = 0.585, Figure 7D). The cytoplasmic redox state did not correlate with changes in IL-1β but had a strong direct relationship with IL-6 (r = 0.816, Figure 7E) and a robust direct correlation with IL-4 (r = 0.713, Figure 7F). No significant relationship was observed between IL-10 levels (Figure 7F). For the mitochondrial redox state, we found a different pattern when correlated with cytokines: it had a significant inverse correlation with fluctuations in IL-1β (r = -0.525, Figure 7G) and no effect on IL-6. Additionally, a moderate inverse correlation was observed with IL-4 (r = -0.412, Figure 7H), and a moderate direct correlation with changes in IL-10 levels (r = 0.428, Figure 7H). Regarding correlations between pancreatic hormones and serum redox state, potentially reflecting liver redox state, insulin did not correlate with the cytoplasmic redox state but showed a significant direct relationship with the mitochondrial redox state (r = 0.464, Figure 7J). Glucagon was inversely correlated with the cytoplasmic redox state (r = -0.437, Figure 7K) and showed a strong direct correlation with the mitochondrial redox state (r = 0.903, Figure 7L).
Figure 7: Correlations among serum levels of insulin, glucagon, cytokines, and NAD/NADH redox state. Scatter plots show the relationship between two sets of data. This relationship is summarized in the Pearson’s correlation coefficient (r) for each comparison in each scatter plot: panel A, insulin vs. IL-1β (r = -0.469, p < 0.05; circles and solid line), and insulin vs. IL-6 (r = -0.157, not significant (n.s.); triangles and dotted line). Panel B, insulin vs. IL-4 (r = -0.083, n.s.; circles and solid line), and insulin vs. IL-10 (r = 0.098, n.s.; triangles and dotted line). Panel C, glucagon vs. IL-1β (r = -0.517, p < 0.01; circles and solid line), and glucagon vs. IL-6 (r = -0.119, n.s.; triangles and dotted line). Panel D, glucagon vs. IL-4 (r = -0.357, p < 0.05; circles and solid line), and glucagon vs. IL-10 (r = 0.585, p < 0.01; triangles and dotted line). Panel E, cytoplasmic redox state vs. IL-1β (r = -0.161, n.s.; circles and solid line), and vs. IL-6 (r = 0.816, p < 0.005; triangles and dotted line). Panel F, cytoplasmic redox state vs. IL-4 (r = 0.713, p < 0.005; circles and solid line), and vs. IL-10 (r = -0.291; triangles and dotted line). Panel G, mitochondrial redox state vs. IL-1β (r = -0.525, p < 0.01; circles), and vs. IL-6 (r = -0.149, n.s.; triangles). Panel H, mitochondrial redox state vs. IL-4 (r = -0.412, p < 0.01; circles), and vs. IL-10 (r = 0.428, p < 0.05; triangles). Panel I, insulin vs. cytoplasmic redox state (r = -0.093, n.s.; circles). Panel J, insulin vs. mitochondrial redox state (r = 0.464, p < 0.05; circles). Panel K, glucagon vs. cytoplasmic redox state (r = -0.437, p < 0.05; circles), and glucagon vs. mitochondrial redox state (r = 0.903, p < 0.001; circles, panel L).
Discussion
Since the introduction of HAART in the mid-1990s, the lifespan of individuals living with HIV has increased significantly. However, the main causes of morbidity and mortality in these patients have shifted towards liver, renal, and cardiovascular diseases [23]. Regarding liver health, AZT has been associated with peroxide production and oxidative stress in hepatocytes [24] and is potentially linked to AZT-induced glutathione depletion [25]. In this study, we conducted prolonged oral administration of AZT at a slightly elevated dose in humans (15 mg ● kg-1) but significantly lower than the considered safe dose in rats, according to dose conversion between animals and humans [26]. This conversion is an empirical method aimed at achieving a maximum recommended starting dose (MRSD) for clinical studies based on body surface area, which is assumed to be related to the metabolic rate of an animal. Therefore, we administered AZT to rats at one-fourth of the no observed adverse effect level (NOAEL) for AZT in rats. Similarly, we selected one-fourth of the NOAEL for ADO, previously used by our group, to treat these animals as MRSD.
Oxidative stress can lead to elevated levels of liver enzymes, which are often considered specific markers of liver toxicity. AZT treatment has been shown to significantly increase liver enzyme levels, including alkaline phosphatase, ALT, and AST [27]. Early initiation of HAART is frequently associated with marked elevations in AST, ALT, and BUN, with some resolution expected as the treatment progresses [28]. Nonetheless, evidence indicates that a significant number of HIV-infected individuals treated with AZT died because of hepatotoxicity [29]. Reports have also indicated that coexisting dyslipidemia and hyperglycemia in individuals starting HAART, including AZT, significantly increase the risk factors for both diseases [30]. In our experimental model, AZT administration led to significant hypoglycemia at 2 and 8 weeks, a phenomenon corrected by combined treatment with AZT and ADO (Table 1). Additionally, AZT reduced serum TG levels without significantly affecting cholesterol levels (Table 1). Thus, we did not observe chronic hyperglycemia or dyslipidemia resulting from AZT administration in this study, likely due to the very low AZT dose used. Conversely, AZT treatment significantly altered the levels of marker enzymes of liver integrity (Figure 1). Nucleoside increased ALT, AST, and LDH levels, primarily after 8 weeks of administration; however, co-administration with ADO normalized these parameters (Figure 1). Furthermore, AZT increased serum arginase activity, whereas OCT activity was reduced (Figure 1). Both type-I arginase and OCT are used to assess hepatocellular damage, including acute hepatitis, cirrhosis, or cancer [31,32]. Drug-induced liver injury (DILI) is characterized by a temporal relationship between drug administration and increased serum levels of liver enzymes and/or alkaline phosphatase [33]. This study demonstrated the occurrence of such metabolic disturbances.
In line with the serum enzymatic patterns induced by AZT, we observed slight liver fat accumulation (measured as TG accumulation) in rats treated with AZT alone, peaking at 8 weeks and possibly resembling liver steatosis (Figure 3). This effect was partially prevented or delayed by the simultaneous administration of ADO (Figure 3). ADO has been linked to lipolysis and glucose clearance and has been proposed to enhance insulin sensitivity through its receptors in adipose tissue [34]. Consequently, improved glucose tolerance has been achieved using adenosine-based pharmacological agents [35]. It has been reported that AZT negatively impacts the oxidation of free fatty acids within hepatic mitochondria, which can be associated with NRTI-induced insulin resistance and dyslipidemia, leading to liver TG accumulation and subsequent hepatic steatosis [23]. In mice, AZT exposure reduces the mtDNA copy number in the liver, which can also affect pancreatic islet size. AZT administration reduced the mean insulin-positive cell area/islets compared to those in control mice. Therefore, reduced mtDNA copy number (caused by AZT treatment) can impair insulin supply and sensing [36].
In our study, ADO administration alone enhanced insulin levels after two weeks, which then normalized. While AZT treatment alone did not result in significant changes, combined treatment with ADO led to a drastic increase in insulin levels compared to both the control and AZT groups (Figure 2A). Additionally, treatment with ADO alone or in combination with AZT increased serum glucagon concentration (Figure 2B), maintaining the insulin/glucagon ratio in animals receiving the combined treatment compared with controls (Figure 2C). Our results showed that AZT did not induce insulin resistance in our rats at any point during treatment, but did produce a transient hypoglycemic response to fasting conditions (Figure 2). In this context, ADO administration has also been linked to pancreatic insulin synthesis, reverse cholesterol transport, prevention of fatty liver disease [35,37], improvement of liver steatosis, and better glucose and insulin clearance [38]. During inflammation, cytokines released by immune cells, such as TNF-α and IL-1β, directly affect the endothelium, increasing the expression of cell adhesion molecules (ICAM-1 and VCAM-1) and E-selectin [39]. Administration of ADO, either alone or with AZT, significantly decreased the levels of inflammatory cytokines IL-1β and IL-6, which were elevated by AZT alone (4 to 8 weeks post-treatment). Treatment with ADO or AZT alone increased the serum IL-4 concentration at 1 - 2 weeks post-administration (Figure 4). It has been reported that IFC-305 (an aspartic-salt derivative of ADO) exhibits antioxidant and anti-inflammatory activities, likely mediated by IL-10 and arginase activity, and prevents or even reverses carbon tetrachloride-induced cirrhosis in rats [40]. Studies have investigated the role of pro-inflammatory cytokines in ADO receptor expression in immune cells, reporting that IL-1 and TNF-α enhance both A2AAR expression and functionality, preventing receptor desensitization in human monocytes [41], which could explain the early increase in TNF-α observed after ADO administration (Figure 4). The anti-inflammatory effect of ARs on macrophages is supported by A2A, A2B, and A3AR activation and extracellular adenosine. Activation of ADO receptors blocks the release of pro-inflammatory mediators such as TNF-α, IL-6, IL-12, nitric oxide, and macrophage inflammatory protein (MIP)-1α [42]. Additionally, treatment with topically selective A2A agonists reduces the inflammatory response, associated with decreased inflammatory cell infiltration and lower LTB4 and TNF-α levels [43,44].
AZT, lamivudine, and other nucleosides used in HIV treatment must cross the plasma membrane to exert therapeutic effects. Human Organic Cation Transporter 1 (hOCT1), encoded by SLC22A1, is responsible for its uptake into target cells [45]. Extracellular ADO enters cells via the SLC28 family of cation-linked concentrative nucleoside transporters (CNTs) and the SLC29 family of energy-independent, equilibrative nucleoside transporters (ENTs), and allows adenosine to move freely across the cell membrane [44,46]. Although different transporters are involved in the cellular entry of AZT and ADO, being nucleosides (pyrimidine and purine, respectively) suggest that predicting interactions with the same receptors or transporters is complex. Previous studies have shown that the adverse effects of nucleoside analogs used in HAART are directly related to mitochondrial damage [47]. Mitochondrial injury manifests as abnormal morphology, depletion of mitochondria-encoded enzymes, and reduced mitochondrial gene numbers [48]. This disruption results in energy loss, electron leakage from the electron transport chain, increased reactive oxygen species (ROS) levels, oxidative damage, and significant imbalances in cellular redox states (i.e., an increased NADH/NAD ratio), shifting the pyruvate/lactate balance toward increased lactate production [49]. Liver and skeletal muscle tissues are targets of mitochondrial damage induced by nucleoside analogs [50,51]. Therefore, we explored the role of changes in cellular redox states and their impact on the liver of AZT-treated animals (Figures 5 and 6). Under our experimental conditions, AZT induced lactic acidosis, although this lactate did not seem to be produced by the liver, as shown in Figure 5. Pyruvate levels showed minimal changes due to either AZT or ADO. The serum NAD/NADH ratio was higher in AZT-treated animals, while the liver cytoplasmic NAD/NADH ratio was transiently oxidized by AZT alone. Co-administration with ADO normalized the serum NAD/NADH ratio and promoted a more oxidized liver cytoplasmic NAD/NADH ratio in AZT-treated animals.
AZT treatment increased serum β-OH-butyrate levels, which were inversely correlated with liver β-OH-butyrate levels, indicating that AZT induced a more reduced serum NAD/NADH ratio of the ketone body redox pair, reflecting the liver mitochondrial state (Figure 6). Changes in cytoplasmic and mitochondrial redox states, with the former being more oxidized and the latter being reduced, particularly after 2 and 4 weeks of treatment, may be linked to progressive mitochondrial dysfunction caused by AZT, like known hepatotoxins [20]. The capacity of the cell to oxidize substrates depends on its redox potential, making the redox state a crucial regulatory factor for many metabolic pathways [21]. An oxidized cytoplasmic redox state could be associated with lower glucagon release and increased serum IL-6 and IL-4 levels, whereas an increased mitochondrial NAD/NADH ratio could promote glucagon and insulin release, producing opposing effects on cytokines (Figure 7).
Conclusion
Oral administration of very low-dose AZT to rats over 1 - 8 weeks increased liver enzyme markers despite no necrotic changes in liver histology or liver function alterations. AZT induced a significant increase in liver TG levels, indicating slight fatty infiltration, suggestive of early micro-vesicular liver steatosis. These findings were associated with hypoglycemia, low serum insulin and glucagon levels, and elevated serum pro-inflammatory cytokines and liver TNF-α. The co-administration of adenosine significantly mitigated AZT-induced adverse effects, including inflammatory molecule production and changes in cellular redox state. This study demonstrates that AZT dosing, even at levels below the NOAEL, can have adverse effects, which could extend to patients receiving this antiretroviral.
Abbreviations List
ADO: Adenosine; AIDS: Acquired Immune Deficiency Syndrome; ALT: alanine aminotransferase; AST: Aspartate aminotransferase; AZT: 3′-Azido-3′-deoxythymidine; HAART: Highly active antiretroviral therapy; IL: Interleukin; LDH: Lactate dehydrogenase; mtDNA: Mitochondrial DNA; NER: Nucleotide Excision Repair; NRTI: Nucleoside-analog reverse transcriptase inhibitor; OCT: Ornithine transcarbamylase; ROS: Reactive oxygen species; TIMP: Tissue inhibitors of metaloproteases; TG: triacylglycerols; TNF-α: Tumor necrosis factor-alpha.
Acknowledgements
We thank Biols. Gerardo Coello, Ana María Escalante, Eng. Juan Manuel Barbosa, and Ivette Rosas for their expert assistance in figure design, Dr. Nicolas Jiménez-Pérez for creating the microscopic images, and Eng. Aurey Galván and Manuel Ortínez for the maintenance and supervision of laboratory equipment. We also acknowledge the valuable assistance of veterinarians Claudia Rivera and Hector Malagón.
Funding
The authors acknowledge the partial funding support for this investigation from PAPIIT grants # IT200420 and # IN210623 of the Dirección General de Asuntos del Personal Académico (DGAPA).
Competing interests:
The authors declare that there are no conflicts of interest.
References
- Bristow C, Barber T. Managing frailty in people with human immunodeficiency virus. British Journal of Hospital Medicine (Lond) 83 (2022): 831-837.
- Singh L, Bell TG, Yousif M, et al. Response of hepatitis B virus to antiretroviral treatment containing lamivudine in HBsAg-positive and HBsAg-negative HIV-positive South African adults. Journal of Medical Virology 91 (2019): 758-764.
- UNAIDS Data 2017.
- Smith CJ, Ryom L, Weber R, et al. D:A:D Study Group. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 384 (2014): 241-248.
- Fang JL, Han T, Wu Q, et al. Differential gene expression in human hepatocyte cell lines exposed to the antiretroviral agent zidovudine. Archives of Toxicology 88 (2014): 609-623.
- Fang JL, Beland FA. Long-term exposure to zidovudine delays cell cycle progression, induces apoptosis, and decreases telomerase activity in human hepatocytes. Toxicological Science 111 (2009): 120-130.
- Rahman MF, Raj R, Govindarajan R. Identification of Structural and Molecular Features Involved in the Transport of 3'-Deoxy-Nucleoside Analogs by Human Equilibrative Nucleoside Transporter 3. Drug Metabolism & Disposition 46 (2018): 600-609.
- Pupure J, Fernandes MA, Santos MS, et al. Mitochondria as the target for mildronate's protective effects in azidothymidine (AZT)-induced toxicity of isolated rat liver mitochondria. Cell Biochemistry and Function 26 (2008): 620-631.
- de la Asunción JG, del Olmo ML, Sastre J, et al. Zidovudine (AZT) causes an oxidation of mitochondrial DNA in mouse liver. Hepatology 29 (1999): 985-987.
- AlMenabawy N, Hassaan HM, Ramadan M, et al. Clinical and genetic spectrum of mitochondrial DNA depletion syndromes: A report of 6 cases with 4 novel variants. Mitochondrion 65 (2022): 139-144.
- Chariot P, Drogou I, de Lacroix-Szmania I, et al. Zidovudine-induced mitochondrial disorder with massive liver steatosis, myopathy, lactic acidosis, and mitochondrial DNA depletion. Journal of Hepatology 30 (1999): 156-160.
- Abebe M, Kinde S, Belay G, et al. Antiretroviral treatment associated hyperglycemia and dyslipidemia among HIV infected patients at Burayu Health Center, Addis Ababa, Ethiopia: a cross-sectional comparative study. BMC Research Notes 2014; 7, Jun21:380.
- Han WM, Apornpong T, Lwin HMS, et al. Non-alcoholic fatty liver disease and non-alcoholic steatohepatitis with liver fibrosis as predictors of new-onset Diabetes Mellitus in people living with HIV: A longitudinal cohort study. Clinical Infectious Diseases 21 (2023): ciad433.
- Butanda-Ochoa A, Hernández-Espinosa DR, Olguín-Martínez M, et al. A single zidovudine (AZT) administration delays hepatic cell proliferation by altering oxidative state in the regenerating rat liver. Oxidative Medicine and Cellular Longevity (2017).
- Hernández-Muñoz R, Santamaría A, García-Sáinz JA, et al. On the mechanism of ethanol-induced fatty liver and its reversibility by adenosine. Archives of Biochemistry and Biophysics 190 (1978): 155-162.
- Hernández-Muñoz R, Díaz-Muñoz M, Suárez-Cuenca JA, et al. Adenosine reverses a pre-established CCl4-induced micronodular cirrhosis through enhancing collagenolytic activity and stimulating hepatocyte cell proliferation in rats. Hepatology 34 (2001): 677-687.
- Morales-González JA, Gutiérrez-Salinas J, Yáñez L, et al. Morphological and biochemical effects of a low ethanol dose on rat liver regeneration. Digestive and Disease Sciences 44 (1999): 1963-1974.
- Colombo JP, Konarska L. Arginase, in: Methods of Enzymatic Analysis (H.U. Bergmeyer, J. Bergmeyer, M. Grassl, Eds), Vol. VII. pp. 285-294, Deerfield Beach, Florida, Verlag Chemie (1984).
- Bowlus CL, Pockros PJ, Kremer AE, et al. Long-term obeticholic acid therapy improves histological endpoints in patients with primary biliary cholangitis. Clinical Gastroenterology and Hepatology. 18 (2020): 1170-8.e6.
- Hernández-Muñoz R, Díaz-Muñoz M, De Sánchez VC. Possible role of cell redox state on collagen metabolism in carbon tetrachloride-induced cirrhosis as evidenced by adenosine administration to rats. Biochimica and Biophysica Acta. 1200 (1994) 93-99.
- Nicholl DG. Quantitative bioenergetics: the measurement of driving forces. In Bioenergetics 2, Eds. Nicholl DG & Ferguson SJ. Academic Press, Harcourt Brace Jovanich, Publishers (1992): 305-312.
- Martiñón-Gutiérrez G, Luna-Castro M, Hernández-Muñoz R. Role of insulin/glucagon ratio and cell redox state in the hyperglycemia induced by exposure to a 60-Hz magnetic field in rats. Scientific Reports 11 (2021): 11666.
- Bakasis AD, Androutsakos T. Liver fibrosis during antiretroviral treatment in HIV-infected individuals. Truth or tale? Cells 10 (2021): 1212.
- Kim JY, Leem J, Kim GM. Kahweol protects against acetaminophen-induced hepatotoxicity in mice through inhibiting oxidative stress, hepatocyte death, and Inflammation. BioMed Research International 27 (2022): 8121124.
- de la Asunción JG, Del Olmo ML, Gómez-Cambronero LG, et al. AZT induces oxidative damage to cardiac mitochondria: protective effect of vitamins C and E. Life Sciences 76 (2004): 47-56.
- Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. Journal of Basic and Clinical Pharmacy 7 (2016) 27-31.
- Abongwa LE, Nyamache AK, Charles F, et al. Risk factors of severe hepatotoxicity among HIV-1 infected individuals initiated on highly active antiretroviral therapy in the Northwest Region of Cameroon. BMC Gastroenterology 22 (2022): 286.
- Tadesse BT, Foster BA, Kabeta A, et al. Hepatic and renal toxicity and associated factors among HIV-infected children on antiretroviral therapy: a prospective cohort study. HIV Medicine 20 (2019) 147-156.
- Mahto SK, Gupta PK, Taneja RS, et al. Zidovudine-induced lactic acidosis with acute pancreatitis and myopathy: Lethal and rare complications. Indian Journal of Pharmacology 50 (2018): 212-214.
- Schulte-Hermann K, Schalk H, Haider B, et al. Impaired lipid profile and insulin resistance in a cohort of Austrian HIV patients. Journal of Infection and Chemotherapy 22 (2016): 248-253.
- Ikemoto M, Tsunekawa S, Toda Y, et al. Liver-type arginase is a highly sensitive marker for hepatocellular damage in rats. Clinical Chemistry 47 (2001): 946-948.
- Díaz-Muñoz M, Hernández-Muñoz R. Molecular and biochemical features of the mitochondrial enzyme ornithine transcarbamylase: a possible new role as a signaling factor. Current Medicinal Chemistry 17 (2010): 2253-2260.
- Kolaric TO, Nincevic V, Kuna L, et al. Drug-induced fatty liver disease: Pathogenesis and treatment. Journal of Clinical Translational Hepatology 9 (2021): 731-737.
- Sundaram V, Björnsson ES. Drug-induced cholestasis. Hepatology Communications 1 (2017): 726-735.
- Johnston-Cox H, Eisenstein AS, Koupenova M, et al. The macrophage A2B adenosine receptor regulates tissue insulin sensitivity. PLoS One 9 (2014): e98775.
- Silva JP, Köhler M, Graff C, et al. Impaired insulin secretion and beta-cell loss in tissue-specific knockout mice with mitochondrial diabetes. Nature Genetics 26 (2000) 336-340.
- Peng Z, Borea PA, Varani K, et al. Adenosine signaling contributes to ethanol-induced fatty liver in mice. Journal of Clinical Investigation 119 (2009): 582-594.
- Johnston-Cox H, Koupenova M, Yang D, et al. The A2b adenosine receptor modulates glucose homeostasis and obesity. PLoS One 7 (2012): e40584.
- Cohen J, D'Agostino L, Tuzer F, et al. HIV antiretroviral therapy drugs induce premature senescence and altered physiology in HUVECs. Mechanisms of Ageing Development 175 (2018): 74-82.
- Pérez-Cabeza de Vaca R, Domínguez-López M, Guerrero-Celis N, et al. Inflammation is regulated by the adenosine derivative molecule, IFC-305, during reversion of cirrhosis in a CCl4 rat model. International Immunopharmacology 54 (2018): 12-23.
- Khoa ND, Postow M, Danielsson J, et al. Tumor Necrosis Factor-α prevents desensitization of Gαs-coupled receptors by regulating GRK2 association with the plasma membrane. Molecular Pharmacology 69 (2006): 1311-1319.
- Ohta A, Sitkovsky M. Extracellular adenosine-mediated modulation of regulatory T cells. Frontiers in Immunology 5 (2014): 304.
- Haskó G, Cronstein B. Regulation of inflammation by adenosine. Frontiers in Immunology 4 (2013): 85.
- Borea PA, Gessi S, Merighi S, et al. Pharmacology of adenosine receptors: The state of the art. Physiological Reviews 98 (2018) 1591-1625.
- Arimany-Nardi C, Minuesa G, Keller T, et al. Role of Human Organic Cation Transporter 1 (hOCT1) polymorphisms in lamivudine (3TC) uptake and drug-drug interactions. Frontiers in Pharmacology 7 (2016): 175.
- Díaz-Muñoz M, Hernández-Muñoz R, Butanda-Ochoa A. Structure-activity features of purines and their receptors: implications in cell physiopathology. Molecular Biomedicine 3 (2022): 5.
- Oliveira NM, Ferreira FA, Yonamine RY, et al. Antiretroviral drugs and acute pancreatitis in HIV/AIDS patients: is there any association? A literature review. Einstein (Sao Paulo) 12 (2014): 112-9.
- Casademont J, Barrientos A, Grau JM, A, et al. The effect of zidovudine on skeletal muscle mtDNA in HIV-1 infected patients with mild or no muscle dysfunction. Brain 119 (1996): 1357-1364.
- Fromenty B. Alteration of mitochondrial DNA homeostasis in drug-induced liver injury. Food Chemistry and Toxicology 135 (2020): 110916.
- Pan-Zhou XR, Cui L, Zhou XJ, et al. Differential effects of antiretroviral nucleoside analogs on mitochondrial function in HepG2 cells. Antimicrobial Agents and Chemotherapy 44 (2000): 496-503.
- Fishman P, Stemmer SM, Bareket-Samish A, et al. Targeting the A3 adenosine receptor to treat hepatocellular carcinoma: anti-cancer and hepatoprotective effects. Purinergic Signaling 19 (2023): 513-22.






 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 