Carvedilol Associated with Ligation For The Prophylaxis of First Esophageal Variceal Bleeding: A Novel Approach of Long-Term Study
Gin-Ho Lo1,3*, Jen-Hao Yeh1,2,3, Hsueh-Chou Lai4, Chi-I Chen5, Chi-Ming Tai1,3, Chih-Wen Lin1,2,3, Hui- Chen Lin1
1Department of Medical Research; Division of Gastroenterology, E-DA Hospital, Taiwan
2Division of Gastroenterology, E-DA Hospital, Da-Chung Branch, Taiwan
3School of Medicine for international students, I-Shou University, Kaohsiung, Taiwan
4China Medical University Hospital, Taichung, Taiwan
5Chia- I Christian Hospital, Chia-I, Taiwan
*Corresponding Author: Gin-Ho Lo, Department of Medical Research; Division of Gastroenterology, E-DA Hospital, Taiwan
Received: 21 June 2022; Accepted: 27 June 2022; Published: 29 June 2022
Article Information
Citation: Gin-Ho Lo, Jen-Hao Yeh, Hsueh-Chou Lai, Chi-I Chen, Chi-Ming Tai, Chih-Wen Lin, Hui- Chen Lin. Carvedilol Associated with Ligation For The Prophylaxis of First Esophageal Variceal Bleeding: A Novel Approach of Long-Term Study. Journal of Surgery and Research 5 (2022): 372-384.
View / Download Pdf Share at FacebookAbstract
Background
The role of combining banding ligation with β-blockers in the prophylaxis of first esophageal variceal bleeding is unsettled. We conducted a trial to assess the value of carvedilol plus banding ligation in the prophylaxis of 1st bleeding.
Subjects and methods
Cirrhotic patients with high risk esophageal varices who have never bled were randomized to 2 groups. The Carvedilol group received regular carvedilol administration alone while Combination group received 3 sessions of banding ligation at 3 and 12 months interval additional to carvedilol administration. End points were first variceal bleeding and survival.
Results
A total of 65 patients were enrolled, 34 patients in the Carvedilol group, and 31 patients in Combination group. Both groups were comparable in baseline data. The mean dose of carvedilol was similar in both groups. A mean of 2.7 session of banding ligation was performed in the Combination group. After a follow-up up to 9 years, 16 patients in the Carvedilol group encountered esophageal variceal bleeding while 3 patients in the Combination group bled (p<0.001, hazard ratio 5.52; confidence interval 2.19-13.9). Upper gastrointestinal bleeding was experienced in 21 patients and 6 patients in the Carvedilol group and Combination group, respectively. Adverse events were similar between both groups. There were 23 deaths (67%) in the Carvedilol group and 11 deaths (35%) in the Combination group (p= 0.03, hazard ratio 2.08; confidence interval 1.06-4.92).
Conclusions
Carvedilol combined with banding ligation proved more effective than carvedilol alone in the prevention of first esophageal v
Keywords
<p>Carvedilol, First esophageal variceal bleeding, Hemorrhage</p>
Article Details
1. Introduction
Hemorrhage from esophageal varices is a formidable complication of portal hypertension. Approximately one third of cirrhotic patients with esophageal varices will bleed. The mortality rate associated with first bleed is still around 20% despite the advancement of therapies in recent years [1,2]. Esophageal varices of medium-large size with red color signs carry a high risk of rupture ranging between 25% and 58% within 2 years [3-6]. Either beta-blockers or endoscopic variceal ligation (EVL) is recommended as a feasible tool for primary prophylaxis of high risk varices [7,8]. The combination of endoscopic therapy and pharmacotherapy has proven to be more effective than either therapy alone in the prevention of variceal rebleeding but not in the prophylaxis of first variceal bleeding [9-12]. This study was undertaken to evaluate the efficacy and safety of carvedilol plus EVL versus carvedilol alone in the prophylaxis of first episodes of esophageal variceal bleeding.
2. Subjects and Methods
Patients presented with chronic liver disease and esophageal varices were selected for possible inclusion in the trial. The inclusion criteria were as follows: 1. the cause of portal hypertension was cirrhosis; 2. the degree of esophageal varices was at least F2 (moderate varices), associated with red color signs (red wale markings, cherry red spots); 3. no history of hemorrhage from esophageal varices or other upper gastrointestinal lesions; and 4. no current treatment with beta-blockers. Cirrhosis was based on results of liver biopsy, or clinical and biochemical examinations and image studies. The exclusion criteria were: 1. age greater than 75 years old or younger than 20 years old; 2. association with malignancy, uremia or other serious medical illness which may reduce the life expectancy; 3. presence of refractory ascites, hepatic encephalopathy or deep jaundice (serum bilirubin > 10 mg/dl); 4. history of shunt operation, transjugular intrahepatic portosystemic stent shunt (TIPS) or endoscopic therapy (EVL); 5. had contraindications to beta-blockers, such as asthma, heart failure, complete atrioventricular block, hypotension (systolic blood pressure < 90mmHg), pulse rates < 60/ min or pregnancy; 6. unable to cooperate; and 7. declined to participate. Patients eligible for the trial were randomized to receive banding ligation plus carvedilol (Combined group) or carvedilol alone (Carvedilol group). The method of randomization was based on opaque-sealed envelopes numbered according to a table of random numbers by a coordinator. Patients were informed about possible benefits and complications. Informed consent was obtained from all the patients. The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of investigators at all the 3 hospitals. The severity of liver disease of each patient was assessed at the time of presentation according to Pugh's modification of Child's classification and MELD scores [13]. The degree of variceal size was based on Beppu’s classification (3). Participants in both groups were advised to abstain from alcohol drinking. Antiviral drugs were administered to individuals with chronic hepatitis B or C when appropriate. Carvedilol was administered from the start of enrollment. The dose of carvedilol given was 6.25 mg (Roche, S.p.A, Segrate, Italy) twice daily. If participants complained of side effects, the dose of carvedilol given was tapered to 6.25 mg per day. Carvedilol was continued until death or the end of the study. After stabilization of the dosage, patients were asked to visit clinic every 3 months to get drugs as well as measurement of blood pressure and pulse rate. The compliance was evaluated by quantifying carvedilol tablets that were not consumed and blood pressure and pulse rates. Among the Combination group, the administration of carvedilol was similar to the Carvedilol group. EVL was initiated 2 weeks after use of carvedilol. Banding ligation was performed under premedication with 20 mg of buscopan intramuscularly. The Saeed Four-Shooter (Wilson-Cook Medical, Winston-Salem, NC, USA) or Speedband Superview Super 7 multiple band ligator (Boston Scientific Corp, Natick, Mass) attached to the video endoscope (Olympus XQ 230) was utilized. Ligation was initiated at the gastroesophageal junction and advanced proximally by experienced endoscopists. Each varix was ligated with one to two rubber bands. Further sessions of banding ligation were performed at intervals of 3 and 12 months. If varices were too small, EVL was not performed.
2.1 Variceal bleeding management and adverse events
All patients suspected of upper gastrointestinal bleeding received emergency endoscopy within 12 hours of presentation. Supportive measures included blood transfusion, vasoconstrictor infusion and prophylactic antibiotics. Acute esophageal or gastric variceal bleeding was based on the Baveno criteria [14]. Emergency EVL was administered for esophageal variceal bleeding and glue injection was performed for gastric variceal bleeding [15,16]. Elective EVL with carvedilol or transjugular intrahepatic porto-systemic shunt (TIPS) for prevention of rebleeding was employed for patients of both groups if indicated [9]. Patients in both groups received follow-ups of abdominal sonogram, serum alpha-fetoprotein, and biochemistry at 3-month intervals. Adverse events were recorded. An adverse event was an event that required a diagnostic or therapeutic intervention. An adverse event was judged severe if it was considered to endanger the health or safety of the participant. Primary end points were first variceal bleeding and upper gastrointestinal bleeding. Secondary end points were adverse events, development of ascites or hepatic encephalopathy and death.
2.2 Statistical analysis and sample size calculation
The data were expressed as mean± standard deviation (S.D.) Statistical analysis was based on an intention-to-treat principle. Continuous variables were compared according to Student's t-test, and categorical variables were compared using the Chi-square test and Fisher's exact test when appropriate. The Kaplan-Meier estimation was applied to examine the time to a first occurrence of bleeding and the time to death. The Log rank test was used to examine the variation of bleeding episodes and survival. The Cox’s regression analysis was used to detect possible prognostic variables other than treatment modality on the rebleeding rate and survival. All P values were two-tailed. A P value < 0.05 was considered significant. Analyses were performed using SPSS statistical package, version 26.0 (IBM Corp, Armonk, NY, USA). Assuming that 20% of the patients in the Carvedilol group may encounter first variceal bleed and which may be reduced to 5% in the Combination group, approximately 77 subjects would be required for each group with a two-tailed test to achieve a beta value of 0.2 and an error of 5% [17,18]. Owing to slow recruitment, the enrollment was terminated after a period of 4 years. All the enrolled subjects were advised to receive medications continuously. A coordinator in each center kept in contact with all the participants closely. We expected that an extension of the study period could compensate for inadequate sample size.
3. Results
The study began on June 28, 2011. The first patient was enrolled on July 8, 2011. Between July 2011 and June 2015, a total of 238 cirrhotic patients were screened for possible inclusion. Ninety-one patients had no esophageal varices and 45 patients had mild varices. Of the 102 eligible patients, 37 patients were excluded for the following reasons: age> 75 years old (5 patients), association with hepatocellular carcinoma (15 patients), other malignancy (2 patients) or uremia (3 patients), ever received EVL (1 patient), deep jaundice (2 patients), refractory ascites (2 patients), bradycardia (1 patient), hypotension (1 patient), refused to participate (5 patients). Allocation of patients is shown in figure 1. Finally 65 consecutive patients were enrolled in the trial, 34 patients in the Carvedilol group and 31 patients in the Combination group. The characteristics of both groups are shown in table 1. Both groups were comparable regarding age, sex, severity of variceal size and liver disease. The majority of patients in both groups were Child-Pugh class A. The median follow-up period was 56 months in Combination group and 55 months in Carvedilol group. Two patients in the Combination group did not receive the third scheduled endoscopy at interval of 12 months. Five patients in the Carvedilol group and 2 patients in the Combination group were not compliant to take carvedilol regularly. Owing to side effects, 2 individuals in Carvedilol group and 1 individual in Combination group were switched to take propranolol since 3 to 6 months after enrollment. Two participants in each group lost follow up at between 13 months and 56 months after enrollment. They were included in the final analysis. The trial was ended till June 2020. In the carvedilol group, the mean dose of carvedilol was 11.3 ± 3.9 mg (range 6.25 - 25 mg) per day. Among the Combination group, the mean dose of carvedilol was 11.6 ± 5.2 mg (range 6.25 - 25 mg) per day. The mean sessions of banding ligation for primary prophylaxis was 2.7± 0.6 (range 2-3). The mean rubber bands applied were 9.5 ± 3.5 (range 7-15). Variceal obliteration was achieved in only 12 patients (39%).
3.1 Hemorrhage from upper gastrointestinal tract
21 patients of the Carvedilol group and 6 patients in the Combination group experienced upper gastrointestinal bleeding (p<0.001, hazard ratio 4.50; 95% confidence interval 2.06-9.82). The origins of upper gastrointestinal bleeding are shown in table 2. Esophageal variceal bleeding was encountered in 16 patients in the Carvedilol group and 3 patients in the Combination group (p<0.001, hazard ratio 5.52; 95% confidence interval 2.19-13.9). The time to first variceal bleeding was 38.2 ± 29.4 months in the Carvedilol group, significantly shorter than the figure of 56.9 ± 33.9 months in the Combination group (p<0.02). All the 5 incompliant individuals in the Carvedilol group encountered bleeding from esophageal varices. Figure 2 shows cumulative upper gastrointestinal bleeding rate. 1-year and 5-year bleeding rates of Carvedilol group vs. Combination group were 14.7% vs. 3.3% and 59.1% vs. 11.5%, respectively. Figure 3 shows cumulative esophageal variceal bleeding rate. 1-year and 5-year bleeding rates of Carvedilol group vs. Combination group were 14.7% vs. 0% and 52.6% vs.4.8%, respectively. Cox regression analysis showed that therapy was the only factor predictive of esophageal variceal bleeding (hazard ratio, 7.79, 95% CI: 1.80-33.6, p=0.006). The development of ascites and hepatic encephalopathy were similar between both groups (Table 2).
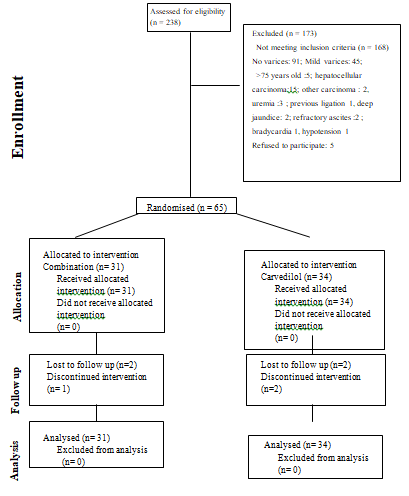
Figure 1: Allocation of eligible patients
|
Carvedilol alone (N = 34) |
Combination (N = 31) |
P value |
|
|
Age |
59.7±6.7 |
56.6±10.6 |
0.16 |
|
Male/female |
23/11 |
21/10 |
0.99 |
|
Etiologies of cirrhosis |
|||
|
Alcohol |
8(23%) |
8(25%) |
0.83 |
|
HBV |
16(47%) |
11(36%) |
0.4 |
|
HCV |
17(50%) |
14(45%) |
0.69 |
|
HBV + HCV |
3(8.5%) |
1(3.2%) |
0.34 |
|
Others |
1(2.9%) |
3(9.7%) |
0.25 |
|
AST (IU/L) |
64.8 ± 33.1 |
69.0 ± 39.2 |
0.64 |
|
ALT (IU/L) |
49.4 ± 24.4 |
56.0 ± 42.6 |
0.43 |
|
Albumin (g/dL) |
3.5 ± 0.5 |
3.6 ± 0.5 |
0.64 |
|
Bilirubin (mg/dL) |
1.8 ± 1.3 |
1.7 ± 0.8 |
0.44 |
|
Prothrombin time prolongation (sec) |
2.2 ± 1.8 |
1.9 ± 1.1 |
0.45 |
|
MELD score |
11.7 ± 3.2 |
12.7 ± 5.4 |
0.36 |
|
Ascites |
7 |
8 |
0.87 |
|
Encephalopathy |
0 |
1 |
0.29 |
|
Child-Pugh score |
6.2 ± 1.6 |
6.2 ± 1.6 |
0.99 |
|
Child-Pugh (A/B/C) |
22-09-2003 |
22-07-2002 |
0.85 |
|
Size of esophageal varices |
|||
|
F1/ F2/ F3* |
0 / 24 / 10 |
0 / 19 / 12 |
0.62 |
|
Gastric varices |
7 |
9 |
0.42 |
|
Hemoglobin (gm/dl) |
12.0± 2.1 |
11.90± 1.7 |
0.9 |
F1/F2/F3: mild, moderate, large varices; MELD: Model For End-Stage Liver Disease
Table 1: Baseline data of both groups
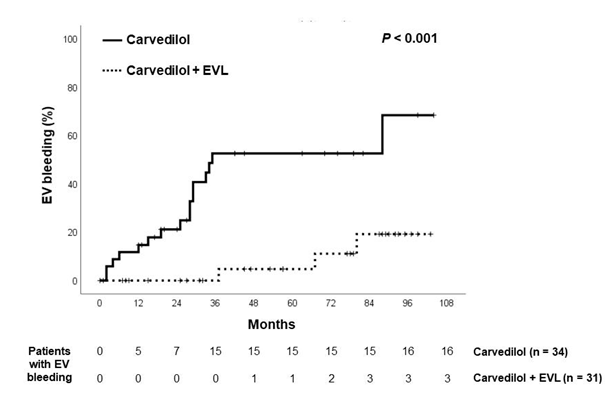
Figure 2: Kaplan-Meier estimates of probability of being free from first bleeding from esophageal varices in both groups.
|
Carvedilol (N = 34) |
Combination (N = 31) |
P value |
|
|
Carvedilol (mg/day) |
11.6 ± 5.2 |
11.3 ± 3.9 |
0.797 |
|
Ligation sessions* |
2.7 ± 0.6 |
||
|
Rubber bands |
9.5 ± 3.5 |
||
|
Time to first bleeding (day) |
38.2 ± 29.4 |
56.9 ± 33.9 |
0.02* |
|
UGI bleeding |
21 (61%) |
6 (19%) |
0.001* |
|
Sources of hemorrhage |
|||
|
EV bleeding |
16 (47%) |
3 (9%) |
0.001* |
|
Gastric varices |
2 |
1 |
|
|
Peptic ulcers |
2 |
2 |
|
|
Gastropathy |
1 |
0 |
|
|
Presence of ascites |
18(53%) |
11(35%) |
0.15 |
|
Presence of encephalopathy |
8(24%) |
6(19%) |
0.28 |
|
Death |
23 (67%) |
11 (35%) |
0.01* |
Ligation session*: required to achieve variceal obliteration;
UGI: upper gastrointestinal; EV: esophageal varices
PHG: portal hypertensive gastropathy
Table 2: Treatment results
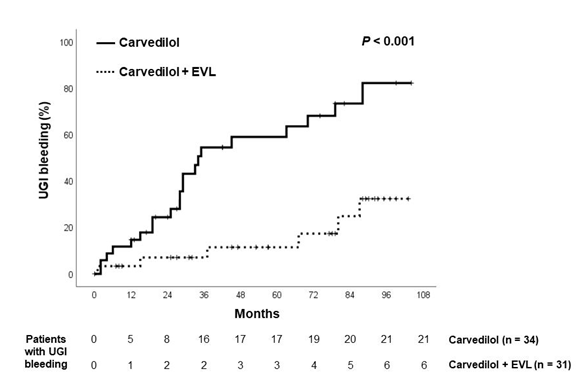
Figure 3: Kaplan-Meier estimates of probability of being free from upper gastrointestinal bleeding in both groups.
|
Adverse events |
Carvedilol (N = 34) |
Combination (N = 31) |
|
Ligation-induced ulcer bleeding |
0 |
1 |
|
Retrosternal pain |
0 |
2 |
|
Transient dysphagia |
0 |
2 |
|
Nausea |
1 |
0 |
|
Shortness of breath |
3 |
3 |
|
Dizziness |
2 |
1 |
|
Bradycardia |
1 |
1 |
|
General weakness |
1 |
0 |
|
Mortality |
||
|
Hepatic failure |
4 |
3 |
|
Sepsis |
5 |
2 |
|
SBP |
4 |
2 |
|
HCC |
2 |
2 |
|
Hepatorenal syndrome |
1 |
0 |
|
EV bleeding |
2 |
0 |
|
CVA |
1 |
1 |
|
Other malignancy |
4 |
1 |
SBP: spontaneous bacterial peritonitis; HCC: hepatocellular carcinoma
EV: esophageal varices; CVA: cerebral vascular accident
Table 3: Adverse events and mortalities
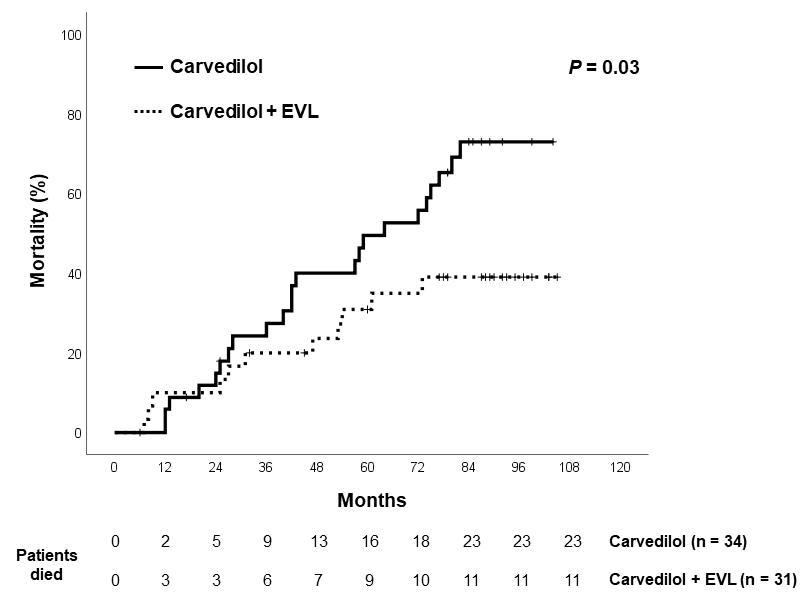
Figure 4: Actuarial of mortality in the 2 groups.
3.2 Adverse events
One episode of ulcer bleeding was encountered in the Combination group at 5 days after first session of EVL and rescued by use of vasoconstrictor and proton pump inhibitor. No serious adverse effects were encountered in Carvedilol group. The adverse events are shown in table 3. No significant differences existed between both groups.
3.3 Mortality rates
There were 23 deaths in the Carvedilol group and 11 deaths in the Combination group (hazard ratio 2.08, 95% CI= 1.06-4.92, p=0.03). Kaplan-Meier survival curve is shown in figure 4. The mortality rates 1-year and 5-year of Carvedilol group vs. Combination group were 5.9% vs. 10.0% and 49.5% vs. 30.9%, respectively. Cox regression analysis revealed that variceal bleeding ((hazard ratio, 2.53, 95% CI: 1.22- 5.25, p=0.012) and therapy were the factors predictive of mortality (hazard ratio, 2.54, 95% CI: 1.03- 6.30, p=0.043). The causes of mortality are shown in table 3. The majority of patients died of hepatic failure, infections and malignancy. One patient in the Carvedilol group received liver transplantation at 55 months after enrollment. Two patients in the Carvedilol group versus no patients in Combination group died of variceal hemorrhage.
4. Discussion
It is well recognized that individuals with high risk varices should receive prophylactic measures to prevent first bleeding [2,7,8]. Beta blockers having the advantages of systemic effects and non- invasiveness, are generally recommended as the first choice [7,8,19]. Moreover, carvedilol, a ß blocker with anti-α1 activity, at daily dose of 6.25-12.5 mg was shown to elicit a greater decrease in hepatic venous pressure gradient (HVPG) than propranolol and combination with isosorbide mononitrate [20-23]. Some meta-analyses showed that EVL is superior to ß blockers in reducing variceal bleeding [24-26], while recent studies disclosed that carvedilol is superior to EVL in the prevention of first variceal bleed [26-28]. On the other hand, carvedilol could achieve significant HVPG reduction in only 54% of subjects [20-23]. Despite the use of ß blockers, at least 15% of individuals still bled from esophageal varices [24,25]. Combination therapy of ß blockers with EVL is hopefully via different mechanisms to achieve an enhanced effect in preventing variceal bleeding. Up to now, 8 trials, including 6 full articles [11-12,29-32] and 2 in abstract form [33,34], have assessed the efficacy of combination therapy. Interestingly, most of the published prospective studies showed negative results while the latest 2 large trials presented in abstract form and a network meta-analysis demonstrated enhanced effect by the combination therapy as compared with ß blockers or EVL alone [26,33,34]. Our trial firstly adopted carvedilol association with limited sessions of ligation as the combination therapy. The esophageal variceal bleeding rates were 0%, and 4.8% in 1-year and 5-year in the Combination group, respectively. By contrast, the corresponding figures were 14.7% and 52.6% in the Carvedilol group. These data suggested the long-term beneficial effects by association with EVL. The frequency of minor complications was similar between both treatment groups. Prior studies in cirrhotic patients using beta blockers showed that the incidences of first variceal bleeding were in the range of 10% and 43% [5,6,24,25]. The slightly higher incidence of variceal bleeding in our Carvedilol group could be ascribed to long-term observation and partly to poor compliance in the long-term study. Due to significant reduction of first variceal bleeding, long-term combination therapy achieved an enhanced survival as compared with individuals treated with carvedilol alone. This significant end point has been rarely achieved in previous trials [5,6,11,12,17,18]. The combination of EVL and beta blockers is currently not recommended by most guidelines, simply owing to inadequate evidence [8,14,19]. Conversely, a study from Germany revealed that up to 64% of clinicians using combination therapy to prevent first bleeding [31]. In this trial, we adopted a unique policy to combine EVL. The interval of EVL was lengthened and sessions of EVL were limited, with a purpose to reduce variceal size instead of complete variceal obliteration. All the previous trials pursued high variceal obliteration rates in a short period [5,6,11,12]. This policy requires bothersome regular endoscopic examinations and therapies to prevent variceal recurrence, potentially resulting in increased incidences of severe adverse events. Our methods could effectively reduce variceal size without hampering the effect. Small residual varices were left to be controlled by combination with carvedilol and treatment of underlying etiologies. Our variceal obliteration was only 39%, lower than previous figures up to 98% [25]. However, the variceal bleeding rate remained only 4.8% at 5-year by combination therapy. The appropriate interval of EVL has been an issue of controversy [34,35]. A trial from Japan with interval up to 2 months showed satisfactory results in primary prophylaxis of variceal bleeding [36]. There were no serious complications in either group in our trial except one episode of ulcer bleeding. The development of ascites and hepatic encephalopathy were similar between both groups. Our trial clearly showed that limited EVL sessions could be a good partner to carvedilol in the decrease of first esophageal variceal bleeding. The weakness of this trial was small sample size. The strength of our trial was multi-center, long term study using carvedilol together with a novelty design in the institution of EVL. This approach could obviate HVPG measurement and regular endoscopic surveillance. Most studies for primary prophylaxis of variceal bleeding usually observed for only 1 to 2 years [24-26]. Short study period and overtreatment with EVL were presumed to be responsible for the negative results of combination studies [30]. This trial with study period extending to 9 years, and a median of 55 months, demonstrated that variceal bleeding rate as well as mortality in cirrhotic patients with high risk varices could be effectively reduced by the combination of EVL and ß blockers. Choices between ß blockers and EVL for primary prophylaxis of variceal bleeding have been an issue of long-term controversy [26,38,39]. While experts prefer ß blockers, 64% of surveyed patients conversely prefer EVL over ß blockers [40]. Our trial suggested that combination therapy is a viable, practical approach. In conclusion, our data revealed that the addition of EVL to carvedilol could significantly enhance the effect in reducing the incidence of first variceal bleeding as well as mortality.
References
- Carbonell N, Pauwels A, Serfaty L, et al. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology 40 (2004): 652-659.
- Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 362 (2010): 823-832.
- Beppu K, Inokuchi K, Koyanagi N, et al. Prediction of variceal hemorrhage by esophageal endoscopy. Gastrointest Endosc 27 (1981): 213-218.
- North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med 319 (1988): 983-989.
- Sarin SK, Lamba GS, Kumar M, et al. Comparison of endoscopic ligation and propranolol for primary prevention of variceal bleeding. N Engl J Med 340 (1999): 988-993.
- Lo GH, Chen WC, Chen MH, et al. Endoscopic ligation vs. nadolol in the prevention of first variceal bleeding in patients with cirrhosis. Gastrointest Endosc 59 (2004): 333-338.
- De Franchis R, Baveno VI Faculty. Expanding consensus in portal hypertension report of the Baveno VI consensus workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol 63 (2015): 743-752.
- Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver disease. Hepatology 65 (2017): 310-335.
- Lo GH, Lai KH, Cheng JS, et al. Endoscopic variceal ligation plus nadolol and sucralfate compared with ligation alone for the prevention of variceal rebleeding: A prospective, randomized trial. Hepatology 32 (2000): 461-465.
- Gonzalez R, Zamora J, Gomez-Camarero J, et al. Meta-analysis: Combination endoscopic and drug therapy to prevent variceal rebleeding in cirrhosis. Ann Intern Med 149 (2008): 109-122.
- Sarin SK, Wadhawan M, Agarwal SR, et al. Endoscopic variceal ligation plus propranolol versus endoscopic variceal ligation alone in primary prophylaxis of variceal bleeding. Am J Gastroenterol 100 (2005): 797-804.
- Lo GH, Chen WC, Wang HM, et al. Endoscopic ligation plus nadolol versus nadolol in the prophylaxis of first variceal bleeding in cirrhosis. Hepatology 52 (2010): 230-237.
- Pugh RNN, Murray-Lyon IM, Dawson JL, et al. Transection of esophagus for bleeding esophageal varices. Br. J Surg 60 (1973): 646-649.
- De Franchis R. Updating consensus in portal hypertension: report of the Baveno III consensus workshop on definitions, methodology and therapeutic strategies in portal hypertension. J Hepatol 33 (2000): 846-852.
- Lo GH, Lai KH, Cheng JS, et al. Emergency banding ligation versus sclerotherapy for the control of active bleeding from esophageal varices. Hepatology 25 (1997): 1101-1104.
- Lo GH, Lai KH, Cheng JS, et al. A prospective, randomized trial of butyl cyanoacrylate injection versus band ligation in the management of bleeding gastric varices. Hepatology 33 (2001): 1060-1064.
- Jutabha R, Jensen DM, Martin P, et al. Randomized study comparing banding and propranolol to prevent initial variceal hemorrhage in cirrhotics with high risk esophageal varices. Gastroenterology 128 (2005): 870-881.
- Schepke M, Kleber G, Niirnberg D, et al. Ligation vs. propranolol for the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology 40 (2004): 65-72.
- Tripathi D, Stanley AJ, Hayes PC, et al. UK guidelines on the management of variceal hemorrhage in cirrhotic patients. Gut 64 (2015): 1680-1704.
- Banares R, Moitinho E, Piqueras B, et al. Carvedilol, a new nonselective beta-blocker with intrinsic anti-alpha1-adrenergic activity, has a greater portal hypotensive effect than propranolol in patients with cirrhosis. Hepatology 30 (1999): 79-83.
- Banares R, Moitinho E, Matilla A, et al. Randomized comparison of long-term carvedilol and propranolol administration in the treatment of portal hypertension in cirrhotic. Hepatology 36 (2002): 1367-1373.
- Lin HC, Yang YY, Hou MC, et al. Acute administration of carvedilol is more effective than propranolol plus isosorbide-5-mononitrate in the reduction of portal pressure in patients with viral cirrhosis. Am J Gastroenterol 99 (2004): 1953-1958.
- Bosch J. Carvedilol for portal hypertension in patients with cirrhosis. Hepatology 51 (2010): 2215-2218.
- Imperiale TF, Chalasani N. A meta-analysis of endoscopic variceal ligation for primary prophylaxis of esophageal variceal bleeding. Hepatology 33 (2001): 802-807.
- Khuroo MS, Khuroo NS, Farahat KLC, et al. Meta-analysis: endoscopic variceal ligation for primary prophylaxis of esophageal variceal bleeding. Aliment Pharmacol Ther 21 (2005): 347-361.
- Sharma M, Singh S, Desai V, et al. Comparison of therapies for primary prophylaxis of esophageal variceal bleeding: A systematic review and network meta-analysis. Hepatology 69 (2019): 1657-1675.
- Tripathi D, Ferguson JW, Kochar N, et al. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology 50 (2009): 825-833.
- Reiberger T, Ulbrich G, Ferlitsch A, et al. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with hemodynamic non-response to propranolol. Gut 62 (2013): 1634-1641.
- Gheorghe C, Gheorghe L, Iacob S, Iacob R, Popescu I. Primary prophylaxis of variceal bleeding in cirrhotics awaiting liver transplantation. Hepatogastroenterology 53 (2006): 552-557.
- Je D, Paik YH, Gwak GY, et al. The comparison of esophageal variceal ligation plus propranolol versus propranolol alone for the primary prophylaxis of esophageal variceal bleeding. Clin Mol Hepatol 20 (2014): 283-290.
- Bonilha DQ, Lenz L, Correia LM, et al. Propranolol associated with endoscopic band ligation reduces recurrence of esophageal varices for primary prophylaxis of variceal bleeding: a randomized-controlled trial. Eu J Gastroenterol Hepatol 27 (2015): 84-90.
- Pfisterer N, Dexheimer C, Fuchs EM, et al. Betablockers do not increase efficacy of band ligation in primary prophylaxis but they improve survival in secondary prophylaxis of variceal bleeding. Aliment Pharmacol Ther 47 (2018): 966-979.
- Seo YS, Kim MY, Yim HJ, et al. Multicenter prospective randomized controlled trial comparing propranolol, endoscopic band ligation, and combination therapy for the primary prophylaxis variceal bleeding in patients with liver cirrhosis. J Hepatol 66 (2017): S35.
- Pande A, Sarin SK, Jindal A, et al. Efficacy of Carvedilol, endoscopic variceal ligation (EVL) or a combination for the prevention of first variceal bleed in Child B and C cirrhosis with high risk varices: A randomized controlled trial. Hepatology (2019): 96A.
- Sheibani S, Khemichian S, Kim JJ, et al. Randomized trial of 1-week versus 2-week intervals for endoscopic ligation in the treatment of patients with esophageal variceal bleeding. Hepatology 64 (2016): 549-555.
- Lo GH. The optimal interval of endoscopic variceal ligation: an issue of controversy. (letter) Gastrointestinal Endoscopy 81 (2015): 774.
- Yoshida H, Mamada Y, Taniai N, et al. A randomized control trial of bimonthly versus biweekly endoscopic variceal ligation of esophageal varices. Am J Gastroenterol 100 (2005): 2005-2009.
- Laine L. Interventions for primary prevention of esophageal variceal bleeding. Hepatology 69 (2019): 1382-1384.
- Burroughs AK, Patch D. Primary prevention of bleeding from esophageal varices. N Engl J Med 340 (2019): 1033-1035.
- Longacre AV, Imaeda A, Garcia-Tsao G, et al. A pilot project examing the predicted preferences of patients and physicians in the primary prophylaxis of variceal hemorrhage. Hepatology 47 (2008): 169-176.


 Impact Factor: * 4.2
Impact Factor: * 4.2 Acceptance Rate: 72.62%
Acceptance Rate: 72.62%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 