Mean Platelet Volume/ Platelet Count Ratio in male and female patients with Iron Deficiency Anemia
Mangyung Kandangwa*,1, Damandeep Kaur Dhillon2,3, Johnson Igwe1, Suma Kaza*1,4, P Ramesh Babu4
1Avalon University of Medicine, Curaçao
2Arjun Kripa Clinic, Raikot, India.
3Goyal Lab and Diagnostic center, Raikot, India
4Aster Ramesh hospitals, Guntur, India
*Corresponding author: Mangyung Kandangwa, Avalon University of Medicine, Curaçao and Suma Kaza, Avalon University of Medicine
Received: 17 July 2025; Accepted: 29 July 2025; Published: 07 August 2025
Article Information
Citation: Mangyung Kandangwa, Damandeep Kaur Dhillon, Johnson Igwe, P Ramesh Babu, Suma Kaza. Mean Platelet Volume/ Platelet Count Ratio in male and female patients with Iron Deficiency Anemia. Fortune Journal of Health Sciences. 8 (2025): 749-755
View / Download Pdf Share at FacebookAbstract
Mean platelet volume (MPV) and platelet counts (PC), are sensitive indicators of inflammation and various pathologies. The MPV/PC ratio (MPR) has emerged as a potentially more sensitive and has been applied in studying iron deficiency anemia (IDA). However, hematological parameters tend to be different in men and women. This study explores the relationship between sex and platelet parameters in the context of IDA. The hematological samples were analyzed on HORIBA 5 part haematology analyser. IDA was classified as Hb< 13.0 g/dL and MCV<76 fl for male and Hb< 12.0 g/dL and MCV< 76 fl for female. Patient were classified as non-IDA when the hematological parameter did not fit for IDA. Samples from healthy population was control. Data analysis was performed using Microsoft Excel and GraphPad Prism 7. Statistical analysis was done with Unpaired t-test with Welch’s correction and Brown-Forsythe and Welch ANOVA test. Our result showed MPR had significant difference in male IDA group. Further analysis showed that, in control, females had higher PC than in male, with no significant difference in MPV. In IDA, the PC increased in both male and females, but MPVdecreased significantly only in males.
Keywords
<p>Platelets count (PC), Mean platelet volume (MPV), Mean platelet volume to Platelet count ratio (MPR), Iron deficiency anemia (IDA)</p>
Article Details
Introduction
WHO defines anemia as blood hemoglobin (Hb) level below 130g/L in men and 120g/L in women (WHO). When the anemia is solely due to iron deficiency, it is termed as iron deficiency anemia (IDA). In IDA there is a defect in the hemoglobin (Hb) synthesis resulting in microcytic red blood cells (RBC) (i.e. < 80 fL). In addition to RBC, IDA has also been reported to affect platelet production [1-3] with thrombocytosis observed at higher rates than thrombocytopenia [4]. Increase in endogenous erythropoietin may play a role in inducing thrombocytosis [5,6], however the actual mechanism behind iron deficiency induced thrombocytosis or thrombocytopenia is unclear [7]. Several studies have been done on the application of MPV/ platelet count ratio (MPR) as a new parameter for different pathologies eg. prediction of long term mortality in patients with myocardial infarction [8], prognosis for mortality in patients with severe sepsis [9,10], possible indicator of hepatocarcinoma [11] diagnosis of colorectal cancer [12] and so on. Similarly, Cho SY et al, 2012 also displayed outstanding performance in distinguishing IDA from other types of anemia and suggested it can be utilized as one of the panels along with conventional biochemical markers. However, studies have shown hematological parameters in male and females are different. Females have lower hemoglobin concentration than male, also have higher total WBC count resulting from higher absolute neutrophil and monocyte counts, neutrophil alkaline phosphatase. Though the relation of sex and platelet count is still controversial, emerging evidence has shown females have higher platelet count than male, even after controlling for iron deficiency[13,14]. The MPV has been found to be either higher in male [15] or have no difference [16]. The variation in the platelet parameters in male and female population made us interested in exploring MPV/Platelet count ratio (MPR) in male and female groups in the context of IDA.
Methods
Blood samples were collected from the anemic patients and collected in tubes with EDTA. The blood samples were specifically analyzed for the hematological parameters: serum hemoglobin (Hb), mean corpuscular volume (MCV), platelet count, mean platelet volume (MPV). EDTA blood samples were processed by HORIBA 5-part Analyser.
Anemic patients were categorized to have iron deficiency anemia (IDA) if hemoglobin (Hb) and mean corpuscular volume (MCV) were below the normal cut-off value i.e. Hb< 13.0 g/dL and MCV<76 fl for male and Hb< 12.0 g/dL and MCV< 76 fl for female. The patients were categorized as non-IDA if Hb>13.0 g/dL (male), 12.0 g/dL(female) and/or MCV > 76fL (male, female). Thrombocytosis was defined as platelet count more than 400 × /l[7] while thrombocytopenia was defined by platelet count <150 × 109 /l. (Kuku, I., et al, 2009)
Statistical Analysis
Data was recorded and analyzed using Microsoft excel. The prognostic significance between MPV/plt between anemic patients and the control group were analyzed using the GraphPad Prism 7. Statistical differences were determined by unpaired t-test with Welch’s correction and Brown-Forsythe and Welch ANOVA test and statistical significance was demonstrated as follows: * if P < 0.05, ** if P < 0.01, *** if P < 0.001, **** if P<0.0001, NS if not significant.
Results
Patients demography
In our study, we had 166 male and 166 females. Twenty-one male (12.65%) with mean age of 44.47±22.5 years and fifty-six females (33.73%) with mean age of 38.62±17.14 years were determined to have IDA. The group of male with IDA had mean Hb 8.73±2.26 g/dL and MCV 66.45±6.80 fL and female with IDA had Hb 8.20±2.02 g/dL and MCV was 65.66±11.95 fL (Table 1). The anemic patients who did not fit into the IDA inclusion category were categorized as non-IDA. Eighty-four male (50.60%) and seventy-two females (43.37%) were determined as non-IDA. Control group had sixty-one male and thirty-eight females.
Table 1: Summary of the findings of male and female patients belonging to control, IDA and non-IDA groups.
|
Mean Values |
Male |
Female |
||||
|
Control |
IDA |
non-IDA |
Control |
IDA |
non-IDA |
|
|
Number of patients (%) |
61 |
21 (12.65%) |
84 (-50.6%) |
38 |
56 (-33.73%) |
72 (-43.37) |
|
Age (years) |
41.46 ±17.14 |
44.47 ±22.5 |
51.79 ±16.03 |
36.36 ±18.11 |
38.62 ±17.14 |
44.62 ±16.42 |
|
Hb (g/dL) |
14.06 ±1.48 |
8.73 ±2.26 |
8.64 ±1.83 |
12.65 ±0.78 |
8.20 ±2.02 |
9.48 ±1.75 |
|
MCV (fL) |
84.07 ±9.79 |
66.45 ±6.80 |
90.08 ±8.12 |
82.64 ±12.58 |
65.66 ±11.95 |
86.79 ±10.88 |
|
MPV (fL) |
10.03 ±0.83 |
8.73 ±2.27 |
9.46 ±2.16 |
9.93 ±0.78 |
10.14 ±1.80 |
9.82 ±1.56 |
Platelet parameters analysis
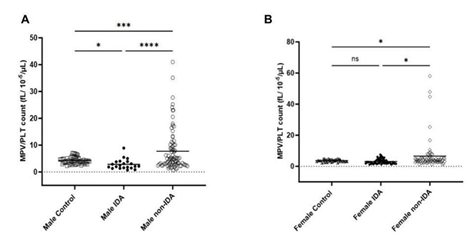
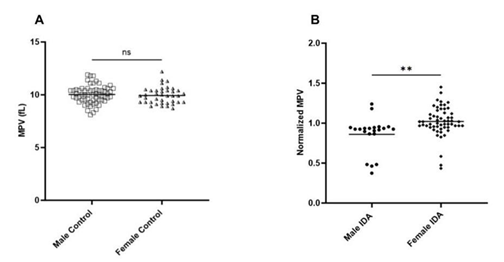
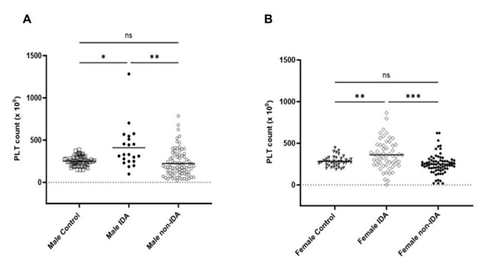
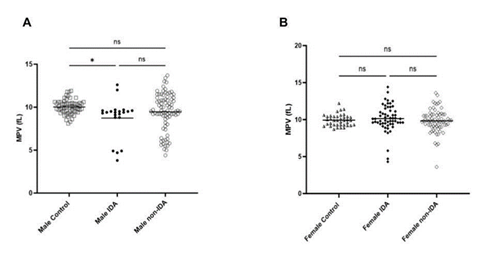
The MPV/ Platelet count ratio (MPR) of IDA was compared with control and non-IDA in both male and female. The MPR of male IDA was significantly lower than in control and non-IDA (Figure 1a) while no significant difference was observed in the female group (Figure 1b). To understand the difference in the trend, the platelet count and MPV were analysed separately in both groups. The mean platelet count (PC) in IDA was found to be slightly elevated than control and non-IDA in both the male (Figure 2a) and female (Figure2b) groups. A biphasic pattern of platelet count i.e. thrombocytosis and thrombocytopenia were observed in both IDA and non-IDA cases. A higher case of thrombocytosis was observed in IDA (M=42.86%, F= 33.93%) compared to non-IDA (M=11.90%, F= 9.72%) (Table 2). A higher case of thrombocytopenia was observed in non-IDA (M= 38.09%, F= 13.89%) compared to IDA (M=4.76%, F=10.71%) (Table 2). The mean MPV of male with IDA were found to be slightly lower than control (Figure 3a). No significant changes in MPV were observed in females with IDA and non-IDA in comparison to control (Figure 3b). The MPV of male and females are compared. There was no difference in the MPV of male and female controls (Figure 4a), however in the case of IDA, female MPV was higher than in male (Figure 4b).
Table 2: Summary of platelet counts observed for male and female patients belonging to control, IDA and non-IDA groups.
|
Platelet count (103/ µL) |
Number of controls (%) |
Number of IDA patients (%) |
Number of non-IDA patients (%) |
|||
|
Male (n=61) |
Female (n=38) |
Male (n=21) |
Female (n=56) |
Male (n=84) |
Female (n=72) |
|
|
<150 |
0 |
0 |
1 (-4.76%) |
6 (-10.71%) |
32 (-38.09%) |
10 (13.89%) |
|
150-400 |
61 (-100%) |
35 (-92.11%) |
11 (-52.38%) |
31 (-55.36%) |
42 (-50%) |
55 (-76.39%) |
|
>400 |
0 |
3 (-7.9%) |
9 (42.86%) |
19 (-33.93%) |
10 (-11.9%) |
7 (-9.72%) |
Discussion
In our study, the MPR was able to distinguish male IDA from non-IDA and control but not in female IDA patients (Figure 1a & 1b). However, in a similar study published with the patient pool that included both male and female showed that MPR was able to differentiate IDA from control and non- IDA group [7]. These anomalies motivated us to look further into the platelet parameters individually in male and female groups and interpret this trend. The parameters of interest for us were platelet count (PC) and mean platelet volume (MPV). According to literature, female populations in general have higher platelet count (PC) than male attributed with chronic menstrual blood loss and hormone differences. The platelet count has been found to be sensitive to IDA and on average are reported to be elevated in both male and female population as in our study (Figure 2). Multiple studies have reported duality in the role of iron in thrombopoiesis and in iron deficiency, thrombocytosis is reported to occur at higher frequency than thrombocytopenia in both male and female [3,4]. In our study we did observe higher cases of thrombocytosis than thrombocytopenia in both IDA groups (Table 2).
Thrombocytosis is said to occur in moderate IDA (Hb> 6.5g/dl) due to loss of inhibitory effect of iron in platelet production [17] and is mediated by moderate elevation of EPO. EPO is proposed to either act synergistically with thrombopoietin (TPO) by signaling at the level of bipotent progenitor (BPU/ E/ MK) [18] or act independent of TPO/MpI-pathway via unknown mechanism [5]. While thrombocytopenia is said to occur in severe iron deficiency i.e. hemoglobin less than 6.5g/dl., It occurs probably due to lack of iron of platelet protein synthesis [17] and/or inhibitory effect of high EPO levels on platelet production [19,20].
Although, the platelet count has been accepted to have a non-linear, inverse relationship to MPV [16, 21],, we observed no difference in male and female MPV despite female PC to be higher. Similar findings have been reported by other studies too [22-24]. While in IDA patients, the MPV was decreased only in male (Figure 3) irrespective of increased PC in both groups (Figure 2). This difference in trend of MPV and PCs to IDA could suggest that platelet response to pathologies may vary within sexes, although the mechanism is unclear. The influence of sex on the response of platelets to pathology has also been reported in other pathologies. Yang, J. J et al 2013, found that male patients with acute appendicitis had lower MPV than female with no significant difference in their platelet count. Similarly, Park, B.J., et. al., 2011, found an increased platelet count and decreased MPV in females with metabolic syndrome while there was no change in male. Hormones can be one of the factors that attribute to the variation in platelet response to pathologies. Estrogen has been reported to be involved in thrombopoiesis via the estrogen receptor beta (ER-B) expressed by platelets and their medullary progenitors, megakaryocytes [25,26]. One study also reported a negative correlation of MPV with total testosterone and free testosterone in isolated male hypogonadotropic hypogonadism (IHH) patients [27]. However, more information on the effect of testosterone on platelet behavior especially in IDA patients is lacking.
Platelets are sensitive to inflammation and oxidative stress [28] and the level of inflammation has been found to vary based on sex. The level of pro-inflammatory markers (IL-6 and high-sensitivity C-reactive protein (hsCRP)) has been found higher in the aging female population than male [29]. Similarly, males have more oxidative stress owing to higher leakage of superoxide anion at the mitochondrial electron transport chain as well as higher activity of pro-oxidant enzymes (eg. xanthine oxidase (XO) and NADPH oxidase) and lower expression and activity of antioxidant enzymes (eg. superoxide dismutase (SOD) and glutathione peroxidase [30]. Body composition also varies within sexes, with higher adipose tissues in females. These adipose tissues secretes a variety of adipokines and cytokines such as leptin, adiponectin, interleukin 6 (IL-6) and tumor necrosis factor-a (TNF-a) and leading to chronic low-grade inflammation which further influence platelets and their response to different pathologies [24]. However, the exact mechanism hormones and inflammation modulate the platelet response to different pathologies is unknown and further study is required. Our study does have some limitations. In our study, we included the outpatients that visited the hospital/ clinic. Thus, we did not have a history of other medical conditions and make our sample more precise. In addition, the population size was smaller. Thus we were not able to categorize and analyse the findings based on different age groups in addition to sex. Ability to this could have provided more insight on the platelet activity throughout different age groups and sex.
Conclusion and future directions
MPV and platelet counts had been found to be sensitive to inflammation and certain pathologies and had been extensively studied as a possible parameter for diagnosis or prognosis. Instead of individual parameters, MPR had been proposed to be more sensitive and attempts had been made to distinguish IDA from non-IDA. However, sex can also highly influence the platelet parameters via hormones and different responses to inflammation and stress. In addition, our findings stated that MPR was able to differentiate male IDA patients from non-IDA and control but not in the female. The exact mechanism and causes is unclear and would require further studies. Our study highlights that we need to take caution while using MPR as a diagnostic tool for IDA and other pathologies in the whole population. We suggest that it would be beneficial to have the insight of the PC and MPV separately in both sexes before using MPR for diagnosis purposes.
Acknowledgements
The authors would like to acknowledge Vinod Sharma and Durgesh Sharma from Arjun Kripa Clinic, Raikot, India. and Goyal Lab and Diagnostic center, Raikot, India, and Dr. Pushkar Admane from HORIBA India Pvt. Ltd. and Dr. Cristo Lamprakos for their help. The research is supported by Avalon University School of Medicine and HORIBA India Pvt. Ltd.
Conflicts of interest
The Authors declares that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Kadikoylu G, Yavasoglu I, Bolaman Z, Senturk T. Platelet parameters in women with Iron deficiency anemia. Journal of the National Medical Association 98 (2006): 398–402.
- Chalise S, Acharya N, Pradhan SB. Correlation between iron parameters and platelet parameters in iron deficiency anemia. Journal of Institute of Medicine Nepal 41 (2019): 35–38.
- Park MJ, Park PW, Seo YH, Kim KH, Park SH, Jeong JH, Ahn JY. The relationship between iron parameters and platelet parameters in women with iron deficiency anemia and thrombocytosis. Platelets 24 (2012): 348–351.
- Kuku I, Kaya E, Yologlu S, Gokdeniz R, Baydin A. Platelet counts in adults with iron deficiency anemia. Platelets 20 (2009): 401–405.
- Hacein-Bey-Abina S, Estienne M, Bessoles S, Echchakir H, Pederzoli-Ribeil M, Chiron A, Aldaz-Carroll L, Leducq V, Zhang Y, Souyri M, Louache F, Abina AM. Erythropoietin is a major regulator of thrombopoiesis in thrombopoietin-dependent and -independent contexts. Experimental Hematology 88 (2020): 15–27.
- Vaziri ND. Thrombocytosis in epo-treated dialysis patients may be mediated by EPO rather than iron deficiency. American Journal of Kidney Diseases 53 (2009): 733–736.
- Cho SY, Yang JJ, Suh JT, Lee WI, Lee HJ, Park TS. Mean platelet volume/platelet count ratio in anemia. Platelets 24 (2012): 244–245.
- Azab B, Torbey E, Singh J, Akerman M, Khoueiry G, Mcginn JT Jr, Widmann WD, Lafferty J. Mean platelet volume/platelet count ratio as a predictor of long-term mortality after non-ST-elevation myocardial infarction. Platelets 22 (2011): 557–566.
- Oh GH, Chung SP, Park YS, Hong JH, Lee HS, Chung HS, You JS, Park JW, Park I. Mean platelet volume to platelet count ratio as a promising predictor of early mortality in severe sepsis. Shock 47 (2017): 323–330.
- Vélez-Páez JL, Legua P, Vélez-Páez P, Irigoyen E, Andrade H, Jara A, López F, Pérez-Galarza J, Baldeón L. Mean platelet volume and mean platelet volume to platelet count ratio as predictors of severity and mortality in sepsis. PLOS ONE 17 (2022): e0262356.
- Cho SY, Yang JJ, You E, Kim BH, Shim J, Lee HJ, Lee WI, Suh JT, Park TS. Mean platelet volume/platelet count ratio in hepatocellular carcinoma. Platelets 24 (2012): 375–377.
- Wu YY, Zhang X, Qin YY, Qin JQ, Lin FQ. Mean platelet volume/platelet count ratio in colorectal cancer: A retrospective clinical study. BMC Cancer 19 (2019): Article 5504.
- Segal JB, Moliterno AR. Platelet counts differ by sex, ethnicity, and age in the United States. Annals of Epidemiology 16 (2006): 123–130.
- Christakoudi S, Tsilidis KK, Evangelou E, Riboli E. Sex differences in the associations of body size and body shape with platelets in the UK Biobank cohort. Biology of Sex Differences 14 (2023): Article 1.
- Panova-Noeva M, Schulz A, Hermanns MI, Grossmann V, Pefani E, Spronk HMH, Laubert-Reh D, Binder H, Beutel M, Pfeiffer N, Blankenberg S, Zeller T, Münzel T, Lackner KJ, ten Cate H, Wild PS. Sex-specific differences in genetic and nongenetic determinants of mean platelet volume: Results from the Gutenberg Health Study. Blood 127 (2016): 251–259.
- Vázquez-Santiago M, Ziyatdinov A, Pujol-Moix N, Brunel H, Morera A, Soria JM, Souto JC. Age and gender effects on 15 platelet phenotypes in a Spanish population. Computers in Biology and Medicine 69 (2016): 226–233.
- Karpatkin S, Garg SK, Freedman ML. Role of iron as a regulator of thrombopoiesis. The American Journal of Medicine 57 (1974): 521–525.
- Racke FK. EPO and TPO sequences do not explain thrombocytosis in iron deficiency anemia. Journal of Pediatric Hematology/Oncology 25 (2003): 919.
- Loo M, Beguin Y. The effect of recombinant human erythropoietin on platelet counts is strongly modulated by the adequacy of iron supply. Blood 93 (1999): 3286–3293.
- Beguin Y. Erythropoietin and platelet production. Haematologica 84 (1999): 541–547.
- Thompson C, Jakubowski J. The pathophysiology and clinical relevance of platelet heterogeneity. Blood 72 (1988): 1–8.
- Ittermann T, Feig MA, Petersmann A, Radke D, Greinacher A, Völzke H, Thiele T. Mean platelet volume is more important than age for defining reference intervals of platelet counts. PLOS ONE 14 (2019): e0213658.
- Yang JJ, Cho SY, Ahn HJ, Lee HJ, Lee WI, Park TS. Mean platelet volume in acute appendicitis: A gender difference. Platelets 25 (2013): 226–227.
- Park BJ, Shim JY, Lee HR, Jung DH, Lee JH, Lee YJ. The relationship of platelet count, mean platelet volume with metabolic syndrome according to the criteria of the American Association of Clinical Endocrinologists: A focus on gender differences. Platelets 23 (2011): 45–50.
- Du C, Xu Y, Yang K, Chen S, Wang X, Wang S, Wang C, Shen M, Chen F, Chen M, Zeng D, Li F, Wang T, Wang F, Zhao J, Ai G, Cheng T, Su Y, Wang J. Estrogen promotes megakaryocyte polyploidization via estrogen receptor beta-mediated transcription of GATA1. Leukemia 31 (2016): 945–956.
- Dupuis S, Noirrit-Esclassan F, Arnal JF, Payrastre B, Valéra MC. Effects of estrogens on platelets and megakaryocytes. International Journal of Molecular Sciences 20 (2019): 3111.
- Carlioglu A, Durmaz SA, Kibar YI, Ozturk Y, Tay A. Mean platelet volume in a patient with male hypogonadotropic hypogonadism. Blood Coagulation and Fibrinolysis 26 (2015): 811–815.
- Naghipour Hamzekolaei M, Jafarisani M, Farajzadeh A, Aghayan SS, Atashi A, Yarmohammadi M, Sadeghi I, Tashakori M. Changes in mean platelet volume and hematologic indices in patients with panic disorder due to oxidative stress. Brain and Behavior 10 (2020).
- Milan-Mattos JC, Anibal FF, Perseguini NM, Minatel V, Rehder-Santos P, Castro CA, Vasilceac FA, Mattiello SM, Faccioli LH, Catai AM. Effects of natural aging and gender on pro-inflammatory markers. Brazilian Journal of Medical and Biological Research 52 (2019).
- Martínez de Toda I, González-Sánchez M, Díaz-Del Cerro E, Valera G, Carracedo J, Guerra-Pérez N. Sex differences in markers of oxidation and inflammation. Implications for ageing. Mechanisms of Ageing and Development 211 (2023): 111797.


 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 