Minimal Invasive Percutaneous Kyphoplasty of C1 Lytic Lesion Using an Intraoperative 3D Imaging-Based Navigation System and Fluoroscopy
Mikael Meyer*, Kaissar Farah, Thomas Graillon, Sébastien Boissonneau, Henry Dufour, Stephane Fuentes
Department of neurosurgery, La Timone university hospital, APHM, Marseille France
*Corresponding Author: Doctor Mikael Meyer, Neurosurgery department, La Timone University Hospital, 264 rue Saint-Pierre, 13385 Marseille, France
Received: 08 November 2020; Accepted: 17 Novemberber 2020; Published: 19 October 2020
Article Information
Citation: Mikael Meyer, Kaissar Farah, Thomas Graillon, Sébastien Boissonneau, Henry Dufour, Stephane Fuentes. Minimal invasive percutaneous kyphoplasty of C1 lytic lesion using an intraoperative 3D imaging-based navigation system and fluoroscopy. Journal of Surgery and Research 3 (2020): 419-427.
View / Download Pdf Share at FacebookAbstract
Background: Percutaneous kyphoplasty is a minimally-invasive techniques that aim to provide pain relief and bone stabilization by the injection of cement. Metastatic lesions of the atlas treated by this technique has been described in only few articles.
Objective: We describe the use of kyphoplasty in a painful osteolytic lesion located in the left mass lateral of C1 with a postero lateral approach using 3D CT-scann intraoperative navigation system and fluoroscopy.
Methods: A 58 year-old woman with metastastic breast neoplastic which admitted for treatment severe left-sided suboccipital exacerbated by head rotation and neck pain refractory to conventional medical treatment painkillers. CT scan and magnetic resonance imaging of the cervical spine revealed osteolytic destruction the left lateral mass of C1 . The patient underwent a percutaneous kyphoplasty managed using polymethylmetacrylate bone cement.
Results: The patient reported post operative substantial pain relief . CT scan showed adequate filling of the osteolytic lesion without the obvious leakage of bone cement. the patient showed no clinical complications, while Clinical follow-up at 3 months revealed that this pain condition was improved and maintained.
Conclusion: Minimally invasive percutaneous kyphoplasty using an intraoperative 3D navigation system and fluroscopy with postero lateral approach is a safe and effective alternative reducing post-operative morbidity and effective possibility in selected patients with C1 metastasis
Keywords
<p>Minimal invasive; Spinal metastasis; Surgical management; Kyphoplasty; AIRO; Intraoperative scann</p>
Article Details
1. Introduction
Spinal metastases have an increasing prevalence and are responsible for pain and neurological complications that aggravate prognosis in oncological patients [1]. Spine is the third most common site of metastasis after lungs and liver and is the most common site in the osteoarticular system [2] ; Only a third of all patients with spinal metastasis are symptomatic and require medical or surgical intervention [3, 4].
Cervical spine is only affected in 8% – 15% [5, 6] of cases of spinal metastases. Pathologic vertebral compression fractures in this region can be associated with a significant amount of mechanical pain, tumor related pain and/or neurological compromise from spinal cord compression. When the management of the metastatic spine is palliative, patients may be treated with opioids and/or radiation therapy. A third of these patients may be resistant to these modalities leading to significant impairment in quality of life and decreased mobility [7, 8] .
Kyphoplasty is a minimally-invasive technique that aims to provide pain relief and bone stabilization by the injection of cement (usually polymethylmethacrylate; PMMA) into the index vertebra [9]. However, metastatic lesions of the atlas [10] are extremely rare and their treatement by percutaneous cement augmentation had been described in only few articles [11-17]. It is considered as a technically challenging procedure due to complex anatomy [15, 17, 18]. We describe the use of percutaneous kyphoplasty in a painful osteolytic lesion located in the left lateral mass of C1 through a postero-lateral approach using 3D CT-scan intra-operative navigation system and fluoroscopy.
2. Methods
A 58 year-old woman with metastastic breast cancer was admitted in our department for treatment of severe left-sided suboccipital pain. Neck pain was refractory to class 3 painkillers consumed for several months and was exacerbated by right head rotation. CT scan and magnetic resonance imaging of cervical spine revealed osteolytic destruction the left lateral mass of C1 (Figure 1) . There was no spinal canal involvement. The C1 bone lesion presented a close relationship with the V3 segment of the left vertebral artery. Due to the widely metastatic nature of this disease, the intractable pain and lack of neurological impairment, a minimal invasive kyphoplasty was proposed. Case was discussed in a multidisciplinary meeting. The goal of surgery was pain relief. Patient consent was sought and obtained.
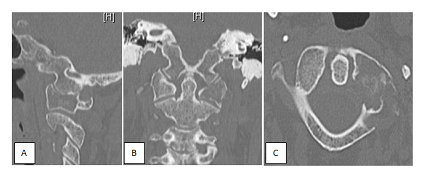
Figure 1: A. Sagittal, B axial and C coronal ct scann slice. Osteolysis of the left lateral mass of the atlas.
2.1 Surgical technique
Under general anesthesia, the patient is placed prone on the Trumpf Medical TrueSystem 7500 (HillromTM), a radiolucent table, with the head maintained in a carbon Mayfield holder (Figure 2A). Required equipment consisted of intraoperative AIRO® CT-scan in conjunction with BrainLab® curve navigation (Brainlab AG Olof-Palme-Straße 9 81829 Munich Germany), biplanar fluoroscopy and KyphonTM Balloon Kyphoplasty (MEDTRONIC® medical device company). After draping, the patient reference array is clipped onto the 2-pin fixator, which is attached to Mayfield holder and tightened into position next to the surgical field.
The field of the acquisition on the selected occipito cervical area was determined pointing with a skin marker on the sterile drapes and was spotted with the laser navigation. Once the scan is completed, images are automatically transferred to the BrainLab® Curve navigation system. Navigation tools were registered and a dedicated pointer was used in order to obtain a perfect agreement between patient position and the navigation system.
The primary surgeon and the rest of the operative team were outside the operating room. Only the radiologic technologist was protected behind a 2-mm-thick mobile lead wall placed 2.5 meters away from the AIRO® CT-scan.
First step of the surgical procedure was performed using a navigated Jamshidi trocar (Figure 2 B) calibrated via the Brainlab Instrument Calibration Matrix (ICM4). A lateral left incision (0.5 cm) was made and under 3D navigation guidance. The tracked Jamshidi trocar was inserted in the C1 left lateral mass according under navigation control avoiding neurological structures and V3 segment of left vertebral artery in the left transverse foramen (Figure 3).
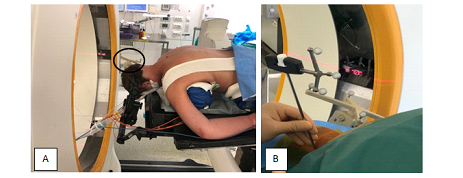
Figure 2: A Patient installation and Reference array (black ellipse). B The navigated jamshidi into C1 left mass lateral.
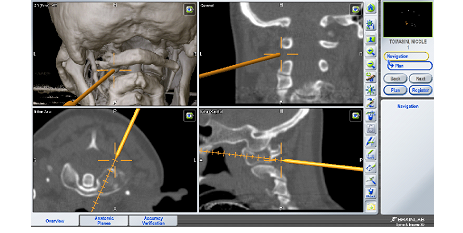
Figure 3: The navigated jamshidi into C1 lateral mass; perioperative screeshots showing real time 3D, coronal, axial and sagittal navigation views.
Second step consisted in K-wire insertion into the Jamshidi trocar before its removal. The Jamshidi trocar is retrieved and a new iCT scan centered on the area of interest was performed in order to assess the positions of the K-wire. Once good positioning is confirmed, biplanar fluoroscopy is used for the third step that consisted in tool introduction and procedure control (reaming, balloon inflation, cementing). With reaming tools one working channel within the lateral mass of the C1 is created and the 10 mm balloon (KyphonTM Balloon Kyphoplasty , MEDTRONIC® medical device company) is inserted. Its placement is checked by two radio-opaque markers. Once inserted, the balloon is then inflated under visual volume and pressure controls. A cavity is therefore created under fluoroscopic control. When the balloon reaches the cortical surface of the left lateral mass, it is then sequentially deflated and removed. The cavity is then filled with polymethylmetacrylate (PMMA) under continuous fluoroscopic control. (Figure 4) About 2ml of cement is slowly injected with a bone filler device. The whole material is retrieved and the wound is closed.
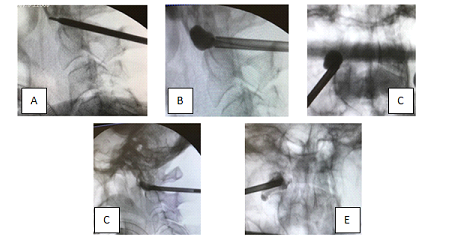
Figure 4: Intraoperative fluoroscopy. A. After Jamshidi trocar and K-wire insertion. B-C. Work tool and balloon toward the right C1 lateral mass D-E. the polymethylmethacrylate injected.
The total radiation dose for the patient during the surgery was 3.7 mSv. The radiation dose received by the primary surgeon and the rest of the operative team outside the operating room, during the imaging acquisition, was considered null. Blood loss was less than 20ml. This study was approved by an IRB n° IRB00011687. Informed consent was obtained from patients or their designated representatives.
3. Results
The patient reported substantial pain relief as VAS was 30mm on postoperative day 1 vs 90mm preoperatively. A CT scan obtained 1 day postoperatively showed adequate filling of the osteolytic lesion without obvious leakage of bone cement (Figure 5). The procedure was uneventful and the patient was discharged home the next day. Clinical follow-up at 3 months postoperatively revealed that her pain condition was improved as VAS was 10mm. No opioid consumption was reported at this time.
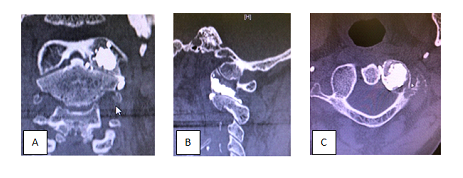
Figure 5: Control scan; A. sagittal, B. axial and C. coronal reconstruction : bone windowing showing the filing of the lateral mass without intracanal leakage.
4. Discussion
Atlas is a rare location for bone tumors [13]. Osteolytic lesions of the lateral mass of C1 may be responsible for severe pain and instability, requiring sometimes aggressive treatment as cranio cervical fixation [19] . This can lead to significant postoperative pain, which is, in large part, secondary to extensive muscle dissection and wound healing. Development of minimal invasive spinal techniques can be of significant interest in order to reduce perioperative morbidity. Percutaneous cement augmentation for lytic metastasis of the spine is considered as a valuable palliative option. It provides pain relief and increases stability. It is often associated to radiation therapy and/or chemotherapy in fractured, painful, or at risk of collapse vertebrae [14, 20-25].
Previous reports described various techniques for percutaneous treatment of C1 metatstasis.Minimally transoral approach requires a large exposition of the posterior wall of the pharynx; eventhough there is a theoretical risk of infection during transoral approach, no bone infection was observed in the previously published case series [26-28]. Yang and al showed that the anterior retropharyngeal approach could be an efficacious alternative to transoral approach; when considering the substantial potential risks. This technically challenging procedure should be performed by experienced operators [ 29]. Two cases of posterior vertebroplasty of C1 lateral mass have been previously reported. The first consisted in a posterior approach [30] for osteolytic metastasis secondary to parotid cancer, whereas the second consisted in a lateral approach for osteolytic metastasis from breast cancer [31]. Both cases reported satisfactory results.
Recent technologic development and especially navigation systems using iCT-scan are advocated mainly to reduce the risk of neurovascular injury. Securing routing procedure placement under navigation guidance offers also the possibility to insert material via a percutaneous approach with reduced radiation exposure for patients, surgical team and operative room staff. To our knowledge, this is the first case presenting a pathological fracture of C1 treated with kyphoplasty using iCT Airo and 3D image-guided navigation. The technique seems secure and reproducible. Unlike percutaneous kyphoplasty performed by radiologist, ergonomy is more appreciable in an OR with 3D navigation system as the donut of the iCT is removed from the working area after image acquisition.
5. Conclusion
Minimally invasive percutaneous postero lateral C1 kyphoplasty using an iCT navigation system and fluroscopy is a major innovation. It is a safe and effective alternative for intractable pain secondary to C1 lateral mass osteolytic metastasis in selected patients. It is associated to an acceptable patient radiation and reduced surgical team exposure. Future studies are needed to assess reproducibility, cement leakage, radiation exposure and cost-effectiveness.
References
- Tabouret E, Gravis G, Cauvin C, Loundou A, Adetchessi T, et al. Long-term survivors after surgical management of metastatic spinal cord compression. Eur Spine J 24 (2015): 209-215.
- Quraishi NA, Rajabian A, Spencer A, Arealis G, Mehdian H, et al. Reoperation rates in the surgical treatment of spinal metastases. Spine J 15 (2015): S37-S43.
- Ibrahim A, Crockard A, Antonietti P, Boriani S, Bünger C, et al. Does spinal surgery improve the quality of life for those with extradural (spinal) osseous metastases?: an international multicenter prospective observational study of 223 patients: invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2007. J Neurosurg Spine 8 (2008): 271-278.
- Sciubba DM, Gokaslan ZL, Suk I, Suki D, Maldaun MV, et al. Positive and negative prognostic variables for patients undergoing spine surgery for metastatic breast disease. Eur Spine J 16 (2007): 1659-1667.
- Constans JP, de Divitiis E, Donzelli R, Spaziante R, Meder JF, et al. Spinal metastases with neurological manifestations. Review of 600 cases. J Neurosurg 59 (1983): 111-118.
- Brihaye J, Ectors P, Lemort M, Van Houtte P. The management of spinal epidural metastases. Adv Tech Stand Neurosurg 16 (1988): 121-176.
- Janjan N. Bone metastases: approaches to management. Semin Oncol 28 (2001): 28-34.
- Schachar NS. An update on the nonoperative treatment of patients with metastatic bone disease. Clin Orthop Relat Res 382 (2001): 75-81.
- Tian QH, Sun XQ, Lu YY, Wang T, Wu CG, et al. Percutaneous vertebroplasty for palliative treatment of painful osteoblastic spinal metastases: a single-center experience. J Vasc Interv Radiol 27 (2016): 1420-1424.
- MouldingHD, Elder JB, Lis E, Lovelock DM, Zhang Z, et al. Local disease control after decompressive surgery and adjuvant high-dose single-fraction radiosurgery for spine metastases. J Neurosurg Spine 13 (2010): 87-93.
- Guo WH, Meng MB, You X, Luo Y, Li J, et al. CT-guided percutaneous vertebroplasty of the upper cervical spine via a translateral approach. Pain Physician 15 (2012): E733-E741.
- Wetzel SG, Martin JB, Somon T, Wilhelm K, Rufenacht DA. Painful osteolytic metastasis of the atlas: treatment with percutaneous vertebroplasty. Spine 27 (2002): E493-E495.
- Mavrogenis AF, Guerra G, Romantini M, Romagnoli C, Casadei R, et al. Tumours of the atlas and axis: a 37-year experience with diagnosis and management. Radiol Med 117 (2012): 616-635.
- Masala S, Anselmetti GC, Muto M, Mammucari M, Volpi T, et al. Percutaneous vertebroplasty relieves pain in metastatic cervical fractures. Clin Orthop Relat Res 469 (2011): 715-722.
- Huegli RW, Schaeren S, Jacob AL, Martin JB, Wetzel SG. Percutaneous cervical vertebroplasty in a multifunctional image-guided therapy suite: hybrid lateral approach to C1 and C4 under CT and fluoroscopic guidance. Cardiovasc Intervent Radiol 28 (2005): 649-652.
- Cianfoni A, Distefano D, Chin SH, Varma AK, Rumboldt Z, et al. Percutaneous cement augmentation of a lytic lesion of C1 via posterolateral approach under ct guidance. Spine J 12 (2012): 500-506.
- Anselmetti GC, Manca A, Chiara G, Regge D. Painful osteolytic metastasis involving the anterior and posterior arches of C1: percutaneous vertebroplasty with local anesthesia. J Vasc Interv Radiol 20 (2009): 1645-1547.
- Mont'Alverne F, Vallee JN, Cormier E, Guillevin R, Barragan H, et al. Percutaneous vertebroplasty for metastatic involvement of the axis. AJNR Am J Neuroradiol 26 (2005): 1641-1645.
- Chung JY , Kim JD , Park GH , Jung ST, Lee KB. Occipitocervical reconstruction through direct lateral and posterior approach for the treatment of primary osteosarcoma in the atlas: a case report . Spine (Phila Pa 1976) 37 (2012): E126-E132.
- Georgy BA. Metastatic spinal lesions: state-of-the-art treatment options and future trends. AJNR Am J Neuroradiol 29 (2008): 1605-1611.
- Krishnaney AA, Steinmetz MP, Benzel EC. Biomechanics of metastatic spine cancer. Neurosurg Clin N Am 15 (2004): 375-380.
- Jakobs TF, Trumm C, Reiser M, Hoffmann RT. Percutaneous vertebroplasty in tumoral osteolysis: a review. Eur Radiol 17 (2007): 2166-2175.
- Bartels RH, van der Linden YM, van der Graaf WT. Spinal extradural metastasis: review of current treatment options. CA Cancer J Clin 58 (2008): 245-259.
- Gerszten PC, Germanwala A, Burton SA, et al. Combination kyphoplasty and spinal radiosurgery: a new treatment paradigm for pathological fractures. J Neurosurg Spine 3 (2005): 296-301.
- Shindle MK, Shindle L, Gardner MJ, Lane JM. Comprehensive review. Supportive care aspects of vertebroplasty and kyphoplasty in patients with cancer. Support Cancer Ther 3 (2006): 214-219.
- Clarençon F, Cormier E, Pascal-Moussellard H, Maldent JB, Pichon S, et al. Transoral approach for percutaneous vertebroplasty in the treatment of osteolytic tumor lesions of the lateral mass of the atlas: feasibility and initial experience in 2 patients. Spine (Phila Pa 1976) 38 (2013): E193-W197.
- Martin JB, Gailloud P, Dietrich PY, Luciani ME, Somon T, et al. Direct transoral approach to C2 for percutaneous vertebroplasty . Cardiovasc Intervent Radiol 25 (2002): 517-519.
- Anselmetti GC, Manca A, Montemurro F, Tutton S, Chiara G, et al. Vertebroplasty using transoral approach in painful malignant involvement of the second cervical vertebra (C2): a single-institution series of 25 patients . Pain Physician 15 (2012): 35-42.
- Yang JS, Chu L, Xiao FT, Zhang DJ, Wang Y, et al. Anterior retopharyngeal approach to CI for percutaneous vertebroplasty under C-armfluoroscopy. Spine. J 15 (2015): 539-545.
- Wetzel SG, Martin JB, Somon T, Wilhelm K, Rufenacht DA. Painful osteolytic metastasis of the atlas: treatment with percutaneous vertebroplasty . Spine (Phila Pa 1976) 27 (2002): E493-E495.
- Anselmetti GC, Manca A, Chiara G, Regge D. Painful osteolytic metastasis involving the anterior and posterior arches of C1: percutaneous vertebroplasty with local anesthesia . J Vasc Interv Radiol 20 (2009): 1645-1647.


 Impact Factor: * 4.2
Impact Factor: * 4.2 Acceptance Rate: 72.62%
Acceptance Rate: 72.62%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 