Overall Survival of Hepatocellular Carcinoma Patients with Associated Diabetes Mellitus - A New Possible Prognostic Score
Mega A1*, De Giorgio M2, Piccin A3,4, Ferro F5, Vittadello F6, Marzi L1, Pelizzaro F7, M. Daves8, Spizzo G9,11, Frena A10, Di Vasto M1, Seeber A11
1Department of Gastroenterology, San Maurizio Regional Hospital, Bolzano, South Tyrol, Italy
2Department of Gastroenterology & Hepatology, Ospedale Papa Giovanni XXIII, Bergamo, Italy
3Department of Internal Medicine V, Medical University of Innsbruck, Innsbruck, Austria
4Northern Ireland Transfusion Service, Belfast, UK
5Department of Radiology, San Maurizio Regional Hospital, Bolzano, South Tyrol, Italy
6Explora Research and Staystical Analysis, Padova, Italy
7Gastroenterology Unit, Department of Surgery, Oncology & Gastroenterology, University of Padua, Padua, Italy
8Biochemical Laboratory, San Maurizio Regional Hospital, Bolzano, South Tyrol, Italy
9Department of Internal Medicine, Hospital of Bressanone, Bressanone, South Tyrol, Italy
10Department of Surgery, San Maurizio Regional Hospital, Bolzano, South Tyrol, Italy
11Department of Hematology and Oncology, Comprehensive Cancer Center Innsbruck, Medical University of Innsbruck, Innsbruck, Austria
*Corresponding Author: Andrea Mega MD, Department of Gastroenterology, San Maurizio Regional Hospital, Bolzano, Italy
Received: 30 May 2022; Accepted: 06 June 2022; Published: 20 February 2023
Article Information
Citation: Mega A, De Giorgio M, Piccin A, Ferro F, Vittadello F, Marzi L, Pelizzaro F, Spizzo G, Frena A, Di Vasto M, Seeber A. Overall Survival of Hepatocellular Carcinoma Patients with Associated Diabetes Mellitus - A New Possible Prognostic Score. Journal of Surgery and Research. 6 (2023): 29-38.
View / Download Pdf Share at FacebookAbstract
Background: Diabetes Mellitus (DM) and Hepatocellular carcinoma (HCC) are conditions with common pathophysiological correlations. Currently, HCC treatment is based on the Barcelona Clinic Liver Cancer algorithm (BCLC). However, no studies have shown that the association of DM with HCC can influence prognosis. The American and European guidelines for Liver Disease (AASLD and EASL) suggest that intermediate stage (BCLC-B) HCC cases, should be treated with trans-arterial chemoembolization (TACE). However, several centers are still using other treatments (liver transplantation, liver resection, percutaneous radiofrequency ablation, percutaneous ethanol injection, radioembolization, sorafenib, etc). In 2012, Bolondi and colleagues suggested a further stratification of BCLC-B patients in 4 sub-groups (B1-B4).
Aim of the study: The aim of this study was to retrospectively validate the Bolondi stratification for BCLC-B patients and to establish the impact of DM on overall survival (OS).
Methods: We conducted a retrospective multicenter study in HCC intermediate stage patients. The study period was from 2000 up to 2015. The median follow up was 6.4 years, with a cumulative OS of 37% at 5 years.
Results: 276 patients with HCC B2 stage were identified. The OS at 5 years for type of treatment (“Bolondi Model“ vs TACE), was better when the Bolondi stratification was used (treated with “Bolondi Model“ n= 57 patients, 20.6%; treated with TACE n= 21 patients, 7.6 %; log rang p<0.001; Ranyi type test p<0.001). Multivariate analysis showed that B2 patients had a better OS compared to all other (p <0.05). According to the “Bolondi Model“, patients stratified in B3 and B4 stage would have had a better outcome if treated with liver trans
Keywords
<p>Hepatocellular Carcinoma, Diabetes Mellitus, Bolondi Model</p>
Article Details
1. Introduction
HCC prognosis depends on tumor stage at diagnosis and the possibility of performing a radical treatment [1]. The BCLC stratifies patients according to tumor stage and stage of liver disease. This allows a more efficacious and tailored therapy [2,3]. The use of this model has resulted in better overall survival (OS). However, this model is not broadly adopted and a significant number of patients with HCC are still treated outside guidelines.
The BCLC classification
BCLC classification includes functional and clinical parameters of liver function. Early stage HCC is stratified into four classes according to: a) number of lesions, b) tumor size, c) porto-caval gradient (hepatic venous pressure gradient, HVPG), d) serum bilirubin levels
Very-early and early HCC (BCLC 0 and A).
Early HCC is a tumor <2cm with compensated cirrhosis [4]. The definition includes patients with a single nodule < 5cm or up to three nodules of 3cm of size or less according to the “Milan criteria” [5]. This group includes heterogeneous tumors, from the very-early HCC (not detectable on CT scan or MRI), to the early HCC (nodular tumor detectable on contrast imaging because neovascularization). In patients with early HCC, orthotopic liver transplantation (OLT) is the best therapeutic option, because it prevents late-onset complications of cirrhosis. Currently, in patients with more than one nodule, multimodal HCC treatment is used. Trans-arterial chemoembolization (TACE) has been used in combination with radiofrequency (RF) or percutaneous ethanol injection (PEI) with promising results [6].
Intermediate HCC (BCLC B).
TACE represents the standard of care for patients not fulfilling “the Milan criteria”. A large meta-analysis study showed that TACE improves OS (> 20 months) in patients with intermediate stage [7]. TACE can only be performed if liver cirrhosis is compensated (Child–Pugh A or B) and in patients with small oesophageal varices. Several centers use different therapeutic strategies for BCLC B patients. The rationale is to deliver radical and not only palliative treatments. These patients are actually treated outside current guidelines.
The “Bolondi Model” in BCLC B patients
The “Bolondi Model“ reclassified BCLC B patients into four subgroups on the basis of: i) impairment of liver function assessed by the Child-Pugh score, ii) tumor burden stage (according to the Milan criteria), iii) patients performance status (PS), iv) tumor-related PS 1 [8]. However, the prognostic capability of this sub-classification has not yet been validated [2,9-11]. Clearly, the Bolondi stratification suggests that TACE should not be considered the “key treatment” for BCLC B HCC patients. By using the Bolondi stratification, the treatment can be more radical and OS can improve.
Advanced HCC (BCLC C)
This group of patients includes subjects with tumors complicated by portal vein thrombosis or extrahepatic tumor-invasion. Patients also have poor PS (PS ≥1). If liver disease (Child–Pugh A) is compensated, the treatment with the multikinase inhibitor Sorafenib© should be considered [12,13].
End-stage hepatocellular carcinoma (Barcelona clinical liver cancer stage D)
These patients have tumors of any size with symptoms of neoplasia and severe liver failure (Child–Pugh C). The average predicted OS is 3 months only. No specific treatment other than palliation is available.
The association between Diabetes mellitus (DM) and HCC
The association between Diabetes mellitus (DM) and HCC is well known. DM has already been identified, per se, as a risk factor for HCC [2]. Metformin, a first-line oral anti-diabetic drug, was first suggested as a candidate anti-cancer agent in 2005 in a cohort study in Scotland [14]. Previous studies, have pointed out the existance of intracellular pathways shared by cancer and DM. The signal transduction mechanisms by which metformin suppresses carcinogenesis in cell lines or xenograft tissues and improves chemoresistance in cancer stem cells, have also been elucidated. Recently, in vitro studies in metastatic HCC cells, have shown that co-treatment of metformin and curcumin induce apoptosis by activating mitochondria pathways [15]. Some authors have demonstrated that DM correlates with intrahepatic HCC recurrence after surgery [16]. Overall, DM has been associated with higher incidence and poorer prognosis of HCC but the influence of DM on patient survival in different HCC stages has not been studied so far [17].
2. Aim of the study
The aim of this study was to retrospectively validate the “Bolondi Model“ for BCLC-B patients and to evaluate the impact of DM on HCC outcome.
3. Material and Methods
We conducted a retrospective study of HCC, in intermediate stage. The OS of patients treated according to the AASLD (American Association for the Study of the Liver) the AISF (Associazione Italiana Studio Fegato) and EASL (European Association for the study of the Liver) guidelines were compared with the OS obtained using the Bolondi stratification.
Ethics
The study protocol was designed in accordance with the ethical guidelines of the Helsinki declaration and was approved by the ethic committee of Bolzano Hospital. A signed informed consent was not requested for this study (because retrospective).
Patients stratification
Consecutive patients with HCC diagnosis treated between 2000-2015 in two large Hospitals in Italy, Bolzano (BZ) and Bergamo (BG) and in the Austrian Hospital of Innsbruck (IBK) were included in the study. In order to establish if the Bolondi stratification has an impact on outcome, we decided to test all patients treated with TACE only (control group) versus patients treated according to the Bolondi stratification. In particular, each stage was compared to the corresponding one in the other group (B1 TACE only versus B1 Bolondi; B2 TACE only versus B2 Bolondi; B3 TACE only versus B3 Bolondi; B4 TACE only versus B4 Bolondi. Patients were subdivided as follows:
- a) control group, b) study group, c) not treated.
- a) Control group
This included all BCLC B patients without distinction and independently from the suggested Bolondi stratification. All cases were treated with TACE only.
- b) Study group
This included BCLC B patients treated in accordance with the suggested Bolondi stratification (B1 = 9 OLT, 17 RF/PEI, 10 resection, 1 Sorafenib; B2= 9 OLT, 27 RF/PEI, 31 resection, 5 Sorafenib; B3 =2 OLT, 2 RF/PEI, 4 resection, 2 Sorafenib; B4 = 1 OLT, 3 Resection)
- c) Not treated
This included all BCLC B patients not treated
Statistical analysis
Continuous covariates were summarized as median and interquartile distance (IQR) and comparison between groups was performed using the non-parametric Mann-Whitney test (two groups) and Kruskal-Wallis test (three or more groups). Categorical covariates were reported as absolute and percentage frequencies. Comparison was made using the Fisher’s exact test or chi Χ2 test, where appropriate. The principal end point of the study was the OS, defined as the time from date of diagnosis to the date of death/last follow up. Survival curves were calculated using the Kaplan-Meier estimates, and statistical comparison between curves was performed using the logrank test [18]. When survival curves crossed each other, the Renyi-type test was utilized [19]. Effect size was reported as a hazard ratio (HR) with a 95% confidence interval (95CI) and estimated using the Cox proportional hazards (PH) regression method [20]. The proportionality of the hazard risk was graphically checked using the scaled Schoenfeld residuals method [21]. Due to the retrospective nature of the study, we did not calculate a sample size. All tests were two sided, and p values < 0.05 were considered statistically significant. P values were not adjusted for multiple comparisons.
DM-HCC Score calculation
A new prognostic score taking into account DM and “Bolondi Model“ was designed and validated (Table 4). This score allowed a specific “power” to the following variables: i) Bolondi stage 1 (B1); ii) Bolondi stage 2 (B2); iii) Bolondi stage 3 (B3); iv) Bolondi stage 4 (B4) and v) DM yes/not.
4. Results
Total Patients
A total of 277 patients with HCC were included into this retrospective study. Patients were prevalently male (M = 234, 84%; F = 43, 16%; p<0.001, M/F ratio was = 5.4). Their median age was 67 years. The main aethiology was HBV/HCV infection (n = 129, 46.7%), followed by alcoholic liver disease (n=97, 35.1%), and other liver diseases (n= 50, 18.1%) (p <0.01). Median MELD score was > 9 in 111 patients (40.2%). Child Pugh score was as follows: A= 196 (75%); B = 61 (23%); C= 5 (2%). There was no significant difference between the three groups for median age, gender, MELD score and Child Pugh score (Table 1). Using the “Bolondi Model“ we identified 53 patients who were stratified as B1 (19.2%), 159 patients as B2 (56.5%), 58 patients as B3 (21.0%), and 26 patients as B4 (9.4%) (Tables 2-4).
- a) Control group (n=123)
Patients treated with TACE only independently of their Bolondi stratification (B1,2,3,4), were used as control group. The demographic data were as follows: mean age 67 (SD +/- 9), M/F ratio= 7.2, male n= 108 (88%), female n= 15 (12%).
- b) Study group (n=123)
This included BCLC B patients treated in accordance with the Bolondi classification (B1 n=37, B2 n=72, B3 n=10 and B4 n=4) (B1 = 9 OLT, 17 RF/PEI, 10 resection, 1 Sorafenib; B2= 9 OLT, 27 RF/PEI, 31 resection, 5 Sorafenib; B3 =2 OLT, 2 RF/PEI, 4 resection, 2 Sorafenib; B4 = 1 OLT, 3 Resection).
- c) Not treated
This included all BCLC B patients not treated (30 patients, 11%).
Diabetes Mellitus and other associated morbidities
DM was screened in 182 patients. DM was present in 63 cases (35%) and absent in 119 patients (65%). Out of those 24 (13%) DM patients were treated (12 with insulin (6.5%); 12 patients with metformine (6.5%) and 39 (21.4%) patients received no antidiabetic treatment.
Statistical Results
The cumulative median follow-up was 6.4 years (range 0.1-17.6 years) (Figure 1a). Subdividing all patients according to the 3 centers (BZ= Bolzano Hospital; BG= Bergamo; IBK= Innsbruck Hospital) we found no difference of OS. OS was 37% (95 Cl 30-44), the median follow-up was 3.4 years (95 Cl 2.9-4.1 years) (Figure 2a). OS analysis for type of treatment (Bolondi stratification versus TACE proposed by EASL and AASLD guidelines) was better when the Bolondi stratification was used (log rang p<0.001, Renyi type test p<0.001) (Figure 1c). Analyzing the stratification of patients in 4 sub-groups as suggested by the Bolondi stratification, there was a significance difference in OS between B1 vs B2, B2 vs B3 and B3 vs B4 (log rank overall p<0.001) (Figure 1d).
The univariate analysis shows that the strongest parameters influencing OS were:
- Child Pugh score (p=0.011)
- the BCLC B1-B4 stage (p<0.001)
These findings are shown in table 3.
Cox regression analysis documented a negligible advantage using the Bolondi stratification, during the first year of observation (HR = 1.11, p=0.757). While over time (prolonged follow-up), the outcome was better than TACE (HR = 0.39, 95CI 0.26-0.58, p<0.001)(Table 5). In the multivariate analysis the BCLC stratification (B1, B2, B3, B4) influenced by far OS, allowing B2 patients to perform better (p <0.05). Patients stratified in B3 and B4 stage would have had a better outcome if treated with liver transplantation, TACE or antitumoral therapy with Sorafenib© (Figures 2a-2c). In multiple regression analysis, the Bolondi stratification showed a better outcome after one year of follow-up (HR = 0.46, 95CI 0.30-0.69, p<0.001) (Tables 5,6). Interestingly, if the OS curves were analysed using the BCLC sub-classification versus type of treatment, we found that the B2 group reached a better OS if treated with other therapeutical strategies (Renyi type test, p < 0.05).
Overall survival taking in relation to Bolondi BCLC classification and Diabetes Mellitus.
A better OS for the BCLC-B2 patients with DM (p = 0.021) was seen (Figure 2b). Furthermore, the influence of DM on OS was confirmed by the Cox regression analysis, where patients without DM showed HR = 1.72 (95CI 1.11-2.67; p=0.015). There were no differences in OS according to type of treatment, aetiology of liver disease and HCC type (p value SD).
DM-HCC Score calculation
We defined a score able to define the influence of DM on OS in HCC BCLC-B patients. This score was taking into account the presence or absence of DM and the severity of BCLC-B stage (where B2 was low risk and B3-B4 high risk). The Cox model on B3 showed a lack of proportionality at 3-years. Further, from the analysis of the z-Wald score, we gave weight 1 for B2 and lack of DM, instead we gave weight 2 for B3 until 3-years of follow-up and weight zero for B3 after 3 years of follow-up. The proposed score straties patients in to three groups; low risk (Score 0-1, n=78, 43%), intermediate risk (Score 2, n=85, 47%) and high risk of death (Score 3, n=19, 10%). The 2-OS% was 90% (95CI 81-55%), 68% (95CI 56-77%) and 8% (95CI 1-29%), intermediate and high risk, respectively (p<0.001), as reported in Table 6 and Figure 2c.
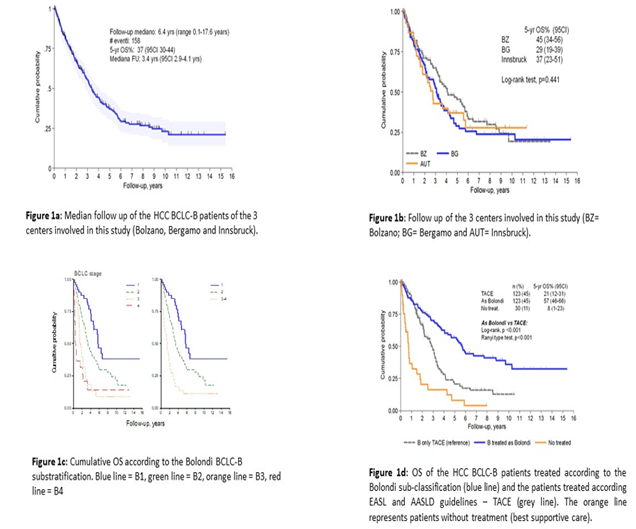
Figure 1:
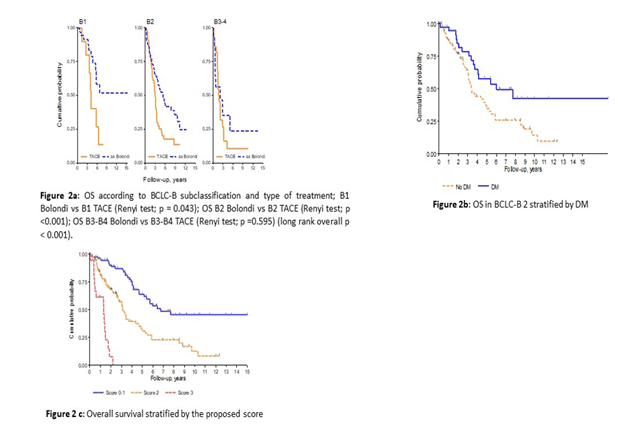
Figure 2:
|
Total |
p value |
||||
|
Variable |
BZ (n=101) |
BG (n=118) |
IBK (n=57) |
276 |
|
|
Median age |
69 (IQR 14) |
63 (IQR 13) |
65 (IQR 14) |
67 (IQR 14) |
0.425 |
|
Gender M |
89 (88) |
95 (81) |
50 (86) |
234 (84) |
0.277 |
|
Child Pugh A |
75 (77) |
87 (74) |
34 (74) |
196 (75) |
0.02 |
|
Child Pugh B |
22 (22) |
31 (26) |
8 (17) |
61 (23) |
|
|
Child Pugh C |
1 (1) |
0 |
4 (9) |
5 (2) |
|
|
MELD > 9 |
46 (47) |
42 (36) |
23 (46) |
111 (42) |
0.231 |
|
Ethiology |
< 0.001 |
||||
|
HCV/HBV |
29 (29) |
83 (70) |
17 (29) |
129 (47) |
|
|
Alcol |
55 (55) |
24 (20) |
18 (31) |
97 (35) |
|
|
Other |
16 (16) |
11 (9) |
23 (40) |
50 (18) |
|
|
Diabete mellitus, yes |
31 (31) |
32 (39) |
- |
63 (35) |
0.349 |
|
Missing value |
1 |
Table 1: Characteristics of the patients of the 3 centers involved in this study (BZ= Bolzano; BG= Bergamo and AUT= Innsbruck) according to type of treatment.
|
Variable |
P value |
||||||
|
Centers |
BZ |
BG |
AUT |
Total |
|||
|
All Therapies |
TACE |
47(47) |
48(41) |
28(49) |
123(45) |
||
|
OLT |
5(5) |
4(3) |
12(21) |
21(8) |
|||
|
RF/PEI |
9(9) |
27(23) |
10(18) |
46(17) |
|||
|
Liver resection |
16(16) |
26(22) |
5(9) |
48(17) |
|||
|
Sorafenib |
6(6) |
1(1) |
1(2) |
8(3) |
|||
|
No therapy |
17(17) |
12(10) |
1(2) |
30(11) |
|||
|
TACE versus other |
0.02 |
||||||
|
TACE |
47(47) |
48(41) |
28(49) |
123(45) |
|||
|
Other |
37(37) |
58(58) |
28(49) |
123(45) |
|||
|
No therapy |
17(17) |
12(12) |
1(2) |
30(11) |
|||
|
Type of treatment |
0.067 |
||||||
|
Curative |
30(36) |
57(54) |
27(48) |
87(46) |
|||
|
Palliative |
53(64) |
49(46) |
29(52) |
102(54) |
|||
|
BCLC-B |
0.937 |
||||||
|
1 |
19 |
22 |
12 |
53 |
|||
|
2 |
61 |
69 |
29 |
159 |
|||
|
3 |
12 |
16 |
10 |
58 |
|||
|
4 |
9 |
11 |
6 |
26 |
Table 2: Characteristics of the patients of the 3 centers involved in this study (BZ= Bolzano; BG= Bergamo and AUT= Innsbruck).
|
n (%) |
5-yrs OS% |
p value |
||
|
Age |
<70 |
162(59) |
41 |
0.165 |
|
>70 |
114(41) |
32 |
||
|
Gender |
M |
233(84) |
38 |
0.291 |
|
F |
43(16) |
33 |
||
|
BCLC |
1 |
95(35) |
47 |
<0.001 |
|
2 |
130(48) |
37 |
||
|
3-4 |
48(18) |
16 |
||
|
MELD |
<10 |
152(58) |
41 |
0.06 |
|
>10 |
110(42) |
33 |
||
|
Child Pugh |
A |
196(75) |
40 |
0.011 |
|
B-C |
66(25) |
30 |
||
|
Diabetes |
No |
119 (65) |
39 |
0.054 |
|
Yes |
63 (35) |
52 |
||
|
Aethiology |
HCV/HBV |
129(47) |
38 |
0.652 |
|
Alcol |
96(35) |
33 |
||
|
Other |
50(18) |
44 |
||
|
Treatment |
TACE |
123 (45) |
21 |
<0.001 |
|
Bolondi |
123 (45) |
56 |
||
|
No treatment |
30 (10) |
8 |
Table 3: Most important characteristics of the patients of the three centers according to the Bolondi et al sub-classification (B1-B4).
|
HR |
95 Cl |
P |
|||
|
STD age |
Continuous |
1.14 |
0.97-1.35 |
0.114 |
|
|
BCLC |
1 |
1 |
|||
|
2 |
1.64 |
1.01-2.64 |
0.044 |
||
|
3-4 |
3.28 |
1.89-5.67 |
<0.001 |
||
|
Treatment |
TACE |
1 |
|||
|
*FU < 1 yr |
Bolondi |
1.35 |
0.67-2.72 |
0.399 |
|
|
FU > 1 yr |
Bolondi |
0.34 |
0.15-0.75 |
0.008 |
|
|
*FU < 1 yr |
No treat |
5.85 |
2.87-11.9 |
< 0.001 |
|
|
FU > 1 yr |
No treat |
1.24 |
0.59-2.62 |
0.566 |
Table 4: Multiple Cox regression in overall survival. Treatment adjusted by age at diagnosis and BCLC staging. FU: time – varying coefficient, follow-up which change the proportionality of hazard ST (age): standardized age (mean=67, SD 10: increase of in HR for increase of 1 standard deviation in age = 10 years).
|
Follow Up (FU) |
Treatment |
HR (95CI) |
P |
|
|
TACE |
1 |
- |
||
|
FU < 1 yr |
Bolondi |
1.13 (0.56-2.26) |
0.738 |
|
|
FU > 1 yr |
Bolondi |
0.39 (0.26-0.58) |
< 0.001 |
|
|
FU < 1 yr |
No |
7.01 (3.48-14.1) |
< 0.001 |
|
|
FU > 1 yr |
No |
1.13 (0.54-2.39) |
0.738 |
|
|
STD age |
1.11 (0.95-1.30) |
0.172 |
Table 5: Overall survival: treatment effect adjusted by age at diagnosis. FU: time – varying coefficient, follow-up which change the proportionality of hazard; ST (age): standardized age (mean=67, SD 10: increase of in HR for increase of 1 standard deviation in age = 10 years)
|
Cox PH model |
HR (95IC) |
Wald z score (weight) |
p |
|
B1 |
1 |
||
|
B2 |
2.07 (1.11-3.87) |
2.29 (1) |
0.022 |
|
B3 < 3yr FU |
10.2 (4.74-21.8) |
5.96 (2) |
<0.001 |
|
B3 > 3yr FU |
0.80 (0.18-3.62) |
-0.30 (0) |
0.765 |
|
No DM |
1.72 (1.11-2.67) |
2.44 (1) |
0.015 |
|
Score |
N (%) |
2-yr OS% |
HR (95IC) |
|
Low (0-1) |
78 (43) |
90 |
1 |
|
Intermediate (2) |
85 (47) |
68 |
2.73 (1.73-4.30) |
|
High (3) |
19 (10) |
8 |
14.9 (7.32-30.5) |
|
High vs Intermediate |
5.47 (2.86-10.5) |
Table 6: Cox proportional hazard model and proposed “DM-HCC score”.
5. Discussion
Despite, new promising treatments (e.g. Sorafenib©) being more widely available, HCC outcome remains poor. For these reasons, a fine-tuning of current clinical management is needed. Reviews of evidence-based staging systems in many national and international centres have suggested that, HCC patients stratified as BCLC B should be treated outside current guidelines. Bolondi et al suggested that staging the patients in a more tailored manner, might improve HCC outcome [8]. However, so far this hypothesis has never been fully confirmed. Piscaglia F et al, looked at OS of Child Pugh B patients in BCLC B stage. However, these authors did not clarify the Bolondi hypothesis. Conversely, our study takes into consideration a large cohort of BCLC B patients only. Furthermore, this cohort was sufficiently strong to verify the Bolondi et al hypothesis, that if by changing patients-stratification (and therefore the associated treatment), OS improves. The OS analysis for type of treatment (Bolondi et al stratification versus TACE as suggested by EASL and AASLD guidelines) showed that OS was statistically significantly better when Bolondi et al stratification was used. We believe that the main reason for this finding is that BCLC B patients undergo a more aggressive approach, such as liver resection and liver transplantation. This allows BCLC-B patients to avoid uneffective treatment and their toxicities (e.g. TACE) and allowing those patients to receive more efficacious therapies. This finding is further supported by our univariate analysis, which showed that the strongest parameters influencing OS are Child Pugh score (p<0.05) and the BCLC B1-B4 stage (p<0.01), exactly what is contemplated within the Bolondi et al stratification. The finding that a follow up of 1 year is needed in order to detect a statistical significant difference of OS, supports our study. In fact, by applying the Bolondi et al sub-stratification, we practically propose a better “tailored therapy”. This allows delivering treatment-toxicity only where is needed. Key data and OS results between each center were overlapping. This finding indirectly validates our patients’ cohort. The pitfalls of our study are the retrospective nature and the fact that data were collected in 3 different centers. For example, it was not possible to evaluate the impact of the liver transplantation per se on outcome. An unexpected finding of our study is the role of DM in the outcome of HCC patients. The association between DM and HCC is well known. The clinical link between these two diseases has been the subject of investigation for over a century, and DM has been established as a risk factor for HCC. Metformin, a first-line oral anti-diabetic, was first proposed as a candidate anti-cancer agent in 2005 in a cohort study in Scotland. Several subsequent large cohort studies and randomized controlled trials have not demonstrated significant efficacy for metformin in suppressing HCC incidence and mortality in diabetic patients. The search for biological links between cancer and diabetes has revealed intracellular pathways that are shared by cancer and diabetes. The signal transduction mechanisms by which metformin suppresses carcinogenesis in cell lines or xenograft tissues and improves chemo-resistance in cancer stem cells have also been elucidated. According to some authors, DM is associated with higher incidence and poorer prognosis of HCC but the influence of DM on patient survival in different HCC stages is unknown (Su YW abstract). Some authors demonstrated that DM is correlated with intrahepatic HCC recurrence after surgery. In conclusion greater attention should be paid to manage patients with HCC and DM to better understand the influence of DM in OS of DM patients and the role of antidiabetic therapy on HCC development [16]. The Kaplan Meier OS curves obtained with the BCLC stratification showed a better OS for the BCLC-B2 patients with DM on treatment (p= 0.05). This suggests that patients with a lower degree of HCC (such as those in stage BCLC 1-2) have a better OS if they also have DM comorbidity on treatment. This finding has never been reported before. Previous studies have already shown that a controlled hyperglycemia might positively impact on cancerogenesis [22]. Interestingly, in our study, DM does not impact positively on OS in patients on BCLC stages 3-4. In fact, those patients have a more advanced disease and therefore a larger HCC tumoral-mass and therefore the positive effect played by DM and DM-treatment may be negligible in these cases. Based on these findings we suggest a new HCC score system to better help clinicians in the management of HCC patients. We have called this new score the MEGA HCC score, derived from the name of the first author. This score needs to be validated in prospective external database.
6. Conclusion
Approximatively one -third of BCLC-B patients can benefit from treatment with TACE in the presence of compensated cirrhosis (Child–Pugh status A or B). Our study confirms the validity of the Bolondi stratification, which allows a more “tailored therapy” and a better OS. A review of the official guidelines and the definition of new treatment models are needed for HCC BCLC-B patients, as OS may change significantly.
References
- Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet 373 (2009): 614-616.
- Ha Y, Shim JH, Kim SO, et al. Clinical appraisal of the recently proposed Barcelona Clinic Liver Cancer stage B subclassification by survival analysis. J Gastroenterol Hepatol 29 (2014): 787-893.
- Forner A, Llovet JM, Bruix J. Chemoembolization for intermediate HCC: is there proof of survival benefit? J Hepatol 56 (2012): 984-986.
- Kojiro M, Roskams T. Early hepatocellular carcinoma and dysplastic nodules. Semin Liver Dis 25 (2005): 133-142.
- Forner A, Reig ME, de Lope CR, et al. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 30 (2010): 61-74.
- Liao M, Huang J, Zhang T, et al. Transarterial chemoembolization in combination with local therapies for hepatocellular carcinoma: a meta-analysis. PLoS ONE 2013; 8: e68453
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 37 (2003): 429-442.
- Bolondi L, Burroughs A, Dufour JF, et al. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis 32 (2012): 348-359.
- Wang JH, Kee KM, Lin CY, et al. Validation and modification of a proposed substaging system for patients with intermediate hepatocellular carcinoma. J Gastroenterol Hepatol 30 (2015): 358-363.
- Weinmann A, Koch S, Sprinzl M, et al. Survival analysis of proposed BCLC-B subgroups in hepatocellular carcinoma patients. Liver Int 35 (2015): 591-600.
- Yamakado K, Miyayama S, Hirota S, et al. Prognosis of patients with intermediate-stage hepatocellular carcinomas based on the Child-Pugh score: subclassifying the intermediate stage (Barcelona Clinic Liver Cancer stage B). Jpn J Radiol 32 (2014): 644-649.
- Llovet JM, Ricci S, Mazzaferro V, et al. SHARP Investigators Study Group Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359 (2008): 378-390.
- Cheng AL, Guan Z, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10 (2009): 25-34.
- Fujita K, Iwama H, Miyoshi H, et al. Diabetes mellitus and metformin in hepatocellular carcinoma. World J Gastroenterol 22 (2016): 6100-613.
- Zang HH, Zhang Y, Cheng YN, et al. Metformin in combination with curcumin inhibits the growth, metastasis and angiogenesis of hepatocellular carcinoma in vitro and in vivo. Mol Carcinog 57 (2018): 44-56.
- Choy Y, Choi Y, Choi CS, et al. Diabetes mellitus increases the risk of intrahepatic recurrence of hepatocellular carcinoma after surgical resection. Tumori 103 (2017): 279-285.
- Su YW, Liu PH, Hsu CY, et al. Prognostic impact of diabetes mellitus on hepatocellular carcinoma: special emphasis from the BCLC perspective. PLoS One 12 (2017): 15-25.
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 53 (1958): 457-481.
- Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. Springer, (2nd edtn), pp: 223-226.
- Cox DR. Regression models and life tables. J R Stat Soc B 34 (1972): 187-202.
- Schoenfeld D. Partial residuals for proportional hazard regression model. Biometrika 69 (1982): 239-241.
- Puhr M, Hoefer J, Eigentler A, et al. Thr glucocorticoid receptor is a key player for prostate cancer cell survival and a target for improved anti-androgen therapy. Clin Cancer Res 20 (2017): 12-15.


 Impact Factor: * 4.2
Impact Factor: * 4.2 Acceptance Rate: 72.62%
Acceptance Rate: 72.62%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 