Reveromycin A Inhibits Bone Loss in Ovariectomized Mice 
Yasuhiro Ozasa1, 2*, Takuro Wada3, Toshihiko Yamashita2 and Yasuo Kokai1??
1Department of Biomedical Engineering, Sapporo Medical University, Sapporo, Japan
2Department of Orthopaedic Surgery, Sapporo Medical University, Sapporo, Japan
3Department of Orthopaedic Surgery, Saiseikai Otaru Hospital, Otaru, Japan
*Corresponding Author: Yasuhiro Ozasa, Department of Orthopaedic Surgery; Sapporo Medical University School of Medicine; S1W16, Chuo-ku, Sapporo, 060-8556, Japan
Received: 22 July 2019; Accepted: 05 August 2019; Published: 12 August 2019
Article Information
Citation: Yasuhiro Ozasa, Takuro Wada, Toshihiko Yamashita, Yasuo Kokai. Reveromycin A Inhibits Bone Loss in Ovariectomized Mice. Journal of Surgery and Research 2 (2019): 87-95.
View / Download Pdf Share at FacebookAbstract
Background and objectives: Reveromycin A inhibits bone metastasis of lung cancer cells in natural killer cell-depleted immunodeficient mice with increasing apoptosis of osteoclasts. This knowledge suggests that reveromycin A is a potent inhibitor for osteoclasts in vivo. To determine the activity of bone loss in acute estrogen deficiency, we studied ovariectomized mice treated with reveromycin A.
Materials and Methods: Eight-week-old female C57BL/6 mice were either ovariectomized or sham-operated. Reveromycin A was continuously administered for the first 2 weeks (Group RA) or the last 2 weeks (Group RP) using a subcutaneous mini osmotic pump. Mice were sacrificed 4 weeks after surgery. Inhibition was detected by images as well as serum carboxyterminal telopeptide of type I collagen (CTX).
Results: BMD reduction in the RA group was significantly inhibited and was comparable with that in the sham group. In contrast, the RP group failed to inhibit BMD reduction. The plasma CTX level at the 28th day of the experiment was significantly increased in the RP and OVX groups compared with that in the sham group, while the increase was inhibited in the RA group. Morphometric analysis revealed that absorption of trabecular bone was strongly inhibited in the RA group.
Conclusion: The inhibitory effects of reveromycin A administered immediately after ovariectomy was the most effective at the early phase of bone loss than at the later stage. A new reagent that inhibits bone loss in acute estrogen deficiency might be a new tool to experimentally study osteoporosis and may have clinical implications.
Keywords
<p>Estrogen deficiency; Osteoporosis; Reveromycin A; Ovariectomy</p>
Article Details
Introduction
Acute bone loss induced by ovariectomy is an important experimental model of osteoporosis in post-menopausal women. Although extensive studies have been performed, the mechanisms of osteoporosis in acute estrogen deficiency have not yet been fully elucidated. A number of treatment strategies, including bisphosphonates, a receptor activator of nuclear factor-kB ligand inhibitor and hormonal reagents, have been introduced to treat this pathology [1-6]. The introduction of a new probe to study this particular condition presents with new possibilities to study osteoporosis. We previously reported that overexpression of granulocyte colony-stimulating factor induced an apparent reduction in trabecular bone mass and increase in bone absorption at the endosteal surface, thereby resulting in severe osteopenia [7, 8].
Reveromycin A was originally identified as an inhibitor of epidermal growth factor (EGF)-dependent cell growth [9, 10]. Subsequently, this reagent was found to be a strong inhibitor for isoleucyl-tRNA synthetase in yeast [11]. Reveromycin A has been shown to inhibit bone metastasis of lung cancer cells in natural killer cell-depleted immunodeficient mice [12]. In a histologic analysis of reveromycin A-treated mice, increased apoptosis of osteoclasts in tumor-implanted mice was detected, suggesting that reveromycin A is a potent inhibitor for osteoclasts in vivo, which affects bone loss with various etiologies, including acute estrogen deficiency. To determine activity of bone loss in acute estrogen deficiency, we studied ovariectomized mice treated with reveromycin A. We hypothesized that reveromycin A strongly inhibits bone loss in ovariectomized mice.
2. Materials and Methods
2.1 Animals
All animals were maintained at our animal facility under specific pathogen-free conditions and were experimented on in accordance with the National Institutes of Health guidelines on the care and use of laboratory animals.
2.2 Reagents
All reagents were purchased from Sigma (St. Louis, MO) unless otherwise stated.
2.3 Ovariectomy
Eight-week-old female C57BL/6 mice were ovariectomized (OVX) or sham-operated under general anesthesia as previously described [13]. Mice were sacrificed 4 weeks after surgery. Blood samples were obtained for hematologic analysis, and the uterus and femur were removed 4 weeks after surgery.
2.4 Administration of reveromycin A
Reveromycin A (1 μg/ml in PBS) was administered using a mini osmotic pump (Alzet Model 2002, Durect Co., Cupertino, CA), which continuously delivered 200 μL for 14 days, resulting in a dose of 700 ng/kg/day of reveromycin A. The osmotic pump was inserted subcutaneously at the day of OVX or sham operation. Because the osmotic pump was effective only for 2 weeks, we administered reveromycin A for the first 2 weeks (Group RA) or the last 2 weeks (starting infusion at the first day of the 3rd week after surgery, Group RP).
2.5 Radiographic analysis (n=10 per group)
The bone mineral density (BMD) of the femur was measured using dual-energy X-ray absorptiometry (DEXA) using a QDR-1000w Plus Densitometer (Hologic, Waltham, MA), calibrated with a hydroxyapatite phantom of the human lumbar spine. The femur excised at 4 weeks after surgery was fixed with 70% ethanol, and soft tissues were removed. The whole bone was scanned, and measurements were performed with a customized rodent whole body software package (Hologic, Waltham, MA). The scans were performed with a 1.270-mm diameter collimator, 0.762-mm line spacing, 0.380-mm point resolution, and an acquisition time of 9 min. Values were expressed as BMD mg/cm2 as described [14]. The extracted bone was also radiologically examined with soft X-rays (Sofron Type SRO-M50; Soken, Tokyo, Japan).
2.6 Measurement of plasma carboxyterminal telopeptide of type I collagen concentration (n=6 per group)
Carboxyterminal telopeptide of type I collagen (CTX) was measured by enzyme-linked immunosorbent assay using the RatLaps kit as according to the manufacturer’s instructions (Nordic Bioscience Diagnosis, Herler, Denmark) [15].
2.7 Bone morphometric analysis (n=5 per group)
Bone morphometric analysis was conducted as described using decalcified bone specimen stained with hematoxylin-eosin (HE) with Bone morphometry software (System Supply, Nagano, Japan), a computer with a digitizing board, and a Nikon Labophot microscope (Nikon, Tokyo, Japan). Data were expressed under the nomenclature of the American Society for Bone and Mineral Research (ASBMR) committee for bone histomorphometry [16] and included the trabecular bone volume (TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular separation (Tb.Sp), periosteal perimeter (Ps.Pm), endosteal perimeter, and cortical area (Ct.A).
2.8 Statistical analysis
Continuous variables are expressed as mean and standard deviation. These data were compared among four groups (sham, OVX, RA, and RP) with one-way factorial analysis of variance. The Tukey–Kramer post hoc test for each pairwise comparison was performed if a significant difference was detected. The significance level was set at p<0.05 in all cases. All statistical analyses were performed using BellCurve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan).
3. Results
3.1 Inhibition of bone loss in ovariectomized mice by reveromycin A
As shown in Figure 1, the X-ray showed apparent inhibition of cancellous bone reduction in the RA group, but not in the RP and OVX groups. This was further confirmed by DEXA (Figure 2). BMD was significantly reduced by almost 10% in the OVX group (0.045 ± 0.003 mg/cm2) compared with that in the sham group (0.05 ± 0.005 mg/cm2, p=0.003). However, reveromycin A administration at the first 2 weeks significantly inhibited BMD reduction in the RA group (0.049 ± 0.005 mg/cm2) and was comparable with that in the sham group. In contrast, the RP group failed to inhibit BMD reduction (0.041 ± 0.005 mg/cm2). The plasma CTX level at the 28th day of the experiment was significantly increased in the RP (29.0 ± 4.82 ng/ml, p=0.01) and OVX groups (24.8 ± 4.66 ng/ml, p=0.05) compared with that in the sham group (19.71 ± 5.50 ng/ml), while the increase in CTX level was inhibited in the RA group (20.33 ± 2.90 ng/ml) (Figure 3).
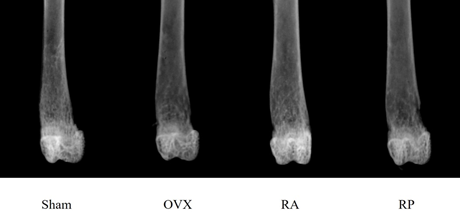
Figure 1: Radiological appearance of the distal femur of each group 4 weeks after surgery. Apparent reduction in cancellous bone mass is observed in the OVX group. Inhibition of cancellous bone loss is evident in the RA group, but not in the RP group.
*Significant difference compared with Sham and RA (p<0.05)
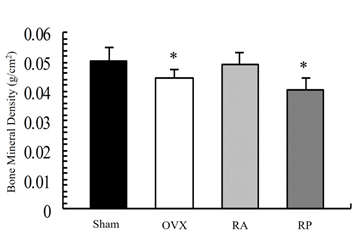
Figure 2: Bone mineral density (BMD) of the femur by dual-energy X-ray absorptiometry. Reduction of BMD is observed in the OVX and RP groups. This reduction is strongly inhibited in the RA group. BMD in the RA group was comparable to that in the sham group.
*Significant difference compared with Sham and RA (p<0.05).
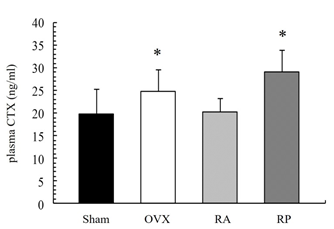
Figure 3: Plasma CTX level at the 28th day of experiment. The RA group exhibited decreased plasma CTX levels. CTX level was significantly increased in the OVX and RP groups compared to that in the Sham group.
3.2 Histologic analysis of bone
Trabecular bone mass was reduced in the OVX group as determined by HE staining (Figure 4). This reduction was strongly inhibited in the RA group, but not in the RP group. Furthermore, morphometric analysis showed that the decrease of TV, Tb.Th, and Tb.N in the OVX group was inhibited in the RA group. Mean Tb.Sp was significantly decreased in the RA group compared with that in the OVX group. In contrast, the RP group did not exhibit detectable inhibition in any parameters (Table 1). Notably, histomorphometric analysis of cortical bone showed little change among these groups (Table 2).
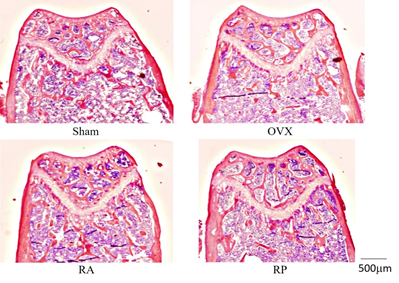
Figure 4: Metaphysis of distal femur. Significant reduction in trabecular bone volume and connection is shown in OVX and RP. The reduction seems to be inhibited in the RA group, similar to the Sham group. (magnification: ×20).
|
TV (%) |
Sham |
OVX |
RA |
RP |
|
10.59 ± 2.90* |
4.69 ± 1.13 |
10.71 ± 1.98* |
5.09 ± 1.56 |
|
|
Tb.Th (μm) |
38.95 ± 5.37 |
31.72 ± 4.37 |
43.50 ± 6.65** |
37.40 ± 8.17 |
|
Tb.N (mm-1) |
2.71 ± 0.62* |
1.46 ± 0.18 |
2.46 ± 0.31* |
1.34 ± 0.18 |
|
Tb.Sp (μm) |
345.0 ± 89.1* |
659.8 ± 92.1 |
367.2 ± 57.0* |
719.3 ± 124.6 |
TV: trabecular bone volume; Tb.Th: trabecular thickness; Tb.N: trabecular number; Tb.Sp: trabecular separation
*Significant difference compared with OVX and RP (p<0.05)
**Significant difference compared with OVX (p<0.05)
Table 1: Histomorphometric measurements of distal femur cancellous bone.
|
Ps.Pm (mm) |
Sham |
OVX |
RA |
RP |
|
4.33 ± 0.25 |
4.57 ± 0.16 |
4.64 ± 0.16 |
4.43 ± 0.27 |
|
|
Es.Pm (mm) |
3.37 ± 0.11 |
3.47 ± 0.03 |
3.54 ± 0.17 |
3.49 ± 0.15 |
|
Ct.A (mm2) |
0.48 ± 0.18 |
0.56 ± 0.11 |
0.62 ± 0.05 |
0.56 ± 0.07 |
Ps.Pm: periosteal perimeter; Es.Pm: endosteal perimeter; Ct.A: cortical area
There is no significant difference between the groups.
Table 2: Histomorphometric measurements of cortical bone of femoral shaft.
3.3 Reveromycin A did not change bone development in ovariectomized mice
Bone length and weight of the uterus were measured as indicators for development and estrogen deficiency. The maximum length of the femur and diameter at the midsection of the femur was measured. Uterine weight was also measured. Reveromycin A did not significantly alter bone length or uterine weight in the OVX, RA, and RP groups (Table 3).
|
Femur length (mm) |
Sham |
OVX |
RA |
RP |
|
14.5 ± 0.14 |
14.66 ± 0.11 |
14.72 ± 0.10 |
14.68 ± 0.19 |
|
|
Femur diameter (mm) |
1.56 ± 0.05 |
1.57 ± 0.05 |
1.57 ± 0.05 |
1.56 ± 0.05 |
|
Uterine weight (mg) |
122 ± 31 |
13 ± 5* |
11 ± 2* |
12 ± 1* |
*Significant difference compared with Sham (p<0.05)
Table 3: Measurements of femur length, diameter, and uterine weight.
4. Discussion
In this report, we explored the inhibitory effect of reveromycin A on acute ovariectomy-induced bone loss. Initial experiments with repeated injections of reveromycin A yielded varying results. Even with relatively high concentrations (i.e., 10 mg/kg/day), inhibition of bone loss could not be reproducibly detected. Failure of inhibition by repeated injection might be due to the very short half-life of reveromycin A (for i.p. administration of 20 mg/Kg reveromycin A, Cmax¬¬ and T1/2 were 6.6 μg/mL and 0.66 h, respectively) [12]. In contrast, continuous administration of reveromycin A by mini osmotic pump resulted in strong inhibition of bone loss with a relatively low dose (700 ng/kg/day of reveromycin A). The inhibition seemed to be specific to the early stage of acute estrogen deficiency, in which administration of reveromycin A during the first 2 weeks showed strong inhibition of bone loss but not when administered in the last 2 weeks. The results suggested that reveromycin A might inhibit bone loss through at least different phase of bone loss, which might be linked to differentiation in osteoclasts. At an early phase of bone loss in ovariectomy, reveromycin A induces apoptosis on mature osteoclasts [12]. However, the decrease in CTX levels at the 28th day of the experiment in the RA group suggested that this reagent might affect the differentiation of osteoclasts at a more immature state. The short half-life of reveromycin A provides researchers with an interesting tool to understand the dynamics of bone loss caused by acute estrogen deficiency.
Reveromycin A was isolated as an activity to inhibit EGF-dependent cell growth from a culture supernatant of Actinomycetes [9, 10]. A later study revealed that this reagent inhibits isoleucyl-tRNA synthetase in Saccharomyces cerevisiae [11]. Isoleucyl-tRNA synthetase is a member of aminoacyl-tRNA synthetase, which plays an essential role in protein translation. This enzyme was widely expressed in cells throughout the body [17, 18]. Therefore, the inhibitory activity of isoleucyl-tRNA synthetase might cause considerable side effects in the host. Reducing the dose might reduce this consideration because we observed no apparent illness, including diarrhea and weight loss, during the study course. Estrogen deficiency leads to several life-threatening diseases in post-menopausal women [19, 20]. In this study, we used ovariectomy as a model to study estrogen deficiency. Although this model is widely accepted to evaluate post-menopausal syndrome, a lack of the perimenopausal period could be critical for the study of estrogen deficiency. Indeed, the present study suggests that reveromycin A has an inhibitory activity on bone loss in the relatively early phase of the experiment. Acute estrogen deficiency induces strong activation of bone resorption, which is controlled by several independent mechanisms [21–23]. The inhibitory activity of reveromycin A in bone resorption can be used as a novel tool to understand the physiological mechanism of bone loss in vivo.
However, there are some limitations in this study. First, the mechanism of inhibition of bone loss remains unknown. Initial studies indicated that reveromycin A increased apoptosis of osteoclasts in vivo as well as in vitro, but it was not shown that this apoptosis was caused by reveromycin A. Development and activation of osteoclasts is a complex physiological event regulated by cytokines interacting with cells, including osteoblasts, as well as physical status. Second, although non-decalcified samples are generally used, we used a decalcified bone specimen for bone morphometric analysis. However, we analyzed only bone structures that were not influenced by the decalcification process. Finally, we only considered administration of reveromycin A at one concentration. This particular concentration has little systemic effects and does not cause side effects. Thus, it is possible that this is an appropriate concentration for bone loss inhibition without side effects.
5. Conclusions
These observations indicate that reveromycin A is a strong inhibitor for bone loss induced by ovariectomy. The effect seemed to be stage-specific as reveromycin A was more effective in bone loss inhibition in early exposure in ovariectomized mice. Furthermore, the inhibitory effect of bone loss might be retained for a relatively long duration, as suggested by the decrease of CTX in the RA group measured on the 28th day of the experiment. Further studies are essential to reveal the physiological mechanism of reveromycin A in bone metabolism.
Acknowledgments
We would like to thank Michihiro Kimura, Mami Yamaguchi, and Seiji Ohtani for their excellent technical support, Hiroyuki Osada for providing reveromycin A, and Enago (www.enago.jp) for the English language editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hooper MJ, Ebeling PR, Roberts AP, et al. Risedronate prevents bone loss in early postmenopausal women: a prospective randomized, placebo-controlled trial. Climacteric 8 (2005): 251-262.
- Reginster JY, Felsenberg D, Cooper C, et al. A new concept for bisphosphonate therapy: a rationale for the development of monthly oral dosing of ibandronate. Osteoporos Int 17 (2006): 159-166.
- Seeman E, Crans GG, Diez-Perez A, et al. Anti-vertebral fracture efficacy of raloxifene: a meta-analysis. Osteoporos Int 17 (2006): 313-316.
- Zizic TM. Pharmacologic prevention of osteoporotic fractures. Am Fam Physician 70 (2004): 1293-1300.
- Tsourdi E, Makras P, Rachner TD, et al. Denosumab effects on bone density and turnover in postmenopausal women with low bone mass with or without previous treatment. Bone 120 (2019): 44-49.
- Diez-Perez A, Marin F, Eriksen EF, et al. Effects of teriparatide on hip and upper limb fractures in patients with osteoporosis: A systematic review and meta-analysis. Bone 120 (2019): 1-8.
- Kuwabara H, Wada T, Oda T, et al. Overexpression of the granulocyte colony-stimulating factor gene impairs bone morphogenetic protein responsiveness in mice. Lab Invest 81 (2001): 1133-1141.
- Takahashi T, Wada T, Mori M, et al. Overexpression of the granulocyte colony-stimulating factor gene leads to osteoporosis in mice. Lab Invest 74 (1996): 827-834.
- Osada H, Koshino H, Isono K, et al. Reveromycin A, a new antibiotic which inhibits the mitogenic activity of epidermal growth factor. J Antibiot 44 (1991): 259-261.
- Takahashi H, Osada H, Koshino H, et al. Reveromycins, new inhibitors of eukaryotic cell growth. I. Producing organism, fermentation, isolation and physico-chemical properties. J Antibiot 45 (1992): 1409-1413.
- Miyamoto Y, Machida K, Mizunuma M, et al. Identification of Saccharomyces cerevisiae isoleucyl-tRNA synthetase as a target of the G1-specific inhibitor Reveromycin A. J Biol Chem 277 (2002): 28810-28814.
- Muguruma H, Yano S, Kakiuchi S, et al. Reveromycin A inhibits osteolytic bone metastasis of small-cell lung cancer cells, SBC-5, through an antiosteoclastic activity. Clin. Cancer Res. 11 (2005): 8822-8828.
- Oda T, Wada T, Kuwabara H, et al. Ovariectomy fails to augment bone resorption and marrow B lymphopoiesis in granulocyte colony-stimulating factor transgenic mice. J Orthop Science 10 (2005): 70-76.
- Kokai Y, Wada T, Oda T, et al. Overexpression of granulocyte colony-stimulating factor induces severe osteopenia in developing mice that is partially prevented by a diet containing vitamin K2 (menatetrenone). Bone 30 (2002): 880-885.
- Hoegh-Andersen P, Tanko LB, Andersen TL, et al. Ovariectomized rats as a model of postmenopausal osteoarthritis: validation and application. Arthritis Res Ther 6 (2004): 169-180.
- Parfitt AM, Drezner MK, Glorieux FH, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Min Res 2 (1987): 595-610.
- Shiba K, Suzuki N, Shigesada K, et al. Human cytoplasmic isoleucyl-tRNA synthetase: selective divergence of the anticodon-binding domain and acquisition of a new structural unit. Proc Natl Acad Sci USA 91 (1994): 7435-7439.
- Park SG, Ewalt KL, Kim S. Functional expansion of aminoacyl-tRNA synthetases and their interacting factors: new perspectives on housekeepers. Trends Biochem Sci 30 (2005): 569-574.
- Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 88 (2003): 2404-2411.
- Rossi R, Grimaldi T, Origliani G, et al. Menopause and cardiovascular risk. Pathophysiol. Haemos Thromb 32 (2002): 325-328.
- Eghbali-Fatourechi G, Khosla S, Sanyal A, et al. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest 111 (2003): 1221-1230.
- Hofbauer LC, Khosla S, Dunstan CR, et al. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Min Res 15 (2000): 2-12.
- Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 115 (2005): 3318-3325.


 Impact Factor: * 4.2
Impact Factor: * 4.2 Acceptance Rate: 72.62%
Acceptance Rate: 72.62%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 