Pyridyl-Substituted Naphthalenes and 3,4-dihydro-1H-quinolin-2-ones as Fluorinated Aldosterone Synthase (CYP11B2) Inhibitors for the Differential Diagnosis of Primary Aldosteronism
Philipp Maier1,2,3, Andreas Schirbel1,*, Magdalena Schneider1,3, Sabine Gabor3, Samario Reese3, Stefanie Hahner3, Britta Heinze3
1Department of Nuclear Medicine, University Hospital of Würzburg, University of Würzburg, 97080 Würzburg, Germany
2University Clinic for Radiology and Nuclear Medicine, Otto von Guericke University (OvGU), 39120 Magdeburg, Germany
3Division of Endocrinology & Diabetes, Department of Medicine I, University Hospital of Würzburg, University of Würzburg, 97080 Würzburg, Germany
*Corresponding authors: Andreas Schirbel, Department of Nuclear Medicine, University Hospital of Würzburg, University of Würzburg, 97080 Würzburg, Germany.
Received: 10 June 2025; Accepted: 17 June 2025; Published: 18 July 2025
Article Information
Citation: Philipp Maier, Andreas Schirbel, Magdalena Schneider, Sabine Gabor, Samario Reese, Stefanie Hahner, Britta Heinze. Pyridyl- Substituted Naphthalenes and 3,4-dihydro-1Hquinolin- 2-ones as Fluorinated Aldosterone Synthase (CYP11B2) Inhibitors for the Differential Diagnosis of Primary Aldosteronism. Journal of Biotechnology and Biomedicine. 8 (2025): 244-260.
DOI: 10.26502/jbb.2642-91280191
View / Download Pdf Share at FacebookAbstract
Aldosterone synthase (CYP11B2) is specifically expressed in aldosterone-producing tissue of the adrenal cortex. It cata-lyzes the final steps in aldosterone synthesis and is pathophysiologically relevant in primary aldosteronism. Therefore, it presents an excellent PET imaging target for the localization of aldosterone excess. To expand compound library for the development of an appropriate F-18 labelled radiotracer, we synthesized fluorinated derivatives of pyridyl-substituted naphthalenes 1 and 3,4-dihydro-1H-quinolin-2-ones 2. Despite the high homology of CYP11B2 with the cortisol-producing enzyme 11β-hydroxylase (CYP11B1) both classes of compounds are well known as selective CYP11B2 inhib-itors. Some of the 30 substances prepared proved to be highly potent CYP11B2 inhibitors (IC50 < 10 nM) in in vitro evaluation using transfected Y1 cells and NCI-H295 cells. Moreover, several compounds exhibited outstanding selec-tivity over CYP11B1 and thus deserve further biological investigations of their F-18 labelled analogues.
Keywords
<p>Primary aldosteronism; Molecular imaging; Naphthyl pyridines; Dihydroisoquinolinones; Fluorinated CYP11B2 inhibi-tors; NCI-H295 cells</p>
Article Details
Introduction
Aldosterone is a steroid hormone that acts on the kidneys to increase sodium reabsorption and potassium excretion, which helps in regulating blood pressure, blood volume, and electrolyte balance. Furthermore, aldosterone can indirectly affect hydrogen ion secretion, aiding in the management of acid-base balance in the body. The biosynthesis of aldosterone is performed by the enzyme aldosterone synthase (CYP11B2) which converts corticosterone into aldosterone in three successive catalytic reactions: 11β-hydroxylation, 18-hydroxylation, and 18-oxidation. Aldosterone synthase is primarily found in the zona glomerulosa of the adrenal cortex and regulated by various factors, namely angiotensin II, plasma potassium levels and to a lesser extent by the adrenocorticotropic hormone (ACTH). Excessive autonomous aldosterone production leads to primary aldosteronism (Conn's syndrome), a condition characterized by treatment-resistant hypertension, hypokalemia and an elevated risk for cardiovascular diseases, kidney damage and stroke [1]. One of the major diagnostic challenges in confirmed primary aldosteronism is localizing the main source of aldosterone excess [2,3]. If the cause is unilateral, typically due to a unilateral aldosterone-producing adenoma (APA), surgery may offer a potential cure and beneficial effects on long-term mortality [4]. In contrast, bilateral aldosterone excess necessitates lifelong medication management, typically involving mineralocorticoid receptor antagonists like spironolactone or eplerenone [2]. These pharmaceuticals act by blocking the mineralocorticoid receptor that is the site of action of aldosterone. Another approach is to inhibit the activity of CYP11B2, thereby reducing the production of aldosterone. However, the development of selective CYP11B2 inhibitors has been challenging due to the similarity between CYP11B2 and CYP11B1, the enzyme relevant for cortisol synthesis. The two enzymes share 93% homology, differing in only 29 amino acids, most of which are not inside the active sites of enzymes. The high selectivity of the CYP11B2 inhibitors is necessary to avoid affecting cortisol production, which can lead to dangerous side effects like adrenal insufficiency.
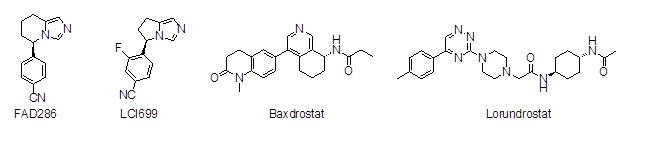
The development of selective CYP11B2 inhibitors was pioneered by the group of R.W. Hartmann, who published many compounds with 3,5-disubstituted pyridines as the pharmacophore [5-10]. Later, other N-containing heterocycles (imidazoles, isoxazoles, pyrimidines and pyrazines) were discovered [11-15]. A recently published review provides a good overview of the very numerous inhibitors [16]. Due to the research activities of big pharmaceutical companies (e.g. Novartis, Roche, Merck, AstraZeneca, Eli Lilly) several CYP11B2 inhibitors have entered clinical trials, aiming to provide a therapeutic option for primary aldosteronism without the side effects of non-selective mineralocorticoid receptor antagonists like spironolactone, namely FAD286 [17,18], LCI699 [19], baxdrostat [20], lorundrostat [21] (Figure 1), LY3045697 [22] and BI 690517 [23] (chemical structures not disclosed). Nevertheless, none of them have approved marketing authorization at the moment, apart from LCI-699 which was repurposed as a CYP11B1 inhibitor and approved for therapy of Cushing´s syndrome under the trade name Isturisa® in the United States and in the European Union [24]. This underlines the difficulty of developing suitable CYP11B2 inhibitors.
In contrast to the numerous CYP11B2 inhibitors for therapeutic purposes described above, only few radiolabelled CYP11B2 inhibitors were described for diagnostic purposes, although there is a great clinical need for these as well: Traditional imaging techniques have limitations when it comes to differentiating between unilateral and bilateral causes of PA, particularly in patients over 40 years old [25]. This limitation arises from the increasing detection of hormonally inactive adrenal masses with age and the potential presence of very small aldosterone-producing adenomas (< 5 mm), which can evade detection through these imaging modalities [26,27].
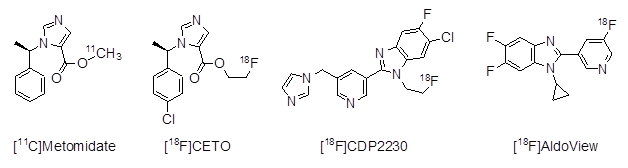
Consequently, the current gold standard for further diagnosis involves bilateral blood sampling and analysis from the adrenal veins [2]. However, this method is invasive, associated with side effects, and requires a skilled clinician. It is only available in a limited number of medical centers [28,29]. Additionally, adrenal vein sampling entails significant radiation exposure, making it an area in need of improvement [30,31]. Molecular imaging of CYP11B2 expression using a radiolabelled inhibitor holds great promise as a non-invasive diagnostic tool for distinguishing between bilateral hyperplasia and APA. Especially positron emission tomography (PET) provides excellent spatial resolution and tracer uptake quantification [32]. Clinical studies involving the non-selective radiotracer [11C]Metomidate have produced varying results regarding its ability to differentiate between unilateral and bilateral disease [33,34] (Figure 2). Due to the short half-life time of carbon-11 (20 min), a radiofluorinated derivative, [18F]CETO was recently introduced into a clinical trial of patients with primary aldosteronism [35,36]. The first selective radiofluorinated CYP11B2 inhibitor, [18F]CDP2230, was introduced, but it showed only moderate selectivity (15.8) towards CYP11B2 and was not further developed for clinical use [37]. [18F]AldoView, on the other hand, seems to be very promising, even if this tracer cannot be labelled using conventional labelling methods [38].
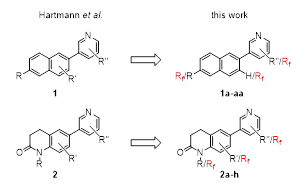
Recently, we presented numerous 3-(4-cyano-3-fluorophenyl)pyridines and 3-(4-cyano-3-fluorophenyl)pyrazines as highly potent and selective CYP11B2 inhibitors [39]. In order to expand the reservoir of compounds that can be considered for the development of a selective radiofluorinated tracer, we decided to prepare fluoro-substituted derivatives of two further compound classes, namely of the pyridyl-substituted naphthalenes 1 and the 3,4-dihydro-1H-quinolin-2-ones 2 that were both investigated by Hartmann et al. [7,40] (Figure 3). Apparently, the 3,4-dihydro-1H-quinolin-2-ones presented in [7] were later developed into Baxdrostat. Favored by the absence of protic functions a one-step radiosynthesis of analogue F-18 labelled PET tracers by nucleophilic aliphatic F-18 fluorination of suitable labelling precursors is viable. Therefore, the fluoro substituent of most of our target compounds was introduced in an alkyl side chain (Rf). All synthesized compounds were analyzed for their potential in the inhibition of CYP11B1 and CYP11B2 enzymes using in vitro assays of transfected Y1 cells, and some were also tested on the human NCI-H295 cell line.
Results and Discussion
Preparation of the fluorinated target compounds
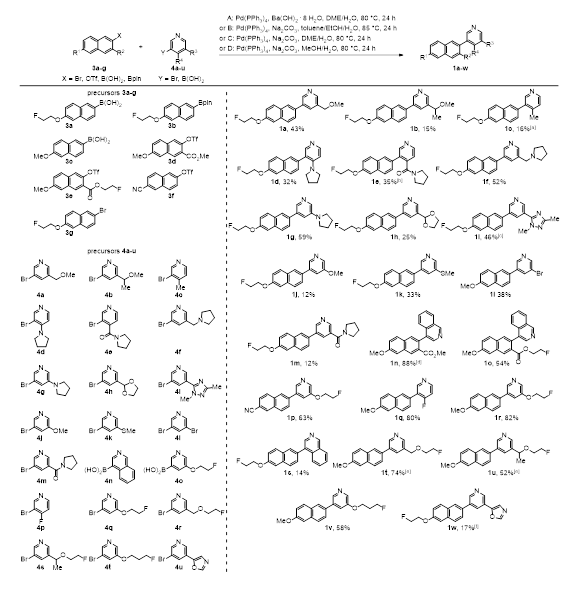
The syntheses of naphthyl pyridines are shown in Schemes 1 and 2. Generally, the target compounds were prepared by Suzuki-Miyaura reactions as a key step. The required naphthalene reactants 3a-g were either commercially available or easily accessible by synthesis according to known procedures [41-43]. Coupling with a variety of pyridines 4a-u provided the biaryl compounds 1a-w (Scheme 1). Almost all of the pyridine precursors are also commercially available or could be prepared in a fast way according to known procedures [10,44-48]. If necessary, the coupling reactions were examined with different combinations of solvents and bases known from the literature, since none of the conditions used provided reliably good yields for all of the target compounds (Methods A-D) [49-51]. Moreover, the yields were apparently independent of the functionalization of both coupling building blocks; neither the boron-containing transmetalation reagents nor the halogen substituted electrophiles proved to be superior for the naphthalene and the pyridine compound species. However, the isolated amounts were sufficient for in vitro studies of the potential new CYP11B2 inhibitors or for further conversion.
Scheme 1: Synthesis of pyridyl-substituted naphthalenes 1a-w by Suzuki cross-coupling reactions of naphthyl compounds 3a-g with different pyridyl bromides or boronic acids 4a-u. Reaction conditions unless otherwise noted: Method A: 1.0 equiv. 3a, 0.8 equiv. 4a-c, 3.2 mol% Pd(PPh3)4, 1.25 equiv. Ba(OH)2 * 8 H2O, or 1.0 equiv. 3b, 1.0 equiv. 4d-l, 3.6 mol% Pd(PPh3)4, 1.4 equiv. Ba(OH)2 * 8 H2O, DME/H2O, 80 °C, 24h, 12-59% for 1a-l; Method B: 1.0 equiv. 3a, 3d or 3e, 1.0-1.6 equiv. 4m or 4n, 7.0-8.1 mol% Pd(PPh3)4, 2.15 equiv. Na2CO3, toluene/EtOH/H2O, 85 °C, 24 h, 12-88% for 1m-o; Method C: 1.0 equiv. 3c, 1.0 equiv. 4o, 2.1 mol% Pd(PPh3)4, 2.2 equiv. Na2CO3 or 1.0 equiv. 3f, 0.8 equiv. 4p, 4.3 mol% Pd(PPh3)4, 3.4 equiv. Na2CO3, DME/H2O, 80 °C, 24 h, 63-80% for 1p/q; Method D: 1.0 equiv. 3c or 3g, 0.4-1.0 equiv. 4o-t, 3.7 mol% Pd(PPh3)4, 6.2-6.5 equiv. Na2CO3, MeOH/H2O, 80 °C, 24 h, 24-82% for 1r-v. [a] 1.5 equiv. Ba(OH)2 * 8 H2O. [b] 0.9 equiv. 4e. [c] 0.8 equiv. 4i. [d] 1.8 equiv. Na2CO3. [e] 3.9 mol% Pd(PPh3)4. [f] reaction conditions: 1.0 equiv. 3b, 1.0 equiv. 4u, 6.0% Pd(PPh3)4, 1.1 equiv. Ag2CO3, benzene, Δ, 48 h.
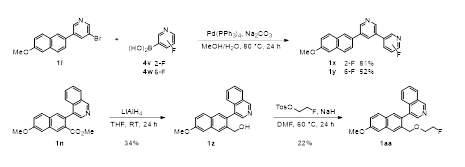
Compound 1l was extended by another Suzuki coupling with pyridine boronic acids 4v and 4w to furnish ortho-fluorine substituted teraryls 1x and 1y, respectively (Scheme 2). With regard to our further research intentions this represents a proper position for F-18 labelling of the corresponding nitro compounds. Methyl ester 1n was transformed in a reduction-alkylation sequence to ether 1aa, using lithium aluminum hydride in tetrahydrofurane to form the benzylic alcohol intermediate 1z and 2-fluoroethyl tosylate with sodium hydride for the subsequent strong base mediated alkylation.
The second type of our target compounds were pyridyl and isoquinolyl substituted 3,4-dihydro-1H-quinolin-2-ones 2a-h. Their preparation was accomplished again by a Suzuki coupling as the key synthetic transformation by means of the connection of 3,4-dihydro-1H-quinolin-2-ones 5a-f with isoquinolyl-4-boronic pinacol ester 4n or pyridines 4p, 4r and 4s, respectively (Scheme 3). Compounds 5a-f were obtained by successive generation of the bicyclic system, bromination and borylation reactions according to known procedures [7,43,52,53].
Scheme 3: Synthesis of pyridyl- and isoquinolyl-substituted dihydro-1H-quinolin-2-ones 2a-h by Suzuki cross-coupling reactions of dihydroquinolinones 5a-f with pyridyl bromides 4p, 4r or 4s or pyridyl boronic acid 4n. Reaction conditions unless otherwise noted: 1.0 equiv. 5, 0.9-1.3 equiv. 4, 3.5-4.2 mol% Pd(PPh3)4, 1.3-1.65 equiv. Ba(OH)2 * 8 H2O, DME/H2O, 80 °C, 24 h. [a] reaction conditions: 1.6 equiv. 4n, 8.2% Pd(PPh3)4, 2.15 equiv. Na2CO3, toluene/EtOH/H2O, 85 °C, 18 h. [b] reaction conditions: 5.8% Pd(PPh3)4, 4.6 equiv. Na2CO3, DME/H2O, 80 °C, 24 h.
Taking into account our original research interest, the majority of the target compounds 1a-w as well as 2a-h contain a fluorinated aliphatic group for which the analogue radioactive form can be expected to be synthesized rapidly in one-step radiolabelling by commonly used nucleophilic F-18 fluorination of corresponding bromo or nitro precursors.
In vitro enzymatic inhibitory assays
For the evaluation of in vitro inhibitory activities of the compounds against either human CYP11B1 or CYP11B2 we applied assays of Y1 cells stably transfected with one of the two genes (Table 1). The cell line Y1 is derived from a murine adrenal adenocarcinoma with naturally low expression levels of both murine CYP11B enzymes and is therefore ideally suited for such investigations. FAD286 was used as positive control agent and displayed inhibitory activities for CYP11B1 and CYP11B2 comparable to reported data, with a selectivity factor of 5.8 [54]. Several of the novel tested compounds were potent and selective CYP11B2 inhibitors, and as expected, the position of substituents in both aryl moieties is one of the decisive factors. In this context, the pyridyl substituted naphthalenes 1c, 1d, 1e and 1q with substituents in 4-position of the pyridine ring showed moderate affinities and no or only very low selectivities towards CYP11B1. Contrarily, substitution in the 5-position provided many compounds exhibiting good or even excellent inhibitory activities at the target enzyme CYP11B2, with 1b being the most potent (IC50 = 6.0 nM). Moreover, heteroaromatic moieties in the 5-position, even with sterically demanding residues, led to reasonable affinities and very good selectivities (1i, 1w). On the other hand, for teraryl derivatives 1x and 1y, representative for compounds with an ortho substituted pyridine ring, no inhibition effect on CYP11B1 could be observed, but the affinities for CYP11B2 were also considerably diminished. Furthermore, replacement of the substituent in 5-position by an annulated aromatic fragment in terms of a isoquinoline resulted in a good IC50 value and strong selectivity for CYP11B2 (1s). The attachment of an additional chain in 3-position of the naphthalene unit, however, caused a significant loss of potency for compounds 1aa and 1o. Regarding the electronic properties of the substituents, a similar tendency was disclosed as for related CYP11B2 inhibitors previously [7,39,55]. Electron-donating residues obviously promote CYP11B2 inhibitory activity, and the best results were achieved for derivatives bearing an electron-donating chain both in 5-position of the pyridine ring and in 6-position of the naphthalene moiety. The affinity and selectivity values are preserved even after a mutual exchange of both chains as demonstrated in the case of naphthyl pyridines 1r and 1j, whereas an exchange of the methoxy group of compound 1r by a cyano substituent decreased both the affinity and the selectivity for 1p towards CYP11B2 confirming the unfavorable effect of electron-withdrawing substituents. The 3,4-dihydro-1H-quinolin-2-ones also exhibited a broad range of IC50 values. Isoquinolyl substituted compounds 2a-c were characterized by a low CYP11B2 inhibition and were only poorly selective towards CYP11B1. As already found for the analogous naphthyl pyridines described above, in particular the alkyl chain in 7-position ortho to the isoquinoline moiety induced almost a complete loss of enzyme inhibition and selectivity. Such an analogy of comparatively low potency could also be determined for compound 2h with a 4-substituted pyridine ring. Likewise the inhibitory effects of compounds 2d-g on CYP11B2 were moderate to very good and partially outstanding selective towards CYP11B1, and therefore consistent with recent studies on 3,5-disubstituted pyridines as well as on 8-chloro-substituted 3,4-dihydro-1H-quinolin-2-ones [7,39]. Encouraged by the good results of several compounds in the inhibition experiments on the transfected Y1 cells, we further assessed the potency of some of the best derivatives on NCI-H295 cells (Table 2). This human cell line is considered a standard tool for adrenal cortical research issues, since it contains the whole cascade of enzymes for producing all adrenocortical steroids [56,57]. In comparison to the transfected Y1 cells, the selectivities towards CYP11B1 were somewhat lower, but binding affinities on CYP11B2 remained essentially on the same level or increased up to the subnanomolar IC50 value for compound 1a.
|
Com-pound |
IC50 (nM)[a] |
sf[b] |
Com-pound |
IC50 (nM)[a] |
sf[b] |
|||
|
CYP11B1 |
CYP11B2 |
CYP11B1 |
CYP11B2 |
|||||
|
1a |
1237 ± 379 |
14.2 ± 4.3 |
87.4 |
1s |
1375 ± 534 |
15.4 ± 3.7 |
89.3 |
|
|
1b |
514 ± 557 |
6.0 ± 4.2 |
86.2 |
1t |
1185 ± 398 |
9.8 ± 3.5 |
121 |
|
|
1c |
56.0 ± 11.6 |
23.6 ± 7.7 |
2.4 |
1u |
241 ± 578 |
7.1 ± 1.2 |
33.8 |
|
|
1d |
2900 |
395 ± 454 |
7.4 |
1v |
585 ± 249 |
36.4± 24.3 |
16.1 |
|
|
1e |
16.4 |
60.4±77.1 |
0.27 |
1w |
1460 ± 181 |
24.9 ± 3.2 |
58.6 |
|
|
1f |
1020 |
105± 24.8 |
9.8 |
1x |
> 5000 |
259 ± 344 |
- |
|
|
1g |
1380± 53.7 |
87.2 |
15.8 |
1y |
> 10000 |
70.0± 21.4 |
- |
|
|
1h |
850 ± 11.6 |
23.2±33.9 |
36.6 |
1aa |
520 ± 683 |
> 10000 |
- |
|
|
1i |
> 1000 |
29.2±23.8 |
- |
2a |
2200 ± 807 |
82.9± 19.5 |
26.5 |
|
|
1j |
1620 ± 151 |
35.0±14.8 |
46.3 |
2b |
> 10000 |
708 ± 356 |
- |
|
|
1k |
1430 ± 149 |
25.2 ± 5.5 |
56.7 |
2c |
2400 ± 1743 |
1100± 531 |
2.2 |
|
|
1m |
581 ± 820 |
480 ± 373 |
1.2 |
2d |
856 ± 320 |
24.4± 17.5 |
38.2 |
|
|
1o |
> 5000 |
210 |
- |
2e |
601 ± 268 |
8.8 ± 9.9 |
68.3 |
|
|
1p |
1130 ± 232 |
76.6±25.6 |
14.9 |
2f |
> 5000 |
61.9± 18.0 |
- |
|
|
1q |
56.0 ± 35.0 |
119 ± 165 |
0.47 |
2g |
655 ± 492 |
15.7± 12.4 |
41.7 |
|
|
1r |
> 1000 |
21.8 ± 4.1 |
- |
2h |
1380 ± 1937 |
192 ± 263 |
7.2 |
|
|
FAD286[c] |
79.1 |
13.5 |
5.8 |
|||||
[a] IC50 values determined from n ≥ 1; for compounds with n > 1 the mean value ± standard deviation is given. [b] sf: selectivity factor = IC50(CYP11B1) / IC50(CYP11B2). [c] FAD286: R-enantiomer of fadrozole.
Table 1: Inhibitory potency (IC50 values for inhibition of CYP11B1 and CYP11B2) and selectivity factors of selected compounds in Y1 cells stably transfected with either human CYP11B1 or human CYP11B2.
|
Com-pound |
IC50 (nM)[a] |
sf[b] |
Com-pound |
IC50 (nM)[a] |
sf[b] |
|||
|
CYP11B1 |
CYP11B2 |
CYP11B1 |
CYP11B2 |
|||||
|
1r |
163 ± 73.6 |
31.3 ± 6.5 |
5.2 |
1a |
18.9 ± 15.8 |
0.95 ± 0.25 |
19.8 |
|
|
1s |
1094± 75.6 |
17.0 ± 6.2 |
64 |
1b |
90.8 ± 23.0 |
8.8 ± 4.8 |
10.3 |
|
|
1t |
435 ± 199 |
64.8 ± 14.6 |
6.7 |
2a |
1260±1330 |
172 ± 43.8 |
7.3 |
|
|
1u |
128 ± 102 |
5.6 ± 1.4 |
23 |
2g |
958 ± 144 |
9.5 ± 7.4[c] |
101 |
|
|
FAD286[d] |
102 ± 38.1 |
50.1 ± 4.4 |
2.0 |
|||||
[a] Mean value ± standard deviation of at least three experiments. [b] sf: selectivity factor = IC50(CYP11B1) / IC50(CYP11B2). [c] n = 2. [d] FAD286: R-enantiomer of fadrozole.
Table 2: Inhibitory potency (IC50 values for inhibition of CYP11B1 and CYP11B2) and selectivity factors of selected compounds in NCI-H295 cells expressing CYP11B1 and CYP11B2.
Materials and Methods
General Chemistry and analytical methods
All chemicals and solvents were purchased from commercial sources (Sigma-Aldrich, Deisenhofen, Germany; Merck, Darmstadt, Germany; ChemPUR, Karlsruhe, Germany) and were used without further purification. The progress of chemical reactions was monitored by thin layer chromatography (TLC) on precoated 0.2 mm silica gel 60 sheets (Macherey-Nagel, Düren, Germany). Developed TLC sheets were visualized with UV light at 254 nm. Separations of organic products by column chromatography were carried out with silica gel 60 (0.03-0.2 mm, Carl Roth, Karlsruhe, Germany). 1H-NMR spectra were recorded on a 250 MHz Bruker NMR spectrometer with tetramethylsilane as internal standard. The chemical shifts δ are presented in parts per million (ppm) using the residual protic solvent as internal reference: CDCl3 (δ = 7.26). Signal multiplicities are characterized as follows: s = singlet, d = doublet, t = triplet, q =quartet, m = multiplet. Mass spectra were obtained on a Bruker Daltonics microtof focus. Melting points were measured on a Büchi Melting Point B-540 apparatus and are uncorrected.
Synthesis of the fluorinated target compounds
General procedure for Suzuki coupling
A suspension of an aryl halide or trifluoromethanesulfonate, an aryl boronic acid or ester, and a base in an appropriate solvent (A: Ba(OH)2 * 8 H2O in DME/H2O; B: Na2CO3 in toluene/EtOH/H2O; C: Na2CO3 in DME/H2O; D: Na2CO3 in MeOH/H2O) was degassed by an argon stream for 15 min. After addition of the catalyst Pd(PPh3)4 the reaction mixture was stirred at elevated temperature for 3-24 h under an argon atmosphere, then cooled to room temperature and the reaction solvent was removed in vacuum. The residue was reconstituted in H2O, the resulting mixture was extracted several times (solvent noted) and the combined organic phases were dried over Na2SO4 and concentrated under reduced pressure. Purification of the residue by column chromatography on silica gel afforded the desired compounds.
Synthesis of Naphthalene Compounds 1a-aa
5-[6-(2-Fluoroethoxy)-2-naphthalenyl]-3-(methoxymethyl)pyridine (1a): Prepared according to the general procedure method A from 6-(2-Fluoroethoxy)-2-naphthaleneboronic acid (3a, 562 mg, 2.40 mmol), 3-Bromo-5-(methoxymethyl)pyridine (4a, 404 mg, 2.00 mmol), Ba(OH)2 * 8 H2O (948 mg, 3.00 mmol) and Pd(PPh3)4 (88.0 mg, 76.2 µmol) in DME (12 mL) and H2O (2 mL); reaction time: 24 h. After work-up (H2O (50 mL), extraction with CHCl3 (5 x 50 mL), column chromatography CH2Cl2/CH3OH = 98:2) compound 1a was obtained as a colorless solid (270 mg, 43%), mp: 111-113 °C. 1H-NMR (CDCl3, 250 MHz): δ = 3.47 (s, 3H, OCH3), 4.29-4.44 (m, 2H, OCH2), 4.57 (s, 2H, ArCH2O), 4.74-4.96 (m, 2H, CH2F), 7.17-7.28 (m, 2H), 7.68-7.73 (m, 1H), 7.81-7.87 (m, 2H), 7.97-8.02 (m, 2H), 8.56 (m, 1H), 8.89 (m, 1H). MS (DIP-APCI, +): m/z (%): calcd. for C19H19FNO2 [M+H]+: 312.1394; found: 312.1396 ([M+H]+).
5-[6-(2-Fluoroethoxy)-2-naphthalenyl]-3-(1-methoxyethyl)pyridine (1b): Prepared according to the general procedure method A from 6-(2-Fluoroethoxy)-2-naphthaleneboronic acid (3a, 562 mg, 2.40 mmol), 3-Bromo-5-(1-methoxyethyl)pyridine (4b, 432 mg, 2.00 mmol), Ba(OH)2 . 8 H2O (948 mg, 3.00 mmol) and Pd(PPh3)4 (88.0 mg, 76.2 µmol) in DME (12 mL) and H2O (2 mL); reaction time: 24 h. After work-up (H2O (50 mL), extraction with CHCl3 (5 x 50 mL), column chromatography CH2Cl2/CH3OH = 98:2) compound 1b was obtained as a yellowish solid (97.4 mg, 15%). 1H-NMR (CDCl3, 250 MHz): δ = 1.54 (d, 3J = 7.6 Hz, 3H, CHCH3), 3.32 (s, 3H, OCH3), 4.29-4.48 (m, 3H, OCH2 and CHCH3 superimposed), 4.74-4.96 (m, 2H, CH2F), 7.17-7.28 (m, 2H), 7.63-7.73 (m, 2H), 7.82-7.87 (m, 2H), 7.94-7.98 (m, 1H), 8.01 (m, 1H), 8.53 (m, 1H), 8.89 (m, 1H). MS (DIP-APCI, +): m/z (%): calcd. for C20H21FNO2 [M+H]+: 326.1551; found: 326.1556 ([M+H]+).
3-[6-(2-Fluoroethoxy)-2-naphthalenyl]-4-methylpyridine (1c): Prepared according to the general procedure method A from 6-(2-Fluoroethoxy)-2-naphthaleneboronic acid (3a, 281 mg, 1.20 mmol), 3-Bromo-4-methylpyridine (4c, 172 mg, 111 µL, 1.00 mmol), Ba(OH)2 . 8 H2O (474 mg, 1.50 mmol) and Pd(PPh3)4 (44.0 mg, 38.1 µmol) in DME (6 mL) and H2O (1 mL); reaction time: 24 h. After work-up (H2O (50 mL), extraction with CHCl3 (5 x 30 mL), column chromatography CH2Cl2/CH3OH = 98:2) compound 1c was obtained as a yellow oil (46.0 mg, 16%). 1H-NMR (CDCl3, 250 MHz): δ = 2.35 (s, 3H, CH3), 4.30-4.45 (m, 2H, OCH2), 4.74-4.97 (m, 2H, CH2F), 7.20 (m, 1H), 7.23-7.28 (m, 2H), 7.41 (dd, 3J = 8.4 Hz, 4J = 1.8 Hz, 1H), 7.72 (m, 1H), 7.79 (m, 1H), 7.83 (m, 1H), 8.48 (d, 3J = 5.2 Hz, 1H), 8.53 (m, 1H). MS (DIP-APCI, +): m/z (%): calcd. for C18H17FNO [M+H]+: 282.1289; found: 282.1290 ([M+H]+).
3-[6-(2-Fluoroethoxy)-2-naphthalenyl]-4-(1-pyrrolidinyl)pyridine (1d): Prepared according to the general procedure method A from 6-(2-Fluoroethoxy)-2-naphthaleneboronic acid pinacol ester (3b, 316 mg, 1.00 mmol), 3-Bromo-4-(1-pyrrolidinyl)pyridine (4d, 227 mg, 1.00 mmol), Ba(OH)2 . 8 H2O (430 mg, 1.36 mmol) and Pd(PPh3)4 (42.0 mg, 36.3 µmol) in DME (5 mL) and H2O (0.9 mL); reaction time: 24 h. After work-up (H2O (200 mL), extraction with CHCl3 (3 x 50 mL), column chromatography CH2Cl2/CH3OH = 98:2) compound 1d was obtained as a brown oil (107 mg, 32%). 1H-NMR (CDCl3, 250 MHz): δ = 1.72-1.78 (m, 2H, NCH2CH2), 2.96-3.03 (m, 2H, NCH2), 4.28-4.43 (m, 2H, OCH2), 4.73-4.95 (m, 2H, CH2F), 6.60 (d, 3J = 6.0 Hz, 1H), 7.17 (m, 1H), 7.24 (dd, 3J = 8.9 Hz, 4J = 2.5 Hz, 1H, superimposed with CDCl3 residual peak), 7.44 (dd, 3J = 8.5 Hz, 4J = 1.8 Hz, 1H), 7.69-7.74 (m, 2H), 7.77 (d, 3J = 9.0 Hz, 1H), 8.18 (s, 1H), 8.22 (d, 3J = 5.9 Hz, 1H). MS (DIP-APCI, +): m/z (%): calcd. for C21H22FN2O [M+H]+: 337.1711; found: 337.1718 ([M+H]+).
2-[6-(2-Fluoroethoxy)-2-naphthalenyl]isonicotinic acid pyrrolidinyl amide (1e): Prepared according to the general procedure method A from 6-(2-Fluoroethoxy)-2-naphthaleneboronic acid pinacol ester (3b, 316 mg, 1.00 mmol), 3-Bromoisonicotinic acid pyrrolidinyl amide (4e, 230 mg, 902 µmol), Ba(OH)2 . 8 H2O (430 mg, 1.36 mmol) and Pd(PPh3)4 (42.0 mg, 36.3 µmol) in DME (5 mL) and H2O (0.9 mL); reaction time: 24 h. After work-up (H2O (200 mL), extraction with CHCl3 (3 x 50 mL), column chromatography CH2Cl2/CH3OH = 98:2) compound 1e was obtained as a yellow oil (116 mg, 35%). 1H-NMR (CDCl3, 250 MHz): δ = 1.38-1.65 (m, 4H, NCH2CH2), 2.71-2.77 (m, 2H, NCH2), 3.38-3.44 (m, 2H, NCH2), 4.29-4.43 (m, 2H, OCH2), 4.73-4.95 (m, 2H, CH2F), 7.17 (m, 1H), 7.25 (dd, 3J = 8.9 Hz, 4J = 2.6 Hz, 1H, superimposed with CDCl3 residual peak), 7.37 (m, 1H), 7.60 (dd, 3J = 8.4 Hz, 4J = 1.8 Hz, 1H), 7.77-7.85 (m, 2H), 7.92 (m, 1H), 8.67 (d, 3J = 4.9 Hz, 1H), 8.81 (s, 1H). MS (DIP-APCI, +): m/z (%): calcd. for C22H22FN2O2 [M+H]+: 365.1660; found: 365.1669 ([M+H]+).
5-[6-(2-Fluoroethoxy)-2-naphthalenyl]-3-(1-pyrrolidinylmethyl)pyridine (1f): Prepared according to the general procedure method A from 6-(2-Fluoroethoxy)-2-naphthaleneboronic acid pinacol ester (3b, 152 mg, 481 µmol), 3-Bromo-5-(1-pyrrolidinylmethyl)pyridine (4f, 116 mg, 481 µmol), Ba(OH)2 . 8 H2O (210 mg, 666 µmol) and Pd(PPh3)4 (20.2 mg, 17.5 µmol) in DME (3 mL) and H2O (0.5 mL); reaction time: 24 h. After work-up (H2O (200 mL), extraction with CHCl3 (3 x 50 mL), column chromatography hexane/EtOAc = 1:1) compound 1f was obtained as a brownish solid (88.0 mg, 52%). 1H-NMR (CDCl3, 250 MHz): δ = 1.79-1.85 (m, 4H, NCH2CH2), 2.55-2.61 (m, 4H, NCH2CH2), 3.73 (s, 2H, ArCH2N), 4.28-4.43 (m, 2H, OCH2), 4.73-4.95 (m, 2H, CH2F), 7.16 (m, 1H), 7.24 (dd, 3J = 9.0 Hz, 4J = 2.8 Hz, 1H, superimposed with CDCl3 residual peak), 7.71 (dd, 3J = 8.5 Hz, 4J = 1.9 Hz, 1H), 7.80-7.85 (m, 2H), 7.99-8.02 (m, 2H), 8.52 (d, 4J = 2.1 Hz, 1H), 8.84 (d, 4J = 2.5 Hz, 1H). MS (DIP-APCI, +): m/z (%): calcd. for C22H24FN2O [M+H]+: 351.1867; found: 351.1884 ([M+H]+).
3-[6-(2-Fluoroethoxy)-2-naphthalenyl]-5-(1-pyrrolidinyl)pyridine (1g): Prepared according to the general procedure method A from 6-(2-Fluoroethoxy)-2-naphthaleneboronic acid pinacol ester (3b, 316 mg, 1.00 mmol), 3-Bromo-5-(1-pyrrolidinyl)pyridine (4g, 227 mg, 1.00 mmol), Ba(OH)2 . 8 H2O (430 mg, 1.36 mmol) and Pd(PPh3)4 (42.0 mg, 36.3 µmol) in DME (5 mL) and H2O (0.9 mL); reaction time: 24 h. After work-up (H2O (200 mL), extraction with CHCl3 (3 x 50 mL), column chromatography hexane/EtOAc = 1:1 → 1:4) compound 1g was obtained as a yellowish solid (200 mg, 59%), mp: 153-155 °C. 1H-NMR (CDCl3, 250 MHz): δ = 2.04-2.11 (m, 2H, NCH2CH2), 3.37-3.43 (m, 2H, NCH2), 4.36 (m, 2H, OCH2), 4.85 (m, 2H, CH2F), 7.11-7.27 (2m superimposed, 3H), 7.62-7.71 (m, 2H), 7.80-7.85 (m, 2H), 7.94-7.99 (m, 2H), 8.24 (m, 1H). MS (DIP-APCI, +): m/z (%): calcd. for C21H22FN2O [M+H]+: 337.1711; found: 337.1723 ([M+H]+).
3-(1,3-Dioxolan-2-yl)-5-[6-(2-fluoroethoxy)-2-naphthalenyl]pyridine (1h): Prepared according to the general procedure method A 6-(2-Fluoroethoxy)-2-naphthaleneboronic acid pinacol ester (3b, 632 mg, 2.00 mmol), 3-Bromo-5-(1,3-dioxolan-2-yl)pyridine (4h, 460 mg, 2.00 mmol), Ba(OH)2 . 8 H2O (860 mg, 2.73 mmol) and Pd(PPh3)4 (84.0 mg, 72.7 µmol) in DME (10 mL) and H2O (1.8 mL); reaction time: 24 h. After work-up (H2O (200 mL), extraction with CHCl3 (3 x 50 mL), column chromatography heptane/EtOAc = 1:1) compound 1h was obtained as a yellowish oil (172 mg, 25%). 1H-NMR (CDCl3, 250 MHz): δ = 4.11-4.19 (m, 4H, OCH2CH2O), 4.30-4.45 (m, 2H, OCH2CH2F), 4.74-4.97 (m, 2H, CH2F), 5.99 (s, 1H, OCHO), 7.19 (m, 1H), 7.27 (dd, 3J = 8.9 Hz, 4J = 2.6 Hz, 1H, superimposed with CDCl3 residual peak), 7.70 (dd, 3J = 8.5 Hz, 4J = 1.8 Hz, 1H), 7.86 (2d superimposed, 3J = 8.9 Hz, 3J = 8.4 Hz, 2H), 8.02 (m, 1H), 8.25 (m, 1H), 8.72 (m, 1H), 8.97 (d, 4J = 2.1 Hz, 1H). MS (DIP-APCI, +): m/z (%): calcd. for C20H19FNO3 [M+H]+: 340.1364; found: 340.1344 ([M+H]+).
3-(1,3-Dimethyl-1,2,4-triazol-5-yl)-5-[6-(2-fluoroethoxy)-2-naphthalenyl]pyridine (1i): Prepared according to the general procedure method A from 6-(2-Fluoroethoxy)-2-naphthaleneboronic acid pinacol ester (3b, 316 mg, 1.00 mmol), 3-Bromo-5-(1,3-dimethyl-1,2,4-triazol-5-yl)pyridine (4i, 196 mg, 774 µmol), Ba(OH)2 . 8 H2O (430 mg, 1.36 mmol) and Pd(PPh3)4 (42.0 mg, 36.3 µmol) in DME (5 mL) and H2O (1 mL); reaction time: 24 h. After work-up (H2O (100 mL), extraction with CHCl3 (3 x 50 mL), column chromatography heptane/EtOAc = 1:1) compound 1i was obtained as a colorless solid (130 mg, 46%), mp: 154-157 °C. 1H-NMR (CDCl3, 250 MHz): δ = 2.47 (s, 3H, CCH3), 4.03 (s, 3H, NCH3), 4.30-4.45 (m, 2H, OCH2), 4.75-4.97 (CH2F), 7.19 (m, 1H), 7.28 (dd, 3J = 9.0 Hz, 4J = 2.6 Hz, 1H, superimposed with CDCl3 residual peak), 7.73 (dd, 3J = 8.5 Hz, 4J = 2.0 Hz, 1H), 7.86 (2d superimposed, 3J = 9.0 Hz, 3J = 8.5 Hz, 2H), 8.06 (m, 1H), 8.34 (m, 1H), 8.87 (m, 1H), 9.07 (d, 4J = 1.8 Hz, 1H). MS (DIP-APCI, +): m/z (%): calcd. for C21H20FN4O [M+H]+: 363.1616; found: 363.1622 ([M+H]+).
5-[6-(2-Fluoroethoxy)-2-naphthalenyl]-3-methoxypyridine (1j): Prepared according to the general procedure method A from 6-(2-Fluoroethoxy)-2-naphthaleneboronic acid pinacol ester (3b, 316 mg, 1.00 mmol), 3-Bromo-5-methoxypyridine (4j, 188 mg, 1.00 mmol), Ba(OH)2 . 8 H2O (430 mg, 1.36 mmol) and Pd(PPh3)4 (42.0 mg, 36.3 µmol) in DME (5 mL) and H2O (1 mL); reaction time: 24 h. After work-up (H2O (100 mL), extraction with CHCl3 (3 x 50 mL), column chromatography heptane/EtOAc = 1:1) compound 1j was obtained as a beige solid (36.0 mg, 12%), mp: 117-120 °C. 1H-NMR (CDCl3, 250 MHz): δ = 3.95 (s, 3H, OCH3), 4.29-4.43 (m, 2H, OCH2), 4.73-4.96 (m, 2H, CH2F), 7.18 (m, 1H), 7.25 (dd, 3J = 8.9 Hz, 4J = 2.7 Hz, 1H, superimposed with CDCl3 residual peak), 7.47 (dd, 4J = 2.8 Hz, 4J = 1.9 Hz, 1H), 7.73 (dd, 3J = 8.4 Hz, 4J = 1.8 Hz, 1H), 7.81 (m, 1H), 7.85 (m, 1H), 7.97 (d, 4J = 1.5 Hz, 1H), 8.31 (d, 4J = 2.9 Hz, 1H), 8.57 (d, 4J = 1.8 Hz, 1H). MS (DIP-APCI, +): m/z (%): calcd. for C18H17FNO2 [M+H]+: 298.1238; found: 298.1248 ([M+H]+).
5-[6-(2-Fluoroethoxy)-2-naphthalenyl]-3-(methylthio)pyridine (1k): Prepared according to the general procedure method A from 6-(2-Fluoroethoxy)-2-naphthaleneboronic acid pinacol ester (3b, 316 mg, 1.00 mmol), 3-Bromo-5-(methylthio)pyridine (4k, 204 mg, 1.00 mmol), Ba(OH)2 . 8 H2O (430 mg, 1.36 mmol) and Pd(PPh3)4 (42.0 mg, 36.3 µmol) in DME (5 mL) and H2O (1 mL); reaction time: 24 h. After work-up (H2O (100 mL), extraction with CHCl3 (3 x 50 mL), column chromatography heptane/EtOAc = 1:1) compound 1k was obtained as a greyish solid (102 mg, 33%), mp: 118-121 °C. 1H-NMR (CDCl3, 250 MHz): δ = 2.59 (s, 3H, SCH3), 4.29-4.44 (m, 2H, OCH2), 4.74-4.96 (m, 2H, CH2F), 7.18 (m, 1H), 7.26 (dd, 3J = 8.9 Hz, 4J = 2.4 Hz, 1H, superimposed with CDCl3 residual peak), 7.66 (dd, 3J = 8.5 Hz, 4J = 1.8 Hz, 1H), 7.81-7.87 (m, 3H), 7.97 (m, 1H), 8.49 (m, 1H), 8.71 (m, 1H). MS (DIP-APCI, +): m/z (%): calcd. for C18H16FNOS [M+H]+: 314.1009; found: 314.1013 ([M+H]+).
3-Bromo-5-(6-methoxy-2-naphthalenyl)pyridine (1l): Prepared according to the general procedure method A from 6-Methoxynaphthalene-2-boronic acid (3c, 2.32 g, 11.48 mmol), 3,5-Dibromopyridine (4l, 2.72 g, 11.48 mmol), Ba(OH)2 . 8 H2O (4.95 g, 15.67 mmol) and Pd(PPh3)4 (482 mg, 417 µmol) in DME (68 mL) and H2O (11 mL); reaction time: 24 h. After work-up (H2O (100 mL), extraction with CH2Cl2 (3 x 100 mL), column chromatography (CH2Cl2/CH3OH = 98:2) compound 1l was obtained as a yellowish solid (687 mg, 38%), mp: 160-162 °C. 1H-NMR (CDCl3, 250 MHz): δ = 3.96 (s, 3H, OCH3), 7.17-7.24 (m, 2H), 7.62-7.67 (dd, 3J = 8.5 Hz, 4J = 2.1 Hz, 1H), 7.80-7.88 (m, 2H), 7.96 (m, 1H), 8.13-8.15 (m, 1H), 8.66 (d, 3J = 2.3 Hz, 1H), 8.87 (d, 3J = 2.0 Hz, 1H).
5-[6-(2-Fluoroethoxy)-2-naphthalenyl]nicotinic acid pyrrolidinyl amide (1m): Prepared according to the general procedure method B from 6-(2-Fluoroethoxy)-2-naphthaleneboronic acid pinacol ester (3b, 632 mg, 2.00 mmol), 3-Bromonicotinic acid pyrrolidinyl amide (4m, 510 mg, 2.00 mmol), Na2CO3 (456 mg, 4.30 mmol) and Pd(PPh3)4 (180 mg, 156 µmol) in toluene (18 mL), EtOH (3 mL) and H2O (3 mL). After work-up (H2O (50 mL), extraction with CHCl3 (5 x 30 mL), column chromatography hexane/EtOAc = 1:1) compound 1m was obtained as brownish solid (88.0 mg, 12%), mp: 152-154 °C. 1H-NMR (CDCl3, 250 MHz): δ = 1.92-2.04 (m, 4H, NCH2CH2), 3.54 (m, 2H, NCH2), 3.70 (m, 2H, NCH2), 4.29-4.44 (m, 2H, OCH2), 4.74-4.96 (m, 2H, CH2F), 7.18 (m, 1H), 7.26 (dd, 3J = 9.0 Hz, 4J = 2.6 Hz, 1H, superimposed with CDCl3 residual peak), 7.69 (dd, 3J = 8.5 Hz, 4J = 1.8 Hz, 1H), 7.81-7.86 (m, 2H), 8.01 (m, 1H), 8.17 (m, 1H), 8.75 (m, 1H), 9.00 (m, 1H). MS (DIP-APCI, +): m/z (%): calcd. for C22H22FN2O2 [M+H]+: 365.1660; found: 365.1665 ([M+H]+).
Methyl 3-(4-isoquinolinyl)-7-methoxy-2-naphthoate (1n): Prepared according to the general procedure method B from Methyl 7-methoxy-3-(trifluoromethylsulfonyloxy)-2-naphthoate (3d, 1.00 g, 2.75 mmol), Isoquinoline-4-boronic acid (4n, 645 mg, 3.73 mmol), Na2CO3 (530 mg, 5.00 mmol) and Pd(PPh3)4 (220 mg, 191 µmol) in a mixture of toluene (20 mL), EtOH (5 mL) and H2O (5 mL); reaction time: 24 h. After work-up (H2O (200 mL), extraction with CHCl3 (5 x 100 mL), column chromatography CH2Cl2/CH3OH = 98:2) compound 1n was obtained as a colorless solid (826 mg, 88%). 1H-NMR (CDCl3, 250 MHz): δ = 3.43 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 7.27-7.32 (m, 2H), 7.52-7.61 (m, 3H), 7.74-7.79 (m, 2H), 7.99-8.06 (m, 1H), 8.47 (s, 1H), 8.56 (s, 1H), 9.28 (s, 1H).
2-Fluoroethyl 3-(4-isochinolinyl)-7-methoxy-2-naphthoate (1o): Prepared according to the general procedure method B from 2-Fluoroethyl 7-methoxy-3-(trifluoromethylsulfonyl)-2-naphthoate (3e, 250 mg, 630 µmol), Isoquinoline-4-boronic acid (4n, 174 mg, 1.00 mmol), Na2CO3 (143 mg, 1.36 mmol) and Pd(PPh3)4 (60.0 mg, 51.0 µmol) in toluene (6 mL), EtOH (1 mL) and H2O (1 mL); reaction time: 24 h. After work-up (H2O (50 mL), extraction with CHCl3 (3 x 25 mL), column chromatography (first column: CH2Cl2/CH3OH = 98:2, second column: hexane/EtOAc = 1:4)) compound 1o was obtained as a colorless solid (128 mg, 54%), mp: 119-121 °C. 1H-NMR (CDCl3, 250 MHz): δ = 3.98-4.07 (m, 2H, OCH2) superimposed with 3.99 (s, 3H, OCH3), 4.13-4.22 (m, 2H, CH2F), 7.29-7.35 (m, 2H), 7.50-7.63 (m, 3H), 7.77-7.82 (m, 2H), 8.02-8.08 (m, 1H), 8.49 (s, 1H), 8.61 (s, 1H), 9.30 (s, 1H). MS (EI): m/z (%): calcd. for C23H18FNO3 [M]+.: 375.1; found: 375.1 (100, [M]+).
3-[6-Cyano-2-naphthalenyl]-5-(2-fluoroethoxy)pyridine (1p): Prepared according to the general procedure method C from 6-Cyano-2-naphthalenyl trifluoromethanesulfonate (3f, 301 mg, 1.00 mmol), 5-(2-Fluoroethoxy)-3-pyridineboronic acid (4o, 185 mg, 1.00 mmol), Na2CO3 (235 mg, 2.22 mmol) and Pd(PPh3)4 (24.0 mg, 20.8 µmol) in DME (11 mL) and H2O (1 mL); reaction time: 3 h. After work-up (H2O (50 mL), extraction with EtOAc (3 x 40 mL), column chromatography hexane/EtOAc = 1:1) compound 1p was obtained as a colorless solid (157 mg, 63%), mp: 139-143 °C. 1H-NMR (CDCl3, 250 MHz): δ = 4.32-4.46 (m, 2H, OCH2), 4.72-4.94 (m, 2H, CH2F), 7.55 (dd, 4J = 2.8 Hz, 4J = 1.9 Hz, 1H), 7.67 (dd, 3J = 8.5 Hz, 4J = 1.6 Hz, 1H), 7.83 (dd, 3J = 8.7 Hz, 4J = 1.8 Hz, 1H), 7.97-8.04 (m, 2H), 8.08 (m, 1H), 8.28 (m, 1H), 8.40 (d, 4J = 2.8 Hz, 1H), 8.62 (d, 4J = 1.8 Hz, 1H). MS (DIP-APCI, +): m/z (%): calcd. for C18H14FN2O [M+H]+: 293.1085; found: 293.1099 ([M+H]+).
4-Fluoro-3-(6-methoxy-2-naphthalenyl)pyridine (1q): Prepared according to the general procedure method C from 6-Methoxynaphthalene-2-boronic acid (3c, 289 mg, 1.43 mmol), 3-Bromo-4-fluoropyridine (4p, 199 mg, 1.13 mmol), Na2CO3 (509 mg, 4.80 mmol) and Pd(PPh3)4 (71.0 mg, 61.4 µmol) in DME (7 mL) and H2O (2 mL); reaction time: 18 h. After work-up (H2O (50 mL), extraction with methyl tert-butyl ether (3 x 25 mL), column chromatography heptane/EtOAc = 1:1) compound 1q was obtained as a greyish solid (230 mg, 80%), mp: 134-136 °C. 1H-NMR (CDCl3, 250 MHz): δ = 3.95 (s, 3H, OCH3), 7.01-7.06 (m, 1H), 7.15-7.24 (m, 2H), 7.62 (dd, 3J = 8.5 Hz, 4J = 2.0 Hz, 1H), 7.77-7.87 (m, 2H), 7.92 (m, 1H), 8.02-8.11 (m, 1H), 8.50-8.54 (m, 1H). MS (DIP-APCI, +): m/z (%): calcd. for C16H13FNO [M+H]+: 254.0976; found: 254.0975 ([M+H]+).
3-(2-Fluoroethoxy)-5-(6-methoxy-2-naphthalenyl)pyridine (1r): Prepared according to the general procedure method D from 6-Methoxynaphthalene-2-boronic acid (3c, 642 mg, 3.18 mmol), 3-Bromo-5-(2-fluoroethoxy)pyridine (4q, 605 mg, 2.75 mmol), Na2CO3 (2.10 g, 19.81 mmol) and Pd(PPh3)4 (137 mg, 119 µmol) in MeOH (31 mL) and H2O (9 mL); reaction time: 24 h. After work-up (H2O (5 mL), extraction with CH2Cl2 (3 x 50 mL), column chromatography CH2Cl2/CH3OH = 100:0 → 98:2) compound 1r was obtained as a colorless solid (669 mg, 82%), mp: 139-141 °C. 1H-NMR (CDCl3, 250 MHz): δ = 3.95 (s, 3H, OCH3), 4.30-4.44 (m, 2H, OCH2), 4.71-4.94 (m, 2H, CH2F), 7.16-7.23 (m, 2H), 7.51-7.54 (m, 1H), 7.67 (dd, 3J = 8.5 Hz, 4J = 1.8 Hz, 1H), 7.79-7.86 (m, 2H), 7.97 (m, 1H), 8.34 (d, 4J = 2.7 Hz, 1H), 8.61 (d, 4J = 1.6 Hz, 1H). MS (DIP-APCI, +): m/z (%): calcd. for C18H17FNO2 [M+H]+: 298.1238; found: 298.1246 ([M+H]+).
4-[6-(2-Fluoroethoxy)-2-naphthalenyl]isoquinoline (1s): Prepared according to the general procedure method D from 2-Bromo-6-(2-fluoroethoxy)naphthalene (3g, 778 mg, 2.89 mmol), Isoquinoline-4-boronic acid (4n, 500 mg, 2.89 mmol), Na2CO3 (1.90 g, 17.93 mmol) and Pd(PPh3)4 (125 mg, 108 µmol) in MeOH (27 mL) and H2O (8 mL); reaction time: 24 h. After work-up (H2O (100 mL), extraction with CH2Cl2 (3 x 80 mL), column chromatography hexane/CH2Cl2 = 1:1 → 100:0) compound 1s was obtained as a brownish solid (132 mg, 14%), mp: 107 °C. 1H-NMR (CDCl3, 250 MHz): δ = 4.32-4.47 (m, 2H, OCH2), 4.76-4.98 (m, 2H, CH2F), 7.23-7.31 (m, 2H), 7.59-7.74 (m, 3H), 7.82-8.00 (m, 4H), 8.04-8.09 (m, 1H), 8.58 (s, 1H), 9.29 (s, 1H). MS (DIP-APCI, +): m/z (%): calcd. for C21H17FNO2 [M+H]+: 318.1289; found: 318.1295 ([M+H]+).
3-(2-Fluoroethoxymethyl)-5-(6-methoxy-2-naphthalenyl)pyridine (1t): Prepared according to the general procedure method D from 6-Methoxynaphthalene-2-boronic acid (3c, 345 mg, 1.71 mmol), 3-Bromo-5-(2-fluoroethoxymethyl)pyridine (4r, 400 mg, 1.71 mmol), Na2CO3 (1.17 g, 11.04 mmol) and Pd(PPh3)4 (77.0 mg, 66.6 µmol) in MeOH (17 mL) and H2O (5 mL); reaction time: 24 h. After work-up (H2O (100 mL), extraction with CH2Cl2 (3 x 80 mL), column chromatography CH2Cl2/CH3OH = 98:2) compound 1t was obtained as a colorless solid (393 mg, 74%), mp: 120-124 °C. 1H-NMR (CDCl3, 250 MHz): δ = 3.75-3.90 (m, 2H, OCH2), 3.95 (s, 3H, OCH3), 4.53-4.76 (m, 2H, CH2F) superimposed with 4.72 (s, 2H, ArCH2O), 7.16-7.23 (m, 2H), 7.70 (dd, 3J = 8.6 Hz, 4J = 1.9 Hz, 1H), 7.80-7.87 (m, 2H), 7.99-8.03 (m, 2H), 8.57 (m, 1H), 8.90 (m, 1H). MS (DIP-APCI, +): m/z (%): calcd. for C19H19FNO2 [M+H]+: 312.1394; found: 318.1399 ([M+H]+).
3-[1-(2-Fluoroethoxy)ethyl]-5-(6-methoxy-2-naphthalenyl)pyridine (1u): Prepared according to the general procedure method D from 6-Methoxynaphthalene-2-boronic acid (3c, 345 mg, 1.71 mmol), 3-Bromo-5-[1-(2-fluoroethoxy)ethyl]pyridine (4s, 170 mg, 685 µmol), Na2CO3 (1.17 g, 11.04 mmol) and Pd(PPh3)4 (77.0 mg, 66.6 µmol) in MeOH (17 mL) and H2O (5 mL); reaction time: 24 h. After work-up (H2O (100 mL), extraction with CH2Cl2 (3 x 80 mL), column chromatography CH2Cl2/CH3OH = 98:2) compound 3u was obtained as a colorless solid (117 mg, 52%), mp: 111 °C. 1H-NMR (CDCl3, 250 MHz): δ = 1.58 (d, 3J = 6.5 Hz, 3H, CHCH3), 3.51-3.78 (m, 2H, OCH2), 3.95 (s, 3H, OCH3), 4.47-4.69 (m, 3H, CH2F and CHCH3 superimposed), 7.17-7.24 (m, 2H), 7.68-7.73 (m, 1H), 7.80-7.89 (m, 2H), 7.99-8.04 (m, 2H), 8.53 (m, 1H), 8.89, 9.14 (m, 1H). MS (EI): m/z (%): calcd. for C20H20FNO2 [M]+.: 325.2; found: 325.1 (100, [M]+.).
3-(2-Fluoropropoxy)-5-(6-methoxy-2-naphthalenyl)pyridine (1v): Prepared according to the general procedure method D from 6-Methoxynaphthalene-2-boronic acid (3c, 321 mg, 1.59 mmol), 3-Bromo-5-(3-fluoropropoxy)pyridine (4t, 372 mg, 1.59 mmol), Na2CO3 (1.05 g, 9.97 mmol) and Pd(PPh3)4 (69.0 mg, 59.7 µmol) in MeOH (15 mL) and H2O (4 mL); reaction time: 24 h. After work-up (H2O (50 mL), extraction with CH2Cl2 (3 x 40 mL), column chromatography CH2Cl2/CH3OH = 100:0 → 95:5) compound 1v was obtained as a colorless solid (287 mg, 58%), mp: 130-131 °C. 1H-NMR (CDCl3, 250 MHz): δ = 2.14-2.34 (m, 2H, OCH2CH2), 3.95 (s, 3H, OCH3), 4.25 (t, 3J = 6.1 Hz, 2H, OCH2), 4.58-4.82 (dt, 2JF,H = 39.5 Hz, 3J = 6.1 Hz, 2H, CH2F), 7.16-7.23 (m, 2H), 7.50 (dd, 4J = 2.7 Hz, 4J = 1.8 Hz, 1H), 7.67 (dd, 3J = 8.6 Hz, 4J = 1.8 Hz, 1H), 7.79-7.86 (m, 2H), 7.97 (d, 4J = 2.0 Hz, 1H), 8.31 (d, 4J = 2.8 Hz, 1H), 8.61 (d, 4J = 1.7 Hz, 1H). MS (DIP-APCI, +): m/z (%): calcd. for C19H19FNO2 [M+H]+: 312.1394; found: 312.1399 ([M+H]+).
5-[6-(2-Fluoroethoxy)-2-naphthalenyl]-3-(1,3-oxazol-5-yl)pyridine (1w): A suspension of 6-(2-Fluoroethoxy)-2-naphthaleneboronic acid pinacol ester (3b, 357 mg, 1.13 mmol), 3-Bromo-5-(1,3-oxazol-5-yl)pyridine (4u, 254 mg, 1.13 mmol) and Ag2CO3 (345 mg, 1.25 mmol) in benzene (15 mL) was degassed with argon. Pd(PPh3)4 (78.0 mg, 67.5 µmol) was added and the reaction mixture was stirred under reflux for 24 h. After cooling to room temperature H2O (100 mL) was added and the mixture was extracted with methyl tert-butyl ether (3 x 50 mL). The combined organic phases were dried over Na2SO4 and the solvent was removed under reduced pressure. Purification of the residue by column chromatography (heptane/EtOAc = 1:1) yielded compound 1w as a colorless solid (64.0 mg, 17%), mp: 143-145 °C. 1H-NMR (CDCl3, 250 MHz): δ = 4.30-4.44 (m, 2H, OCH2), 4.74-4.96 (m, 2H, CH2F), 7.19 (d, 4J = 2.6 Hz, 1H), 7.27 (dd, 3J = 8.9 Hz, 4J = 2.5 Hz, 1H, superimposed with CDCl3 residual peak), 7.56 (s, 1H), 7.63 (d, 4J = 1.7 Hz, 1H), 7.86, 7.87 (2d superimposed, 3J = 9.0 Hz, 3J = 8.9 Hz, 2H), 8.03 (m, 2H), 8.24 (m, 1H), 8.91 (m, 2H). MS (EI): m/z (%): calcd. for C20H15FN2O2 [M]+.: 334.1; found: 334.1 (100, [M]+.).
5-(6-Methoxy-2-naphthalenyl)-3-(2-fluoro-3-pyridyl)pyridine (1x): Prepared according to the general procedure method D from 3-Bromo-5-(6-methoxy-2-naphthalenyl)pyridine (1l, 314 mg, 1.00 mmol), 2-Fluoropyridine-3-boronic acid (4v, 155 mg, 1.10 mmol), Na2CO3 (657 mg, 6.20 mmol) and Pd(PPh3)4 (45.0 mg, 38.9 µmol) in MeOH (10 mL) and H2O (3 mL); reaction time: 24 h. After work-up (H2O (50 mL), extraction with CH2Cl2 (3 x 40 mL), column chromatography (CH2Cl2/CH3OH = 98:2) compound 1x was obtained as a beige solid (267 mg, 81 %), mp: 192 °C. 1H-NMR (CDCl3, 250 MHz): δ = 3.96 (s, 3H, OCH3), 7.18-7.24 (m, 2H), 7.38 (ddd, 3J = 7.5 Hz, 3J = 4.9 Hz, 4J = 1.8 Hz, 1H), 7.72 (dd, 3J = 8.7 Hz, 4J = 1.8 Hz, 1H), 7.82-7.90 (m, 2H), 7.96-8.04 (m, 2H), 8.22 (dd, 3J = 4.0 Hz, 4J = 2.1 Hz, 1H), 8.31 (ddd, 3J = 4.9 Hz, 4J = 2.0 Hz, 5J = 1.2 Hz, 1H), 8.79 (m, 1H), 9.00 (d, 3J = 2.3 Hz, 1H). MS (DIP-APCI, +): m/z (%): calcd. for C21H16FN2O [M+H]+: 331.1241; found: 331.1268 ([M+H]+).
5-(6-Methoxy-2-naphthalenyl)-3-(6-fluoro-3-pyridyl)pyridine (1y): Prepared according to the general procedure method D from 3-Bromo-5-(6-methoxy-2-naphthalenyl)pyridine (1l, 314 mg, 1.00 mmol), 6-Fluoropyridine-3-boronic acid (4w, 155 mg, 1.10 mmol), Na2CO3 (657 mg, 6.20 mmol) and Pd(PPh3)4 (45.0 mg, 38.9 µmol) in MeOH (10 mL) and H2O (3 mL); reaction time: 24 h. After work-up (H2O (50 mL), extraction with CH2Cl2 (3 x 40 mL), column chromatography (CH2Cl2/CH3OH = 98:2) compound 1y was obtained as a beige solid (267 mg, 52%), mp: 219 °C. 1H-NMR (CDCl3, 250 MHz): δ = 3.97 (s, 3H, OCH3), 7.09-7.14 (m, 1H), 7.19-7.26 (m, 2H), 7.71-7.75 (m, 1H), 7.84 (d, 3J = 8.9 Hz, 1H), 7.90 (d, 3J = 8.7 Hz, 1H), 8.05-8.12 (m, 2H), 8.17 (m, 1H), 8.54 (m, 1H), 8.79 (d, 4J = 2.1 Hz, 1H), 9.0 (d, 4J = 2.0 Hz, 1H). MS (DIP-APCI, +): m/z (%): calcd. for C21H16FN2O [M+H]+: 331.1241; found: 331.1252 ([M+H]+).
4-(3-Hydroxymethyl-6-methoxy-2-naphthalenyl)isoquinoline (1z): To a suspension of LiAlH4 (1.33 g, 35.0 mmol) in THF (10 mL) a solution of 1n (826 mg, 2.40 mmol) in THF (10 mL) was added dropwise. After stirring at room temperature for 24 h H2O (20 mL) and H2SO4 (5 mL) were added successively. The resulting solution was neutralized with 1 N NaOH and extracted with CHCl3 (5 x 25 mL). The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure. Purification by column chromatography CH2Cl2/CH3OH = 98:2) afforded compound 1z as a brownish solid (260 mg, 34%). 1H-NMR (CDCl3, 250 MHz): δ = 2.50 (s, 1H, OH), 3.96 (s, 3H, OCH3), 4.51 (s, 2H, CH2OH), 7.17-7.26 (m, 2H), 7.45-7.50 (m, 1H), 7.55-7.65 (m, 2H), 7.66 (s, 1H), 7.73 (d, 3J = 9.4 Hz, 1H), 7.99-8.06 (m, 2H), 8.40 (s, 1H), 9.22 (s, 1H).
4-[3-(2-Fluoroethoxymethyl)-6-methoxy-2-naphthalenyl]isoquinoline (1aa): A solution of 1z (260 mg, 824 µmol), 2-Fluoroethyl p-toulenesulfonate (358 mg, 1.64 mmol) and NaH (60% dispersion in mineral oil, 150 mg, 3.75 mmol) in DMF (4 mL) was stirred at 60 °C for 24 h. The reaction mixture was allowed to come to room temperature and the solvent was removed in vacuo. The residue was taken up in H2O (50 mL). Extraction with CHCl3 (3 x 50 mL), drying over Na2SO4, evaporation of the solvent in vacuo and column chromatography CH2Cl2/CH3OH = 95:5) compound 1aa was obtained as a brownish solid (65.0 mg, 22%). 1H-NMR (CDCl3, 250 MHz): δ = 3.38-3.55 (m, 2H, OCH2), 3.97 (s, 3H, OCH3), 4.25-4.49 (m, 2H, CH2F) superimposed with 4.39 (s, 2H, ArCH2O), 7.20 (dd, 3J = 8.9 Hz, 4J = 2.5 Hz, 1H), 7.26 (m, 1H), 7.48-7.51 (m, 1H), 7.59-7.64 (m, 2H), 7.70-7.76 (m, 2H), 8.01 (s, 1H), 8.04-8.08 (m, 1H), 8.50 (s, 1H), 9.31 (s, 1H). MS (DIP-APCI, +): m/z (%): calcd. for C23H21FNO2 [M+H]+: 362.1551; found: 362.1559 ([M+H]+).
Synthesis of Dihydroquinolinone Compounds 2a-h
1-(2-Fluoroethyl)-6-(isoquinolin-4-yl)-3,4-dihydro-2(1H)-quinolinone (2a): Prepared according to the general procedure method A from 6-Bromo-1-(2-fluoroethyl)-3,4-dihydro-2(1H)-quinolinone (5a, 268 mg, 985 µmol), Isoquinoline-4-boronic acid (4n, 190 mg, 1.10 mmol), Ba(OH)2 . 8 H2O (474 mg, 1.50 mmol) and Pd(PPh3)4 (44.0 mg, 38.1 µmol) in DME (6 mL) and H2O (1 mL); reaction time: 24 h. After work-up (H2O (50 mL), extraction with CHCl3 (5 x 30 mL), column chromatography CH2Cl2/CH3OH = 95:5) compound 2a was obtained as an orange oil (145 mg, 46%). 1H-NMR (CDCl3, 250 MHz): δ = 2.73-2.79 (m, 2H, CH2), 2.98-3.04 (m, 2H, CH2), 4.11-4.25 (dt, 3JF,H = 24.6 Hz, 3J = 5.0 Hz, 2H, CH2CH2F), 4.58-4.81 (dt, 2JF,H = 47.4 Hz, 3J = 5.0 Hz, 2H, CH2F), 7.28-7.33 (m, 2H), 7.37-7.42 (m, 1H), 7.62-7.69 (m, 2H), 7.90-7.95 (m, 1H), 8.02-8.06 (m, 1H), 8.46 (s, 1H), 9.24 (s, 1H).
1-(3-Fluoropropyl)-6-(isoquinolin-4-yl)-3,4-dihydro-2(1H)-quinolinone (2b): Prepared according to the general procedure method A from 6-Bromo-1-(2-fluoropropyl)-3,4-dihydro-2(1H)-quinolinone (5b, 260 mg, 909 µmol), Isoquinoline-4-boronic acid (4n, 208 mg, 1.20 mmol), Ba(OH)2 . 8 H2O (474 mg, 1.50 mmol) and Pd(PPh3)4 (44.0 mg, 38.1 µmol) in DME (6 mL) and H2O (1 mL); reaction time: 24 h. After work-up (H2O (50 mL), extraction with CHCl3 (5 x 30 mL), column chromatography CH2Cl2/CH3OH = 98:2) compound 2b was obtained as a yellowish resin (166 mg, 55%). 1H-NMR (CDCl3, 250 MHz): δ = 2.03-2.24 (m, 2H, NCH2), 2.69-2.75 (m, 2H, CH2), 2.95-3.01 (m, 2H, CH2), 4.12-4.18 (m, 2H, CH2CH2F), 4.46-4.70 (dt, 2JF,H = 47.1 Hz, 3J = 5.6 Hz, 2H, CH2F), 7.18 (d, 3J = 8.4 Hz, 1H), 7.32 (m, 1H), 7.40 (dd, 3J = 8.4 Hz, 3J = 2.2 Hz, 1H), 7.64-7.69 (m, 2H), 7.90-7.95 (m, 1H), 8.01-8.05 (m, 1H), 8.45 (s, 1H), 9.23 (s, 1H).
7-(2-Fluoroethoxy)-6-(isoquinolin-4-yl)-3,4-dihydro-2(1H)-quinolinone (2c): Prepared according to the general procedure method B from 6-Bromo-7-(2-fluoroethoxy)-3,4-dihydro-2(1H)-quinolinone (5c, 134 mg, 465 µmol), Isoquinoline-4-boronic acid (4n, 129 mg, 746 mmol), Na2CO3 (106 mg, 1.00 mmol) and Pd(PPh3)4 (44.0 mg, 38.1 µmol) in toluene (6 mL), EtOH (1 mL) and H2O (1 mL); reaction time: 18 h. After work-up (H2O (50 mL), extraction with CHCl3 (5 x 30 mL), column chromatography CH2Cl2/CH3OH = 98:2) compound 2c was obtained as a salmon solid (99.0 mg, 63%). 1H-NMR (CDCl3, 250 MHz): δ = 2.04-2.11 (m, 2H, NCH2CH2), 3.37-3.43 (m, 2H, NCH2), 4.36 (m, 2H, OCH2), 4.85 (m, 2H, CH2F), 7.11-7.27 (2m superimposed, 3H), 7.62-7.71 (m, 2H), 7.80-7.85 (m, 2H), 7.94-7.99 (m, 2H), 8.24 (m, 1H).
8-[5-(2-Fluoroethoxymethyl)pyridine-3-yl]-1,2,5,6-tetrahydro-4H-pyrrolo[3,2,1-ij]-4-quinoli-none (2d): Prepared according to the general procedure method A from 1,2,5,6-tetrahydro-4H-pyrrolo[3,2,1-ij]-4-quinolinone-8-boronic acid pinacol ester (5d, 471 mg, 1.57 mmol), 3-Bromo-5-(2-fluoroethoxymethyl)pyridine (4r, 322 mg, 1.38 mmol), Ba(OH)2 . 8 H2O (645 mg, 2.04 mmol) and Pd(PPh3)4 (64.0 mg, 55.4 µmol) in DME (8.5 mL) and H2O (1.3 mL); reaction time: 24 h. After work-up (H2O (50 mL), extraction with CHCl3 (3 x 25 mL), column chromatography CH2Cl2/CH3OH = 95:5) compound 2d was obtained as an off-white solid (277 mg, 54%), mp: 138-140 °C. 1H-NMR (CDCl3, 250 MHz): δ = 2.69-2.76 (m, 2H, CH2), 3.02-3.08 (m, 2H, CH2), 3.23-3.29 (m, 2H, CH2), 3.72-3.88 (OCH2CH2F), 4.11-4.17 (m, 2H, CH2), 4.52-4.74 (m, 2H, CH2F) superimposed with 4.68 (s, 2H, ArCH2O), 7.22 (m, 1H), 7.30 (m, 1H), 7.85 (m, 1H), 8.52 (d, 4J = 2.1 Hz, 1H), 8.72 (d, 4J = 2.4 Hz, 1H). MS (DIP-APCI, +): m/z (%): calcd. for C19H20FN2O2 [M+H]+: 327.1503; found: 327.1519 ([M+H]+).
6-[5-(2-fluoroethoxymethyl)pyridin-3-yl]-1-methyl-3,4-dihydro-2(1H)-quinolinone (2e): Prepared according to the general procedure method A from 1-Methyl-3,4-dihydro-2(1H)-quinolinone-6-boronic acid pinacol ester (5e, 369 mg, 1.28 mmol), 3-Bromo-5-(2-fluoroethoxymethyl)pyridine (4r, 301 mg, 1.28 mmol), Ba(OH)2 . 8 H2O (605 mg, 1.92 mmol) and Pd(PPh3)4 (60.0 mg, 51.9 µmol) in DME (8 mL) and H2O (1.3 mL); reaction time: 24 h. After work-up (H2O (50 mL), extraction with CHCl3 (3 x 25 mL), column chromatography (first column: CH2Cl2/CH3OH = 95:5, second column: hexane/EtOAc = 1:3) compound 2e was obtained as an off-white solid (163 mg, 40%). 1H-NMR (CDCl3, 250 MHz): δ = 2.67-2.73 (m, 2H, CH2), 2.96-3.02 (m, 2H, CH2), 3.40 (s, 3H, NCH3), 3.73-3.88 (OCH2CH2F), 4.52-4.74 (m, 2H, CH2F) superimposed with 4.68 (s, 2H, ArCH2O), 7.08 (d, 3J = 8.4 Hz, 1H), 7.39-7.43 (m, 1H), 7.49 (dd, 3J = 8.4 Hz, 4J = 2.3 Hz, 1H), 7.90 (m, 1H), 8.53 (d, 4J = 2.1 Hz, 1H), 8.76 (d, 4J = 2.2 Hz, 1H).
8-Chloro-6-[5-(2-fluoroethoxymethyl)pyridin-3-yl]-1-methyl-3,4-dihydro-2(1H)-quinoli-none (2f): Prepared according to the general procedure method A from 8-Chloro-1-methyl-3,4-dihydro-2(1H)-quinolinone-6-boronic acid pinacol ester (5f, 322 mg, 1.00 mmol), 3-Bromo-5-(2-fluoroethoxymethyl)pyridine (4r, 216 mg, 923 µmol), Ba(OH)2 . 8 H2O (432 mg, 1.37 mmol) and Pd(PPh3)4 (44.0 mg, 38.1 µmol) in DME (6 mL) and H2O (0.9 mL); reaction time: 24 h. After work-up (H2O (50 mL), extraction with CHCl3 (3 x 25 mL), column chromatography CH2Cl2/MeOH = 95:5) compound 2f was obtained as a yellowish oil (139 mg, 43%). 1H-NMR (CDCl3, 250 MHz): δ = 2.60-2.66 (m, 2H, CH2), 2.90-2.96 (m, 2H, CH2), 3.49 (s, 3H, NCH3), 3.72-3.88 (m, 2H, OCH2CH2F), 4.51-4.74 (m, 2H, CH2F), 4.68 (s, 2H, ArCH2O), 7.32 (m, 1H), 7.50 (d, 4J = 2.1 Hz, 1H), 7.86 (m, 1H), 8.56 (d, 4J = 2.1 Hz, 1H), 8.73 (d, 4J = 2.3 Hz, 1H).
8-Chloro-6-{5-[1-(2-fluoroethoxy)ethyl]pyridin-3-yl}-1-methyl-3,4-dihydro-2(1H)-quinolino-ne (2g): Prepared according to the general procedure method A from 8-Chloro-1-methyl-3,4-dihydro-2(1H)-quinolinone-6-boronic acid pinacol ester (5f, 322 mg, 1.00 mmol), 3-Bromo-5-[1-(2-fluoroethoxy)ethyl]pyridine (4s, 218 mg, 879 mmol), Ba(OH)2 . 8 H2O (432 mg, 1.37 mmol) and Pd(PPh3)4 (44.0 mg, 38.1 µmol) in DME (6 mL) and H2O (0.9 mL); reaction time: 24 h. After work-up (H2O (50 mL), extraction with CHCl3 (3 x 25 mL), column chromatography CH2Cl2/MeOH = 95:5) compound 2g was obtained as a yellowish oil (112 mg, 35%). 1H-NMR (CDCl3, 250 MHz): δ = 1.55 (d, 3J = 6.4 Hz, 3H, CHCH3), 2.62-2.67 (m, 2H, CH2), 2.92-2.97 (m, 2H, CH2), 3.51 (s, 3H, NCH3), 3.54-3.72 (m, 2H, OCH2CH2F), 4.45-4.69 (m, 2H, CH2F) superimposed with 4.61 (q, 3J = 6.4 Hz, 1H, CHCH3), 7.33 (m, 1H), 7.51 (d, 4J = 2.3 Hz, 1H), 7.86 (m, 1H), 8.54 (d, 4J = 2.0 Hz, 1H), 8.74 (d, 4J = 2.1 Hz, 1H). MS (EI): m/z (%): calcd. for C19H20ClFN2O2 [M]+.: 362.1; found: 277.0 (100, [M-CH2CH2CONCH3]+), 362.0 (29, [M]+.).
8-Chloro-6-(4-fluoropyridin-3-yl)-1-methyl-3,4-dihydro-2(1H)-quinolinone (2h): Prepared according to the general procedure method C from 8-Chloro-1-methyl-3,4-dihydro-2(1H)-quinolinone-6-boronic acid pinacol ester (5f, 338 mg, 1.05 mmol), 3-Bromo-4-fluoropyridine (4p, 199 mg, 1.13 mmol), Na2CO3 (509 mg, 4.80 mmol) and Pd(PPh3)4 (71.0 mg, 61.4 µmol) in DME (7 mL) and H2O (2 mL); reaction time: 24 h. After work-up (H2O (50 mL), extraction with methyl tert-butyl ether (3 x 25 mL), column chromatography heptane/EtOAc = 1:1) compound 2h was obtained as a colorless solid (191 mg, 63%), mp: 127-129 °C. 1H-NMR (CDCl3, 250 MHz): δ = 2.61-2.67 (m, 2H, CH2), 2.91-2.97 (m, 2H, CH2), 3.50 (s, 3H, NCH3), 6.99-7.05 (m, 1H), 7.26 (m, 1H, superimposed with CDCl3 residual peak), 7.45 (d, 4J = 2.1 Hz, 1H), 7.90-7.97 (m, 1H), 8.37-8.41 (m, 1H).
Cell Cultures
The primary human adrenocortical cell line NCI-H295 was purchased commercially from ATCC via LGC Standards GmbH (Wesel, Germany) and was cultured in RPMI 1640 medium supplemented with 10% FCS, 5 µg/ml insulin, 100 µg/ml transferrin and 5.2 ng/ml sodium selenite at 37 °C in a humidified atmosphere (95%) with 5% CO2. The medium was exchanged every 48 hours, with approximately 30% of the old medium being reused to preserve synthesized growth factors. Alternately, the cells were passaged every four to five days. The murine adrenocortical tumour Y1 cell line was purchased commercially from CLS Cell Lines Service GmbH (Eppelheim, Germany). Y1 cells stably transfected with human CYP11B1 (Y1-CYP11B1) and Y1 cells stably transfected with CYP11B2 (Y1-CYP11B2) were cultured in DMEM AQ (Dulbeccos Modified Eagle Medium) supplemented with 4.5 g/l glucose, 10% FCS and 1 mg/ml of the cell culture antibiotic zeocin at 37 °C in a humidified atmosphere (95%) with 5% CO2. The Y1-CYP11B1 and the Y1-CYP11B2 cells were passaged every 48 hours. All cell culture media and supplements were obtained from Sigma-Aldrich (Deisenhofen, Germany).
Evaluation of CYP11B1 and CYP11B2 inhibition in NCI-H295 cells and Y1 cells
To evaluate CYP11B1 and CYP11B2 inhibition of all synthesized compounds, Y1-CYP11B1 and Y1-CYP11B2 cells were subcultured on 6-well plates (5 x 105 cells per well) in 2 ml of culture medium. The enzyme reaction was started after 24 h by the addition of 1 ml culture medium containing either 11-deoxycortisol (RSS) or deoxycorticosterone (DOC) (obtained from Sigma, Deisenhofen, Germany) as enzyme substrate and the corresponding inhibitor. RSS and DOC were added because the Y1 cells do not contain the whole cascade of enzymes to produce cortisol and aldosterone, and were dissolved in ethanol prior to addition to the cell culture medium. The final concentrations were 1 µM for the substrate and, for the determination of IC50 values, 0.1 nM - 10 µM for the corresponding inhibitor before incubating for 48 h at 37 °C in a humidified atmosphere (95%) with 5% CO2. Y1-CYP11B1 and Y1-CYP11B2 cells treated in the same way but without inhibitors served as negative controls.
Selected inhibitors were evaluated for their CYP11B1 and CYP11B2 inhibition using cells of the adrenocortical cell line NCI-H295 subcultured on 6-well plates (1 x 106 cells/well) in culture medium. NCI-H295 cells contain the whole cascade of enzymes to produce cortisol and aldosterone. For determination of IC50 values, the inhibitors were added to the culture medium at final concentrations between 0.1 nM - 10 µM before incubating for 48 h at 37 °C in a humidified atmosphere (95%) with 5% CO2. NCI-H295 cells treated in the same way without inhibitors served as negative controls.
At the end of incubation time, cortisol and aldosterone concentrations were determined in the cell supernatant by commercially available radioimmunoassays (DPC Biermann, Bad Nauheim, Germany; IBL international, Hamburg, Germany). The intra-assay variance of both assays was <8%, the inter-assay variance was <12%.
Conclusions
In conclusion, we report the synthesis and in vitro evaluation of fluorinated pyridyl-substituted naphthalenes and 3,4-dihydro-1H-quinolin-2-ones. Several compounds from both classes were found to act as highly potent aldosterone synthase (CYP11B2) inhibitors up to low nanomolar affinities using assays of stably transfected murine Y1 cells and the human adrenocortical NCI-H295 cell line. In addition, many of these inhibitors showed good or even excellent selectivity values owing to weak binding affinities on CYP11B1. The observed correlations between their substitution patterns and potency largely aligns with recent findings for similar compounds, such as the beneficial substitution of the 5-position of the pyridine moiety compared to the 4-position [39]. Based on these promising results, the organic synthesis of suitable precursors for F-18 fluorination, along with the respective labelling chemistry and preclinical evaluation of the most promising radiotracers are well underway.
Acknowledgments
The authors thank Martina Zink, Antonia Dohles and Katja Marienfeld (Department of Medicine I, Endocrinology and Diabetology) for their excellent technical assistance.
Author Contributions
Conceptualization, A.S., S.H. and B.H.; methodology, P.M., M.S., S.G., S.R. and B.H.; formal analysis, P.M., A.S. and B.H.; writing – original draft preparation: P.M. and A.S.; writing - review and editing, A.S., P.M. and S.H.; supervision, A.S. and S.H.; project administration, A.S. and B.H.; funding acquisition, A.S. and S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) (within the CRC/Transregio 205/1 “The Adrenal: Central Relay in Health and Disease”), IZKF Würzburg (Grant No. F-24 to A.S. and S.H.), and the Else Kröner-Fresenius Stiftung (Grant No. EKFS2013 A213 to S.H.).
Declarations
Competing interests
AS and SH filed a patent application: PET radiopharmaceuticals for the differential diagnosis between bilateral and unilateral conditions of primary hyperaldosteronism. WO/2011/151411. All other authors declare no conflict of interest.
References
- Wu X, Yu J, Tian H. Cardiovascular risk in primary aldosteronism. Medicine 98 (2019): e15985.
- Funder J W, Carey R M, Mantero F, et al. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 101 (2016): 1889-1916.
- Williams T A, Reincke M. Pathophysiology and histopathology of primary aldosteronism. Trends Endocrinol Metab 33 (2022): 36-49.
- Wu V C, Wang S M, Huang K H, et al. Long-term mortality and cardiovascular events in patients with unilateral primary aldosteronism after targeted treatments. Eur J Endocrinol 186 (2022): 195-205.
- Voets M, Antes I, Scherer C, et al. Heteroaryl-Substituted Naphthalenes and Structurally Modified Derivatives: Selective Inhibitors of CYP11B2 for the Treatment of Congestive Heart Failure and Myocardial Fibrosis. J Med Chem 48 (2005): 6632-6642.
- Voets M, Antes I, Scherer C, et al. Synthesis and Evaluation of Heteroaryl-Substituted Dihydronaphthalenes and Indenes: Potent and Selective Inhibitors of Aldosterone Synthase (CYP11B2) for the Treatment of Congestive Heart Failure and Myocardial Fibrosis. J Med Chem 49 (2006): 2222-2231.
- Lucas S, Heim R, Ries C, et al. In Vivo Active Aldosterone Synthase Inhibitors with Improved Selectivity: Lead Optimization Providing a Series of Pyridine Substituted 3,4-Dihydro-1H-quinolin-2-one Derivatives. J Med Chem 51 (2008): 8077-8087.
- Voets M, Müller-Vieira U, Marchais-Oberwinkler S, et al. Synthesis of Amidinohydrazones and Evaluation of Their Inhibitory Effect towards Aldosterone Synthase (CYP11B2) and the Formation of Selected Steroids. Arch Pharm 337 (2004): 411-416.
- Lucas S, Heim R, Negri M, et al. Novel Aldosterone Synthase Inhibitors with Extended Carbocyclic Skeleton by a Combined Ligand-Based and Structure-Based Drug Design Approach. J Med Chem 51 (2008): 6138-6149.
- Heim R, Lucas S, Grombein C M, et al. Overcoming Undesirable CYP1A2 Inhibition of Pyridylnaphthalene-Type Aldosterone Synthase Inhibitors: Influence of Heteroaryl Derivatization on Potency and Selectivity. J Med Chem 51 (2008): 5064-5074.
- Hoyt S B, Park M K, London C, et al. Discovery of Benzimidazole CYP11B2 Inhibitors with in Vivo Activity in Rhesus Monkeys. ACS Med Chem Lett 6 (2015): 573-578.
- Sparks S M, Danger D P, Hoekstra W J, et al. Development of Highly Selective Pyrimidine-Based Aldosterone Synthase (CYP11B2) Inhibitors. ACS Med Chem Lett 10 (2019): 1056-1060.
- Meyers K, Cogan D A, Burke J, et al. Dihydrobenzoisoxazole-4-one compounds are novel selective inhibitors of aldosterone synthase (CYP11B2) with in vivo activity. Bioorg Med Chem Lett 28 (2018): 979-984.
- Meguro M, Miyauchi S, Kanao-Arisumi Y, et al. Identification of sulfonylpyrimidines as novel selective aldosterone synthase (CYP11B2) inhibitors. Bioorg Med Chem 108 (2024): 117775.
- Petrilli W L, Hoyt S B, London C, et al. Discovery of Spirocyclic Aldosterone Synthase Inhibitors as Potential Treatments for Resistant Hypertension. ACS Med Chem Lett 8 (2017): 128-132.
- Guo C, Zhang G, Wu C, et al. Emerging trends in small molecule inhibitors targeting aldosterone synthase: A new paradigm in cardiovascular disease treatment. Eur J Med Chem 274 (2024): 116521.
- Meredith E L, Ksander G, Monovich L G, et al. Discovery and in Vivo Evaluation of Potent Dual CYP11B2 (Aldosterone Synthase) and CYP11B1 Inhibitors. ACS Med Chem Lett 4 (2013): 1203-1207.
- Lenzini L, Zanotti G, Bonchio M, et al. Aldosterone synthase inhibitors for cardiovascular diseases: A comprehensive review of preclinical, clinical and in silico data. Pharmacol Res 163 (2021): 105332.
- Papillon J P, Adams C M, Hu Q Y, et al. Structure-activity relationships, pharmacokinetics, and in vivo activity of CYP11B2 and CYP11B1 inhibitors. J Med Chem 58 (2015): 4749-4770.
- Freeman M W, Halvorsen Y D, Marshall W, et al. Phase 2 Trial of Baxdrostat for Treatment-Resistant Hypertension. N Engl J Med 388 (2023): 395-405.
- Irfan H, Ahmed A, Nawani K D. Hypertension and Lorundrostat: Key Discoveries From the TARGET-HTN Trial. Curr Probl Cardiol 49 (2024): 102144.
- Sloan-Lancaster J, Raddad E, Flynt A, et al. LY3045697: Results from two randomized clinical trials of a novel inhibitor of aldosterone synthase. J Renin Angiotensin Aldosterone Syst 18 (2017): 1470320317717883.
- Bornstein S R, de Zeeuw D, Heerspink H J, et al. Aldosterone synthase inhibitor (BI 690517) therapy for people with diabetes and albuminuric chronic kidney disease: A multicentre, randomized, double-blind, placebo-controlled, Phase I trial. Diabetes Obes Metab 26 (2024): 2128-2138.
- Bertagna X, Pivonello R, Fleseriu M, et al. LCI699, a potent 11beta-hydroxylase inhibitor, normalizes urinary cortisol in patients with Cushing's disease: results from a multicenter, proof-of-concept study. J Clin Endocrinol Metab 99 (2014): 1375-1383.
- Zhou Y, Wang D, Jiang L, et al. Diagnostic accuracy of adrenal imaging for subtype diagnosis in primary aldosteronism - systematic review and meta-analysis. BMJ Open 10 (2020): e038489.
- Kempers M J, Lenders J W, van Outheusden L, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Int Med 151 (2009): 329-337.
- Williams T A, Burrello J, Sechi L A, et al. Computed Tomography and Adrenal Venous Sampling in the Diagnosis of Unilateral Primary Aldosteronism. Hypertension 72 (2018): 641-649.
- Vonend O, Ockenfels N, Gao X, J et al. Adrenal venous sampling: evaluation of the German Conn's registry. Hypertension 57 (2011): 990-995.
- Stewart P M, Allolio B. Adrenal vein sampling for Primary Aldosteronism: time for a reality check. Clin Endocrinol 72 (2010): 146-148.
- Fuss C T, Treitl M, Rayes N, et al. Radiation exposure of adrenal vein sampling: a German Multicenter Study. Eur J Endocrinol 179 (2018): 261-267.
- Augustin A M, Dalla Torre G, Fuss C T, et al. Reduction of Radiation Exposure in Adrenal Vein Sampling: Impact of the Rapid Cortisol Assay. RöFo - Fortschr Röntgenstr 193 (2021): 1392-1402.
- Shukla A K, Kumar U. Positron emission tomography: An overview. J Med Phys 31 (2006): 13-21.
- Soinio M, Luukonen A K, Seppänen M, et al. Functional imaging with 11C-metomidate PET for subtype diagnosis in primary aldosteronism. Eur J Endocrinol 183 (2020): 539-550.
- Wu X, Senanayake R, Goodchild E, et al. [11C]metomidate PET-CT versus adrenal vein sampling for diagnosis surgically curable primary aldosteronism: a prospective, within-patient trial. Nat Med 29 (2023): 190-202.
- Gillett D, Senanayake R, MacFarlane J, et al. A PhaseI/IIa Clinical Trial to Evaluate Safety and Adrenal Uptake of Para-Chloro-2-[18F]Fluoroethyletomidate in Healthy Volunteers and Patients with Primary Aldosteronism. J Nucl Med 66 (2025): 434-440.
- Silins I, Sundin A, Lubberink M, et al. First-in-human evaluation of [18F]CETO: a novel tracer for adrenocortical tumours. Eur J Nucl Med Mol Imaging 50 (2023): 398-409.
- Abe T, Naruse M, Young W F, et al. A Novel CYP11B2-Specific Imaging Agent for Detection of Unilateral Subtypes of Primary Aldosteronism. J Clin Endocrinol Metab 101 (2016): 1008-1015.
- Sander K, Gendron T, Cybulska K A, et al. Development of [18F]AldoView as the First Highly Selective Aldosterone Synthase PET Tracer for Imaging of Primary Hyperaldosteronism. J Med Chem 64 (2021): 9321-9329.
- Maier P, Heinze B, Gabor S, et al. Fluorinated aldosterone synthase (CYP11B2)-inhibitors for differential diagnosis between bilateral and unilateral conditions of primary aldosteronism. Bioorg Med Chem Lett 96 (2023): 129501.
- Ries C, Lucas S, Heim R, et al. Selective aldosterone synthase inhibitors reduce aldosterone formation in vitro and in vivo. J Steroid Biochem Mol Biol 116 (2009): 121-126.
- Allolio B, Hahner S, Schirbel A, et al. PET Radiopharmaceuticals for the Differential Diagnosis Between Bilateral and Unilateral Conditions of Primary Hyperaldosteronism. Patent WO2011151411A1.
- Ono S, Inoue Y, Yoshida T, et al. Preparation and Pharmacological Evaluation of Novel Glycoprotein (Gp) IIb/IIIa Antagonists. 1. The Selection of Naphthalene Derivatives. Chem Pharm Bull 67 (1999): 1685-1693.
- Hartmann R W, Heim R, Lucas S. 6-Pyridin-3-yl-3,4-Dihydro-1H-Quinolin-2-one Derivatives and Related Compounds as Inhibitors of the Human Aldosterone Synthase CYP11B2. Patent WO2009135651A1.
- Ackerley N, Brewster A G, Brown G R, et al. A Novel Approach to Dual-Acting Thromboxane Receptor Antagonist/Synthase Inhibitors Based on the Link of 1,3 Dioxane-Thromboxane Receptor Antagonists and -Thromboxane Synthase Inhibitors. J Med Chem 38 (1995): 1608-1628.
- Zhao D, Chen C Y, Xu F, et al. Preparation of [R-(R,S)]-β-Methyl-α-Phenyl-1-Pyrrolidineethanol. Org Synth Coll Vol 10 (2004): 556-561.
- Hertzog-Ronen C, Borzin E, Gerchikov Y, et al. Detection and Identification of Alkylating Agents by Using a Bioinspired "Chemical Nose". Chem Eur J 15 (2009): 10380-10386.
- Castanedo G M, Seng P S, Blaquiere N, et al. Rapid Synthesis of 1,3,5-Substituted 1,2,4-Triazoles from Carboxylic Acids, Amidines, and Hydrazines. J Org Chem 76 (2011): 1177-1179.
- Van Leusen A M, Wildeman J, Oldenziel O H. Base-Induced Cycloaddition of Sulfonylmethyl Isocyanides to C,N Double Bonds. Synthesis of 1,5-Disubstituted and 1,4,5-Trisubstituted Imidazoles from Aldimines and Imidoyl Chlorides. J Org Chem 42 (1977): 1153-1159.
- Sawai K, Tatumi R, Nakahodo T, et al. Asymmetric suzuki-miyaura coupling reactions catalyzed by chiral palladium nanoparticles at room temperature. Angew Chem Int Ed 47 (2008): 6917-6919.
- Chan F, Magnus P, McIver E G. Synthesis of the 4-arylindole portion of the antitumor agent diazoamide and related studies. Tetrahedron Lett 41 (2000): 835-838.
- Voets M, Antes I, Scherer C, et al. Heteroaryl-Substituted Naphthalenes and Structurally Modified Derivatives: Selective Inhibitors of CYP11B2 for the Treatment of Congestive Heart Failure and Myocardial Fibrosis. J Med Chem 48 (2005): 6632-6642.
- Bass R J, Koch R C, Richards C, et al. Tricyclic Amides: A New Class of Systemic Fungicides Active against Rice Blast Disease. J Agric Food Chem 29 (1981): 576-579.
- Occhiato E G, Ferrali A, Menchi G, et al. Synthesis, Biological Activity, and Three-Dimensional Quantitative Structure-Activity Relationship Model for a Series of Benzo[c]quinolizin-3-ones, Nonsteroidal Inhibitors of Human Steroid 5-Reductase 1. J Med Chem 47 (2004): 3546-3560.
- LaSala D, Shibanaka Y, Jeng A Y. Coexpression of CYP11B2 and CYP11B1 with adrenotoxin and adrenotoxin reductase for assessing the potency and selectivity of aldosterone synthase inhibitors. Anal Biochem 394 (2009): 56-61.
- Hoyt S B, Petrilli W, London C, et al. Discovery of triazole CYP11B2 Inhibitors with in Vivo Activity in Rhesus Monkeys. ACS Med Chem Lett 6 (2015): 861-865.
- Gazdar A F, Oie H K, Shackleton C H, et al. Establishment and characterization of a human adrenocortical carcinoma cell line that expresses multiple pathways of steroid biosynthesis. Cancer Res 50 (1990): 5488-5496.
- Rainey W E, Bird I M, Mason J I. The NCI-H295 cell line: a pluripotent model for human adrenocortical studies. Mol Cell Endocrinol 100 (1994): 45-50.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 