Unspecific Peroxygenases - Functional Hybrids between P450 Monooxygenases and Heme Peroxidases
Katrin Scheibner1*, Martin Hofrichter2
1Head of Enzyme Technology, Institute of Biotechnology, Faculty of Environment and Natural Sciences, Brandenburg University of Technology Cottbus-Senftenberg, BTU University place, 101968, Senftenberg, Germany
2Chair of Environmetal Biotechnology, International Institute Zittau, Dresden University of Technology, TUD, Germany
*Corresponding authors 1: Katrin Scheibner, Head of Enzyme Technology, Institute of Biotechnology, Faculty of Environment and Natural Sciences, Brandenburg University of Technology Cottbus-Senftenberg, BTU University place, 101968, Senftenberg, Germany.
*Corresponding authors 2: Martin Hofrichter, Chair of Environmetal Biotechnology, International Institute Zittau, Dresden University of Technology TU Dresden, Germany.
Received: 10 December 2024; Accepted: 20 December 2024; Published: 16 October 2025
Article Information
Citation: Katrin Scheibner, Martin Hofrichter. Unspecific Peroxygenases – Functional Hybrids between P450 Monooxygenases and Heme Peroxidases Journal of Biotechnology and Biomedicine. 8 (2025): 328-330.
DOI: 10.26502/jbb.2642-91280200
View / Download Pdf Share at FacebookAbstract
Unspecific peroxygenases (UPOs) secreted by fungi represent an intriguing enzyme type that selectively transfers peroxide-borne oxygen with high efficiency to diverse substrates including unactivated hydrocarbons. They contain a cysteine-ligated heme and catalyze hydroxylation, epoxidation, dealkylation, deacylation as well as hetero atom, halide and one-electron oxidations. Substrate spectra of UPOs resemble both those of P450 monooxygenases and heme peroxidases. Non-specific peroxygenases (UPOs, EC 1.11.2.1) belong to the heme-thiolate proteins and behave "promiscuously" with respect to demanding oxygen transfer reactions. The first UPO was discovered in 2004 in the southern field mushroom (Agrocybe aegerita), a hardwood-colonizing edible mushroom from the wider mushroom family (order Agaricales) [1]. Further enzymes of this type were found in cultures of other fungi (e.g. Marasmius rotula, Chaetomium globosum) [2,3] and the long-known chloroperoxidase (CPO, EC 1.11.1.10) turned out to be a special case of UPOs [4]. The heterologous expression of UPOs is associated with major difficulties and has so far only been successful in a few cases (e.g. in Sacharomyces, Pichia); the complex folding and the formation of disulfide bridges may play a role in this [3,6]. The crystal structures of the UPOs of A. aegerita and M. rotula have been solved and reveal a compact spherical shape dominated by ? helices and containing a heme-stabilizing magnesium and a highly conserved PCP motif. The latter ideally exposes a cysteine as proximal heme ligand towards the iron. The heme access channels of UPOs are lined with hydrophobic amino acid residues (Phe or Leu/Ile/Val) and their molecular architecture is of crucial importance for the substrate specificity of the respective enzyme [3,6].
Article Details
UPO reactions and mechanism
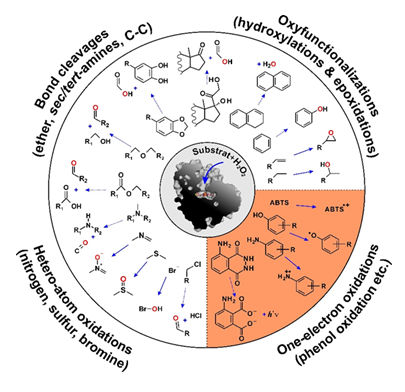
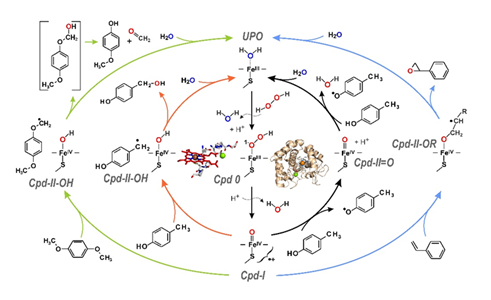
Functionally, UPOs are monooxygenases acting outside the fungal hyphae, which transfer a peroxide-borne oxygen atom (H-O-O-R) to various organic substrates. The target substrates are subject to hydroxylation, epoxidation, dealkylation, deacylation and heteroatom oxygenation; in addition, one-electron oxidations are catalyzed analogous to classical peroxidases (Figure 1) [4,7]. The product spectra of UPOs are often similar to those of cytochrome P450 monooxygenases, which are active as universal detoxification enzymes in the human liver [8,9,10], e.g. with regard to the conversion of active pharmaceutical ingredients or xenobiotics. Figure 2 illustrates the mechanisms of UPO-catalyzed conversions using the substrates p-cresol, 1,4-dimethoxybenzene and styrene. The former is both benzylic hydroxylated and oxidized to the corresponding phenoxy radical, the aromatic diether is O-dealkylated and styrene is epoxidized. In each case, the key intermediate is the so-called UPO compound I (Cpd-I), an oxoferryl cation radical complex of heme, which is formed after binding and heterolytic cleavage of H2O2 [4,11]. Cpd-I is an extremely strong oxidizing agent that attacks C-H bonds, double bonds and phenolic OH groups, among others, forming radicals. Subsequently, rebound mechanisms lead to the transfer of oxygen (peroxygenation of a C atom or epoxide formation) or to a second radical bond (phenol oxidation). The reactions that take place thus correspond to a combination of the so-called "peroxide shunt" of certain P450 enzymes and the catalytic cycle of classic heme peroxidases [4].
Figure 1: Reactions of non-specific peroxygenases (UPOs). The reactions in the lower right quarter are one-electron oxidations, while all other reactions are oxygen transfer reactions. In the center of the figure, the UPO from Agrocybe aegerita (AaeUPO) is shown in cross-section; modified after [4,12].
Figure 2: Catalytic cycles of unspecific peroxygenases (UPOs) with four possible routes that branch off at the UPO compound I (Cpd I) stage depending on the substrate. The inner cycles describe the oxygenation (red arrows) and oxidation (black arrows) of p-cresol, the outer cycles the epoxidation of styrene (green arrows) and the O-dealkylation of 1,4-dimethoxybenzene (blue arrows); modified and rearranged according to [4,12].
Distribution in the fungal kingdom and phylogeny
Several thousand putative UPO sequences have now been found in fungal genomes. They suggest the wide distribution of these enzymes throughout the fungal kingdom, including all strains of true fungi (Eumycota) and some fungus-like stramenopiles [4,12]. Within the basal sister group of the other fungi, the Cryptomycota, a UPO gene was detected in Rozella allomycis. Other basal fungal groups (Microsporidia, Neocallimastigomycota, Blastocladiomycota, Kickxellomycotina) lack UPO genes; the same applies to the sister group of fungi, the Holozoa, which comprises the animals (Metazoa) and their closest relatives (Choanoflagellata, Ichthyosporea). Representatives of the Chytridiomycota (flagellate fungi), on the other hand, possess up to seven UPO genes; their enzymes may have been the starting point for the evolutionary development of the UPO multigene family, which ultimately led to the possession of 70 and more UPO genes in individual species of higher fungi. Apart from the Mortierellomycotina, representatives of the other groups of polyphyletic Zygomycota and Glomeromycota possess one or two UPO genes.
Phylogenetically, the UPO sequences can be divided into two families. Family I - the "short" UPO sequences - comprises representatives of all the above-mentioned UPO-positive fungal groups. Family II contains the "long" UPOs, whose occurrence is restricted to the Ascomycota and Basidiomycota (Fig. 3). Some distinct basal subgroups in family I contain UPO sequences without recognizable signal peptides, which is why it seems plausible that the UPO archetype was a similar intracellular enzyme. This applies to about a quarter of the UPO sequences analyzed. Whether these enzymes work freely in the cytosol or are active in specific hyphal compartments is unclear. Since UPOs are found exclusively in the fungal kingdom, including the basal Cryptomycota, and no evidence of their presence in the Holozoa has been found to date, it can be postulated that they arose over 600 million years ago, after the separation of fungi and animals. It is possible that the drastic conditions of the developing oxygen atmosphere, similar to the case of the P450 enzymes, played an evolutionary role. Whether UPOs and P450s have a common origin is still unclear. Although both enzyme types have structural and catalytic similarities (cysteine as proximal heme ligand, compounds 0, I, II), there is no homology at the sequence level.
Outlook
Despite their high relevance for enzyme technology applications, only a few UPOs are currently available and almost nothing is known about their physiological significance. Considering their wide distribution in the fungal kingdom, their high number in the genomes of some fungi and their versatility in terms of catalyzed reactions, various functions are conceivable. First and foremost, detoxification reactions should certainly be mentioned, as UPOs attack structures that frequently occur in plant and microbial secondary metabolites and environmental pollutants [9,12]. Considering the high diversity of UPO genes, an involvement in pathogenicity or in lignin and humus transformation should not be excluded. The latter would require a molecular architecture that allows aromatic polymer structures to be attacked superficially via heme access channels that are as broad and shallow as possible. In this context, it should be noted that the crystalline cellulose-cleaving LPMOs (lytic polysaccharide monooxygenases) discovered only a few years ago are, according to recent publications, also surface-active, extracellular peroxygenases (albeit based on copper-dependent catalysis) [13].
Modern molecular techniques such as genome editing using CRISPR/Cas could help to answer the question of the function of UPOs. To this end, UPO knock-out mutants of suitable model organisms would have to be generated and subsequently tested physiologically. Molecular and enzymatic field studies (e.g. in the context of the Biodiversity Exploratories, DFG SPP-1374) could also help to clarify the functions of UPOs (e.g. in a similar way to what has recently been achieved in the case of manganese peroxidases [14]). In addition to the further development of microbial expression systems (yeasts, molds, bacteria), cell-free expression with isolated ribosomes could be an innovative approach to accelerate the production of recombinant UPOs [15].
Acknowledgments
The authors would like to thank all colleagues who have contributed to the research on peroxygenases in the laboratory facilities in Zittau and Senftenberg over the past ten years. Research was supported by the Federal Ministry of Education and Research (BMBF, Germany, StStG Kohle Elimik, NGD ). We gratefully acknowledge the Ministry of Science, Research and Culture (MWFK, Brandenburg, Germany).
References
- Ullrich R, Nüske J, Scheibner K, et al. Novel haloperoxidase from the agaric basidiomycete Agrocybe aegerita oxidizes aryl alcohols and aldehydes. Appl Environ Microbiol 70 (2004): 4575-4581.
- Gröbe G, Ullrich R, Pecyna MJ, et al. High-yield production of aromatic peroxygenase by the agaric fungus Marasmius rotula. AMB Express 1 (2011): 31.
- Kiebist J, Schmidtke KU, Zimmermann J, et al. A Peroxygenase from Chaetomium globosum Catalyzes the Selective Oxygenation of Testosterone. Chembiochem 18 (2017): 563-569.
- Hofrichter M, Kellner H, Pecyna M, et al. Fungal unspecific peroxygenases: heme-thiolate proteins that combine peroxidase and cytochrome P450 properties. Adv Exp Med Biol 851 (2015): 341-368.
- Piontek K, Strittmatter E, Ullrich R. Structural basis of substrate conversion in a new aromatic peroxygenase: cytochrome P450 functionality with benefits. J Biol Chem 288 (2013): 34767-34776.
- Ullrich R, Poraj-Kobielska M, Scholze S. Side chain removal from corticosteroids by unspecific peroxygenase. J Inorg Biochem 183 (2018): 84-93.
- Hofrichter M, Ullrich. Oxidations catalyzed by fungal peroxygenases. Curr Opin Chem Biol 19 (2014): 116-125
- Poraj-Kobielska M, Kinne M, Ullrich R, et al. Preparation of human drug metabolites using fungal peroxygenases. Biochem Pharmacol 82 (2013): 789-796.
- Kiebist J, Hofrichter M, Zuhse R, et al. Oxyfunctionalization of pharmaceuticals by fungal peroxygenases. In: Pharmaceutical Biocatalysis - Chemoenzymatic Synthesis of Active Pharmaceutical Ingredients. Grunwald P (Hrsg.). Jenny Stanford Publishing Pte. Ltd., (2019).
- Karich A, Ullrich R, Scheibner K, et al. Fungal unspecific peroxygenases oxidize the majority of organic EPA priority pollutants. Front Microbiol 8 (2017): 1463.
- Wang X, Ullrich R, Hofrichter M, et al. Heme-thiolate ferryl of aromatic peroxygenase is basic and reactive. Proc Natl Acad Sci USA 112 (2015): 3686-3691.
- Hofrichter M, Kellner H, Herzog R, et al. Fungal peroxygenases: A phylogenetically old superfamily of heme enzymes with promiscuity for oxygen transfer reactions. In: Nevalainen H (Hrsg.) Grand Challenges in Fungal Biotechnology. Springer International Publishing, in press (2019).
- Forsberg Z, Sørlie M, Petrović D, et al. Polysaccharide degradation by lytic polysaccharide monooxygenases. Curr Opin Struct Biol 59 (2019): 54-64.
- Leonhardt S, Hoppe B, Stengel E, et al. Molecular fungal community and its decomposition activity in sapwood and heartwood of 13 temperate European tree species. PLoS One 14 (2019): e0212120.
- Thoring L, Zemella A, Kubick S. Accelerating the production of druggable targets: eukaryotic cell-free systems come into focus. Methods Protoc 2 (2019): 30.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 