Debilitating Pain of Endometriosis: Could Anti-Tumour Necrosis Factor Alpha Be a Saviour?
Salma Sultana1,3*, Aiman Hassan3, Iana Malasevskaia2,3, Stacey E. Heindl3
1Shadan Institute of Medical Sciences, Hyderabad, India.
2Private Clinic of Obstetrics and Gynecology, Sana’a, Republic of Yemen
3California Institute of Behavioral Neurosciences and Psychology, Fairfield, USA
*Corresponding Author: Salma Sultana, Shadan Institute of Medical Sciences, Hyderabad, India, California Institute of Behavioral Neurosciences and Psychology, Fairfield, USA
Received: 24 April 2021; Accepted: 10 May 2021; Published: 12 May 2021
Article Information
Citation: Salma Sultana, Aiman Hassan, Iana Malasevskaia, Stacey E. Heindl. Debilitating Pain of Endometriosis: Could Anti-Tumour Necrosis Factor Alpha Be a Saviour?. Fortune Journal of Health Sciences 4 (2021): 346-358.
View / Download Pdf Share at FacebookAbstract
Backgrounds: Endometriosis is a chronic, debilitating gynecological disorder causing pelvic pain, infertility, and emotional despair. Endometrial tissue production is based on estrogen provided by the ovaries and, thus, conventional management focuses on suppression of ovarian function. Current drug regimens are used to control the condition by causing the hypoestrogenism state. While this lack of circulating estrogen levels contribute to a regression of the disorder, this hypoestrogenicity often produces many negative and undesirable side effects. These drawbacks of existing drug therapies such as vaginal atrophy, dryness, bone loss, abnormalities in the lipid profile show their shortcomings and the need to produce new endometriosis treatments. The latest treatment for the medical management of endometriosis, and its limitations, is discussed in this review. Potential target areas that may be an enticing alternative to them also analyzed the conventional therapies and include aromatase inhibitors, gonadotropin-releasing hormone (GnRH) agonist, and tumor necrosis factor (TNF)-α inhibitors.
Aim of the study: The objective of the present comprehensive review was to determine the potency and safety of Anti-TNF-α in pelvic pain due to endometriosis and patient satisfaction with treatment in women with symptomatic recurrent endometriosis. The aim was to identify reports of studies including patients with endometriosis undergoing advanced non-hormonal, hormonal, surgical treatment, and evaluation of the effect of treatment on pain, recurrence, and spread of the endometriosis in premenopausal women.
Methods: Medline, PubMed, and Cochrane systemic review databases were searched using keywords: endometriosis, pelvic pain, dysmenorrhea, GnRH, Anti-TNF α, infliximab. A MeSH search through PubMed was conducted using combinations of keywords like endometriosis, advanced medical treatment, com
Keywords
<p>Endometriosis; Pelvic pain; Dysmenorrhea; GnRH; Anti-TNF α; Infliximab</p>
Article Details
Introduction & Background
Endometriosis is a debilitating, inflammatory disease in which endometrial glands or stroma are present outside the uterus, and it is found in women of reproductive age group [1]. Commonly affected areas are the peritoneum and pelvic organs, and sometimes other organs of the body such as the lungs are infrequently affected [1]. The symptoms that can be found in endometriosis based on clinical experience are dysmenorrhea, chronic pelvic pain, infertility, perimenstrual symptoms (e.g., bowel or bladder associated) with or without heavy bleeding, and dyspareunia [1]. The mechanism behind the endometriosis-related pelvic pain is not yet substantiated. Although extensive research has been performed to move forward our understanding of endometriosis, its natural history remains dubious, with an inconclusive etiology, eccentric clinical introduction, hard determination, and ineffectively standardized treatment. Approximately 80% of teenage girls with chronic pelvic pain who does not respond to traditional medical treatment have endometriosis [2,3]. In Figure 1, are summarized the signs and symptoms encountered in patients suffering of endometriosis.
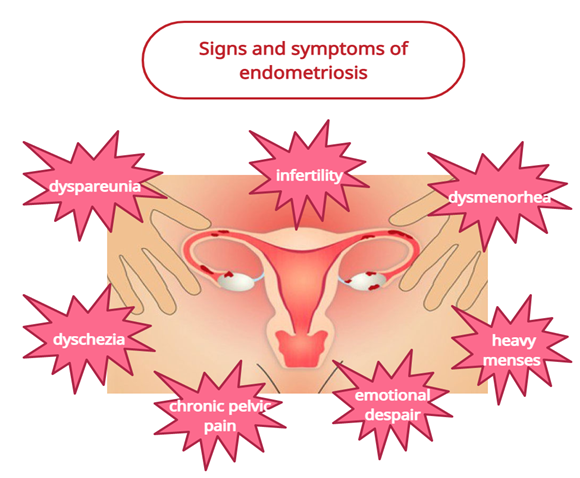
Figure 1: Signs and symptoms encountered in patients with endometriosis.
The diagnosis of endometriosis based on signs alone can be hard since the presentation is so complex and there is significant overlap with other disorders such as pelvic inflammatory disorder and irritable bowel syndrome. As a result, there is a period of delay between the onset of symptoms and diagnosis [1]. However, there is no agreement in the field that adjuvant medical care is appropriate for all adolescent patients or that long-term conditions such as recurrence, infertility, or chronic pain can be prevented [3,4]. The diagnosis of endometriosis can be made if acutely infiltrating nodules are found in the pouch of Douglas, or on the uterosacral ligaments, and if there are obvious lesions in the cervix or vagina. however, laparoscopy remains the gold standard investigation for the diagnosis of endometriosis [1].
Approximately 30 to 50% of women who have endometriosis is infertile and 25 to 50% of infertile women have endometriosis [5]. Until now, it has not been clear whether a medical approach is less expensive than a surgical approach in patients with endometriosis [5].
Treatment strategies
Unfortunately, all existing therapies for endometriosis (hormonal, non-hormonal) inhibit ovulation. In addition, severe side effects and potential long-term osteoporosis is associated with certain medical treatment types, such as gonadotropin-releasing hormone (GnRH) agonists [6,7]. Besides, recurrence of pain is common after the cessation of GnRH-agonist treatment. Data showed that the median pain recurrence period for patients treated with GnRH agonists was 5.2 months, and for patients treated with danazol was 6.1 months [7,8]. As shown in the Table 1, current medication used to cure endometriosis are combined oral contraceptives (COCs), gonadotroph releasing hormone agonists (GnRH agonists), nonsteroidal anti-inflammatory drugs (NSAIDs), aromatase inhibitors and tumour necrosis alpha factor inhibitors (TNF-alpha).
|
|
MEDICAL THERAPY OF ENDOMETRIOSIS
|
|
1. |
Combined Oral Contraceptives (COCs) |
|
2. |
NSAIDs |
|
3. |
GnRH agonists |
|
4. |
Aromatase inhibitors |
|
5. |
TNF-α inhibitors |
Table 1: Medication used for the treatment of endometriosis.
NSAIDs- nonsteroidal anti-inflammatory drugs, (TNF-α) inhibitors - Tumour necrosis factor-alpha Inhibitors, GnRH agonists- gonadotropin-releasing hormone agonist.
The treatment of endometriosis is aimed to control pain, maintain fertility, prevent a recurrence of disease, reduce operational intervention and improve the quality of life. Estrogen is the most potent stimulus of inflammation and survival in ectopic and eutopic endometrial tissues [9,10]. Because of this reason, treatment of endometriosis is more directed towards the suppression of estrogen production and ovulation [9]. The purpose of hormone therapy is to induce amenorrhea, thus producing a moderately hypoestrogenic state that prevents the progression of the disease and inhibits the inflammatory mechanism [10,11].
Conventionally, ovulatory processes associated with retrograde menstruation and estrogen release have been inhibited at the hypothalamus/pituitary stage by GnRH agonists, aromatase inhibitors, progestins, oral contraceptives, Cytokines [10,12]. Hormonal treatment of endometriosis which relies on suppression of estrogen includes gonadotropin-releasing hormone (GnRH) agonists, progesterone-only contraceptives, combined oral contraceptives, gonadotropin-releasing hormone (GnRH) agonists, aromatase inhibitors, and danazol [10,13]. Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most widely used first-line agents in the treatment of endometriosis-related pain. It works by inhibiting the enzyme COX, which is vital for the production of inflammatory mediators like cytokines, prostaglandins, and interleukins [13]. While both COX1 and COX2 receptors are present, studies have shown that COX 2 receptors are more abundant in ectopic endometrial tissues [13].
Long-term use of a selective COX2 inhibitor with fewer side effects decreases chronic pelvic pain, but the increased cardiovascular risk has restricted its use [10]. Despite the insufficient evidence about the efficacy of NSAIDs (naproxen, ibuprofen,) in the treatment of endometriosis-associated pain, the most common first-line drug in patients with pelvic pain is NSAIDs [13]. The suppression of the ovary along with the suppression of disease activity provides a basis for the use of hormonal contraception in endometriosis [13]. Progesterone alone or the combination of estrogen and progesterone contribute to decidualization of the endometriotic tissue and should slow the progression of the endometriosis [13]. In contrast to cyclical administration, continuous therapy with combined hormonal contraceptives (COC) have been found to have improved pain management [14].
Advantages of COC include a reasonable cost of treatment (compared to other hormonal drugs), the potential to lower the risk of ovarian, and endometrial cancer, can be administered on a long-term basis, and a contraception advantage [14,15]. GnRH agonists, synthetic peptide analogs of GnRH, are efficacious in the treatment of symptomatic endometriosis, and a reduction in the size of some eutopic or ectopic endometrial lesions [16]. Successful use of GnRH agonists depends on the certainty that it contributes to significant hypoestrogenism by inhibiting the synthesis of ovarian estrogen [13]. They are a better choice for women who haven't had success with COC or who cannot take COC because of their medical history [13]. Because of certain side effects like vaginal atrophy and dryness, bone loss, hot flashes, and abnormalities in lipid profile, GnRH agonists continuous use is limited to only up to six months [13]. Based on their composition GnRH agonists (nafarelin, buserelin, gonadorelin, leuprorelin, goserelin, triptorelin,) can be administered subcutaneously, intranasally, or intramuscularly [16].
In recent years, aromatase inhibitors (AIs), a novel class of drugs, have been recognized as a promising agent in the management of endometriosis. The primary sources of aromatase, an enzyme, are adipose cells in postmenopausal women and ovarian granulosa cells in premenopausal women [17]. AIs stop the production of estrogen in both the periphery and the ovaries and eliminates all estrogen production [10,13]. In postmenopausal women with endometriosis, the drug of choice is AIs such as letrozole or anastrozole [10]. Since AIs alone can cause ovarian folliculogenesis in premenopausal women, they're usually paired with a GnRH agonist or COC [10]. AIs have been shown to increase the quality of life and reduce pelvic pain in patients with endometriosis when used with oral contraceptives [17]. Ovarian follicular cysts and an increased risk of bone fractures and osteoporosis are among the side effects of AIs [13,17].
Treatment of endometriosis must be adjusted, taking into consideration the clinical condition of the patient in its entirety and its effect on the quality of life [1]. As expected, newer forms of endometriosis treatment have been suggested for therapies that interfere with a tumor necrosis factor-alpha (TNF-α) activity, such as chimeric Anti-TNF-α monoclonal antibody, infliximab, or TNF-α immunoglobulin fusion protein, etanercept. Recent studies have shown that the extent of spontaneous active endometriosis in baboons is reduced effectively by etanercept [7,18]. TNF-α is an inflammatory cytokine that plays a vital role in immune response initiation and regulation. It triggers inflammatory leukocytes and stimulates macrophages to produce interleukins, such as interleukin (IL) -1, IL-6, and TNF-derivatives [19]. It is produced to a great extent by macrophages and conjointly by several other cell types including lymphoid cells, pole cells, endothelial cells, fibroblasts, and nerve cells [20]. The peritoneal fluid in endometriosis patients are higher in TNF-α concentration than women with no endometriosis, and in active endometriosis, their concentrations were greater than in inactive endometriosis. With the highest concentrations being found in women with severe endometriosis [21]. In baboons with laparoscopically confirmed endometriosis, TNF-α blockade of p55 soluble TNF-α receptors results in inhibition of the production and growth of endometriotic implants [22,23].
As no clinical trials have been published on TNF-α blocker's efficacy in human endometriosis, we reviewed the impact of Anti-TNF-α like infliximab and etanercept on pain in women with endometriosis at a dosage showed to be active in IBD and rheumatoid arthritis [23].
Review
Pathophysiology:
Inflammation, fibrosis, and the development of adhesions and ovarian cysts are the major pathological processes involved in endometriosis [24]. However, 90 percent of women have retrograde menstruation, while just 10%-20% of women have endometriosis [24]. While extensive research has been conducted to enhance our understanding of endometriosis, this disease's etiology and pathogenesis is not clear. Animal models with endometriosis are helpful for the understanding of disease pathogenesis and the effectiveness of treatment strategies. The Olive Baboon (Papio Anubis) is the best specimen for the study of the pathogenesis of endometriosis [25,26]. Baboons develop endometriosis spontaneously when induced, and their menstrual cycle is identical to that of humans [25,27]. The baboon model enables us to analyze the development of the early phase of the disease and gain insights into how therapeutic interventions (such as lesion excision) can affect the immune response and modulate the progression of the disease. To develop more effective early intervention techniques, it is important to first consider the initial responses [25]. Sampson's hypothesis that endometrial tissue cells infiltrate the peritoneal cavity along the fallopian tubes by retrograde menstruation is broadly accepted [28]. The major factors in the pathogenesis of endometriosis are retrograde menstruation, peritoneal adhesion, and outgrowth of endometrial tissue [28,29].
According to this hypothesis, endometrial tissue fragments from an ectopic endometrial lesion and also, from the mucosa lining the uterus may be circulated into the venous circulation during menstruation. Embolic and metastatic endometriosis develops from the implantation of this emboli (endometrial tissue fragments) in adjacent veins [28]. Endometrial tissue that has been released during menstruation may continue to grow if it gets located to sites suitable for its existence [30]. This also explains the existence of endometriosis in different areas outside the pelvis, including the lymph nodes, brain, lung, extremities, and abdominal wall [28,29]. The involvement of endometrial tissue in the uterine vasculature, which has been reported in patients with adenomyosis, has scientifically confirmed this hypothesis [28]. Hasting et al. concluded in their study that, in a baboon induced with endometriosis there is a decreased apoptotic potential, estrogen hyper- responsiveness, progesterone resistance, and an increased angiogenic capacity and failing to respond adequately to embryonic signals leads to the decreased fertility associated with endometriosis [27].
A profound study conducted by Kyama et al. stated that women with endometriosis have more pronounced proteolytic and inflammatory properties in their menstrual endometrium compared to women without endometriosis [31]. The study conducted by Cunningham et al. gives indications of gradual but important structural immune changes in the non-human primate model of induced endometriosis. They have also reported an increased presence of immune cells in the peritoneal cavity and immune cells into Lymph nodes, draining the uterus in animals with induced endometriosis [25]. Endometrial mRNA expressions of IL-8, TNF-alpha, were higher during the menstrual phase relative to the luteal phase in women with endometriosis, while there was no such discrepancy in women with no endometriosis [31]. Only menstrual endometrium with elevated levels of inflammatory cytokines such as IL-8 and TNF-alpha can implant ectopically leading to the development of endometriosis. The amount of IL-8 and TNF-alpha can predict the natural development and severity of endometriosis in the menstrual endometrium [31]. Baboons with induced endometriosis exhibited functional molecular variations in the ectopic lesions and eutopic endometrium that are like those found in women with endometriosis [25,27].
Estrogen and Progesterone Receptors in Endometriosis
Endometriosis exhibits resistance to progesterone whereas dependency on estrogen. The key regulators in endometrial tissue are estrogen and progesterone, which control the expression of hundreds of genes during different phases of the menstrual cycle [32]. Intracellular estrogen production plays an important role in the pathophysiology of endometriosis, especially in postmenopausal females [33]. There are estrogen and progesterone receptors in both endometriotic and endometrial tissues, and they respond to these hormones by remarkably similar histological variations. In endometriosis, however, an attenuated or dysregulated progesterone resistance is reported at the molecular level, and, notably, progestin-based therapy of endometriosis is variably effective [34,35].
Progesterone resistance is a feature of endometriotic tissue relative to ectopic endometrium, while it has been identified in the ectopic endometrium of affected women compared to controls [36]. The symbiotic counterpart of estrogen overproduction and over-expression is the resistance of progesterone in endometriotic tissue that inhibits the modulation of genes involved in decidualization, control of the cell cycle, and inhibition of estrogen response [37]. In women with endometriosis, the expression of aromatase mRNA is higher in epithelial cells than in stromal cells, independent of the cycle, and higher in endometriosis lesions than in ectopic endometrium [38,39].
Immunology and Endometriosis
The association between the immune system and endometriosis was discovered almost 15 years ago, with the first description of TNF-alpha in peritoneal fluid in women with endometriosis [40]. Cytokines such as TNF-alpha and IL-8 facilitate the adhesion and proliferation of endometrial cells [31]. During the menstrual phase, the peritoneum is immunologically more active in contrast to the luteal phase and more so the peritoneum of women with endometriosis both in the luteal and menstrual phase varies biologically from that of controls (women without endometriosis) [31]. Kyama et al. also reported that during the menstrual phase there is up-regulation of IL-6 and transforming growth factor-beta whereas during the luteal phase there is up to regulation of TNF-alpha in the peritoneum of women with endometriosis when compared to controls [31].
Noble et al. demonstrated p450 transcription of aromatase in the ectopic endometrium of women with endometriosis and conversely, ectopic endometrium in women without endometriosis did not express the transcript of p450 aromatase [41]. In this respect, the most commonly studied cytokines are interleukin-1 (IL-1), IL-6, and TNF-α. Therefore, TNF-α is a sound choice for studies linked to endometriosis growth and progression. Certolizumab pegol (Cimzia), etanercept (Enbrel), adalimumab (Humira), and infliximab are the commercially active Anti-TNF-α drugs (Remicade). In this area, clinical trials and laboratory research are rare to date. D’hooghe et al. have showed that Anti-TNF-α treatment in baboons has decreased the progression of the disease and prevented the development of ndometriotic lesions and related adhesions without inducing the hypoestrogenic symptoms seen with conventional treatments [22].
One analysis showed that etanercept had reduced endometriotic implants. However, while Anti-TNF-α offers the potential to treat the disease, they are at an initial phase of development and have not yet been fully tested in clinical trials. Anti-TNF-α therapies may also have a negative impact on host defense, such as causing infections and malignancies, and necessary measures should therefore be taken [42]. More recently, Falconer et al. found that Anti-TNF mAb c5N decreases the number and location of active endometrial lesions and prevents the normal growth of peritoneal endometriosis seen in baboons [43]. The unique inhibitory effect on subtle red lesions means that in the treated population, not only the size but also development of the endometriotic lesions is decreased [43]. A study found that endometriosis and peritoneal and serum IL-6 and TNF-α levels of endometrial implants is decreased in size in the etanercept-treated population in rats [44].
Koninckx et al. in their study concluded that there seems to be no major beneficial effect of infliximab on pain associated with deep endometriosis. In two-thirds of women, they found an average placebo effect of 25%, which reached over 50%, stressing the need for well-conducted, double-blind, randomized placebo-controlled trials when testing experimental pain therapies. The effectiveness of surgical excision on pain has been confirmed, as has the efficacy of infliximab therapy during the pre-operative phase [23].
One study reports that the efficacy of infliximab did not raise the chances of adverse effects in the short-term for endometriosis participants [45]. They included one trial involving 21 subjects in the analysis. The findings revealed no evidence of improved pain score or decreased use of pain killers following treatment with infliximab, one of the known Anti-TNF-α medications. While there was no proof of a rise in adverse effects in the infliximab population relative to placebo, no therapeutic advantage of infliximab was reported concerning endometriotic lesions, dysmenorrhea, or pelvic tenderness and It is difficult to draw any conclusions regarding the cost-effectiveness of Anti-TNF-α medications in the treatment of endometriosis-related pelvic pain [45].
Further Research
Extensive research and studies can be done to explore the efficacy of TNF-α blockers in relation to pain, recurrence, and spread of endometrial tissue. Further studies are required to understand the potential of these drugs, to discover new treatment regimens in the management of endometriosis as it adversely affects the quality of life of a woman and also has a significant economic effect because of delays in diagnosis. The need for continuing care and high recurrence rates we think more experimental studies of Anti-TNF-α could change the management of symptoms related to endometriosis and they are significant as endometriosis is a chronic condition and patients need new and long-term benefits to avoid both worsening of the disease and recurrence of pain that are sustained following termination of treatment. Increased understanding of endometriosis pathophysiology would allow for the creation of new objectives in order to avoid adverse side effects and particularly to target lesions without affecting ovary function.
Currently, there are many therapeutic options available, both hormonal and non- hormonal, to provide symptomatic relief and to monitor disease progression. Most of the above treatment approaches have been fairly successful in managing pelvic pain in women with endometriosis but conversely, their adverse effects and negative effects on fertility restrain their long-term use. These drugs may be used either alone or with surgery in the selected women.
Conclusions
The primary aim of this review article was to shed light on the correlation of endometriosis and TNF-α receptors and how TNF-α blockers can help in relieving the pelvic pain of endometriosis. The presence of TNF-α receptors in the peritoneum of women with endometriosis has been elicited in this article. The recurrence of pain associated with endometriosis after the termination of treatment is normal, even after the brief follow-up periods recorded in these studies. Many studies have been conducted eliciting TNF-α receptors in the endometriosis patient, however, there is no sound evidence which shows complete cessation of pain or arrest of ectopic endometrial implants.
Treatment with these agents has shown that it reduced the size and number of endometriotic implants in animal models, with decreased inflammatory cytokines. However, there is insufficient evidence to support the use of Anti-TNF-α drugs like etanercept in the treatment of pain relief in women with endometriosis. Not much evidence of the clinical benefit of infliximab has been found for endometriosis-induced pelvic pain or no recurrence of endometriosis after cessation of TNF-α blockers. Anti-TNF-α therapy may be a potential non-hormonal therapeutic alternative to treat endometriosis in humans. There is not enough evidence to substantiate the use of Anti-TNF-α drugs in women with endometriosis to ease pelvic pain.
Conflict of Interest:
The authors have no conflict of interest to declare.
Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Kennedy S, Bergqvist A, Chapron C, et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Human Reproduction 20 (2005): 2698-2704.
- Yeung Jr P, Gupta S, Gieg S. Endometriosis in adolescents: a systematic review. Journal of Endometriosis and Pelvic Pain Disorders 9 (2017): 17-29.
- Sieberg CB, Lunde CE, Borsook D. Endometriosis and pain in the adolescent-striking early to limit suffering: A narrative review. Neuroscience & Biobehavioral Reviews 108 (2020): 866-876.
- Tandoi I, Somigliana E, Riparini J, et al. High rate of endometriosis recurrence in young women. Journal of Pediatric and Adolescent Gynecology 24 (2011): 376-379.
- Bulletti C, Coccia ME, Battistoni S, et al. Endometriosis and infertility. Journal of Assisted Reproduction and Genetics 27 (2010): 441-447.
- Olive DL. Medical therapy of endometriosis. Endometriosis in Clinical Practice (2004): 257-283.
- Shakiba K, Bena JF, McGill KM, et al. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstetrics & Gynecology 111 (2008): 1285-1292.
- Miller JD, Shaw RW, Casper RF, et al. Historical prospective cohort study of the recurrence of pain after discontinuation of treatment with danazol or a gonadotropin-releasing hormone agonist. Fertility and Sterility 70 (1998): 293-296.
- Bedaiwy MA, Allaire C, Yong P, et al. Medical management of endometriosis in patients with chronic pelvic pain. InSeminars in Reproductive Medicine 35 (2017): 038-053.
- Bulun SE, Yilmaz BD, Sison C, et al. Endometriosis. Endocr Rev 40 (2019): 1048.
- Barbieri RL. Hormone treatment of endometriosis: the estrogenthreshold hypothesis. American Journal of Obstetrics and Gynecology 166 (1992): 740-745.
- Bulun SE. Endometriosis. The New England Journal of Medicine 360(2009): 268-279.
- Rafique S, Decherney AH. Medical management of endometriosis. Clinical Obstetrics and Ggynecology 60 (2017): 485.
- Zorbas KA, Economopoulos KP, Vlahos NF. Continuous versus cyclic oral contraceptives for the treatment of endometriosis: a systematic review. Archives of Gynecology and Obstetrics 292 (2015): 37-43.
- Jensen JT, Schlaff W, Gordon K. Use of combined hormonal contraceptives for the treatment of endometriosis-related pain: a systematic review of the evidence. Fertility and Sterility 110 (2018): 137-152.
- Georgiou EX, Melo P, Baker PE, et al. Long-term GnRH agonist therapy before in vitro fertilisation (IVF) for improving fertility outcomes in women with endometriosis. Cochrane Database of Systematic Reviews (2019).
- Slopien R, Meczekalski B. Aromatase inhibitors in the treatment of endometriosis. Przeglad Enopauzalny= Menopause Review 15 (2016): 43.
- Barrier BF, Bates GW, Leland MM, et al. Efficacy of anti-tumor necrosis factor therapy in the treatment of spontaneous endometriosis in baboons. Fertility and Sterility 81 (2004): 775-779.
- Lee MK, Park AJ, Kim DH. Tumor necrosis factor-alpha and interleukin-6 promoter gene polymorphisms are not associated with an increased risk of endometriosis. Fertil Steril 77 (2002): 1304-1305.
- Zito G, Luppi S, Giolo E, et al. Medical treatments for endometriosis-associated pelvic pain. Biomed Research International 2014 (2014): 191967.
- Küpker W, Felberbaum R, Bauer O, et al. Significance of tumor necrosis factor alpha (TNF-alpha) in endometriosis. Geburtshilfe und Frauenheilkunde 56 (1996): 239-242.
- D’Hooghe TM, Nugent NP, Cuneo S, et al. Recombinant human TNFRSF1A (r-hTBP1) inhibits the development of endometriosis in baboons: a prospective, randomized, placebo-and drug-controlled study. Biology of Reproduction 74 (2006): 131-136.
- Koninckx PR, Craessaerts M, Timmerman D, et al. Anti-TNF-α treatment for deep endometriosis-associated pain: a randomized placebo-controlled trial. Human Reproduction 23 (2008): 2017-2023.
- Montgomery GW, Nyholt DR, Zhao ZZ, et al. The search for genes contributing to endometriosis risk. Human Reproduction Update 14 (2008): 447-457.
- Hey-Cunningham AJ, Fazleabas AT, Braundmeier AG, et al. Endometrial stromal cells and immune cell populations within lymph nodes in a nonhuman primate model of endometriosis. Reproductive Sciences 18 (2011): 747-754.
- Gashaw I, Hastings JM, Jackson KS, et al. Induced endometriosis in the baboon (Papio anubis) increases the expression of the proangiogenic factor CYR61 (CCN1) in eutopic and ectopic endometria. Biology of Reproduction 74 (2006): 1060-1066.
- Hastings JM, Fazleabas AT. A baboon model for endometriosis: implications for fertility. Reproductive Biology and Endocrinology 4 (2006): 1-4.
- Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. American Journal of Obstetrics & Gynecology 14 (1927): 422-469.
- Sampson JA. Heterotopic or misplaced endometrial tissue. American Journal of Obstetrics and Gynecology 10 (1925): 649-664.
- Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. The American Journal of Pathology 3 (1927): 93.
- Kyama CM, Overbergh L, Debrock S, et al. Increased peritoneal and endometrial gene expression of biologically relevant cytokines and growth factors during the menstrual phase in women with endometriosis. Fertility and Sterility 85 (2006): 1667-1675.
- Kao LC, Tulac S, Lobo SA, et al. Global gene profiling in human endometrium during the window of implantation. Endocrinology 143 (2002): 2119-2138.
- de Almeida Asencio F, Ribeiro HA, Ribeiro PA, et al. Symptomatic endometriosis developing several years after menopause in the absence of increased circulating estrogen concentrations: a systematic review and seven case reports. Gynecological Surgery 16 (2019): 1-11.
- Metzger DA, Olive DL, Haney AF. Limited hormonal responsiveness of ectopic endometrium: histologic correlation with intrauterine endometrium. Human Pathology 19 (1988): 1417-1424.
- Winkel CA, Scialli AR. Medical and surgical therapies for pain associated with endometriosis. Journal of Women's Health & Gender-Based Medicine 10 (2001): 137-162.
- Patel BG, Rudnicki M, Yu J, et al. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstetricia et Gynecologica Scandinavica 96 (2017): 623-632.
- McKinnon B, Mueller M, Montgomery G. Progesterone resistance in endometriosis: an acquired property?. Trends in Endocrinology & Metabolism 29 (2018): 535-548.
- Zeitoun K, Takayama K, Sasano H, et al. Deficient 17β-hydroxysteroid dehydrogenase type 2 expression in endometriosis: failure to metabolize 17β-estradiol. The Journal of Clinical Endocrinology & Metabolism 83 (1998): 4474-4480.
- Matsuzaki S, Canis M, Pouly JL, et al. Analysis of aromatase and 17β-hydroxysteroid dehydrogenase type 2 messenger ribonucleic acid expression in deep endometriosis and eutopic endometrium using laser capture microdissection. Fertility and Sterility 85 (2006): 308-313.
- Eisermann J, Gast MJ, Pineda J, et al. Tumor necrosis factor in peritoneal fluid of women undergoing laparoscopic surgery. Fertility and Sterility 50 (1988): 573-579.
- Noble LS, Simpson ER, Johns A, et al. Aromatase expression in endometriosis. The Journal of Clinical Endocrinology & Metabolism 81 (1996): 174-179.
- Ceyhan ST, Onguru O, Fidan U, et al. Comparison of aromatase inhibitor (letrozole) and immunomodulators (infliximab and etanercept) on the regression of endometriotic implants in a rat model. European Journal of Obstetrics & Gynecology and Reproductive Biology 154 (2011): 100-104.
- Falconer H, Mwenda JM, Chai DC, et al. Treatment with anti-TNF monoclonal antibody (c5N) reduces the extent of induced endometriosis in the baboon. Human Reproduction 21 (2006): 1856-1862.
- Zulfikaroglu E, K?l?c S, Islimye M, et al. Efficacy of anti-tumor necrosis factor therapy on endometriosis in an experimental rat model. Archives of Gynecology and Obstetrics 283 (2011): 799-804.
- Lu D, Song H, Shi G. Anti-TNF-α treatment for pelvic pain associated with endometriosis. Cochrane Database of Systematic Reviews (2013).


 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 