Efficacy and Safety of a Polyherbal Unani drug as an Adjuvant Therapy in type-2 diabetes mellitus: a double blind, Randomized, Placebo Controlled Study
Yasmeen Shamsi1, *, Manju Sharma2, Azhar Jabeen3, Wasi Akhtar4
1Department of Moalajat, School of Unani Medical Education & Research, Jamia Hamdard, New Delhi
2Department of Pharmacology, School of Pharmaceutical Education & Research, Jamia Hamdard, New Delhi
3Department of Moalajat, School of Unani Medical Education & Research, Jamia Hamdard, New Delhi
4Department of Moalajat, School of Unani Medical Education & Research, Jamia Hamdard, New Delhi
*Corresponding author: Yasmeen Shamsi, Department of Moalajat, School of Unani Medical Education & Research, Jamia Hamdard, New Delhi, India
Received: 14 December 2021; Accepted: 05 January 2022; Published: 18 February 2022
Article Information
Citation: Yasmeen Shamsi, Manju Sharma, Azhar Jabeen, Wasi Akhtar. Efficacy and Safety of a Polyherbal Unani drug as an Adjuvant Therapy in type-2 diabetes mellitus: a double blind, Randomized, Placebo Controlled Study. Fortune Journal of Health Sciences 5 (2022): 43-57
View / Download Pdf Share at FacebookAbstract
Background This double-blind, randomized, placebo-controlled study was conducted to assess the efficacy and safety of a polyherbal Unani drug as adjuvant therapy in Patients with Type-2 Diabetes who were inadequately controlled with Metformin monotherapy. Methods Patients who had inadequate glycaemic control (HbA1c between 7% to 10%) despite Metformin therapy were randomized into Unani Adjuvant therapy (n=40) and placebo (n = 38) groups. Patients were administered either Unani drug or Placebo at a dose of 02 capsules (each capsule = 500 mg) twice daily in addition to Metformin (500 mg twice daily) therapy. The primary endpoint was the change in glycosylated haemoglobin (HbA1c) levels from the baseline to week 12. Result There was a statistically significant decrease in HbA1c levels from the baseline in the Unani drug group (-1.89 ± 0.05 %, p=.001) compared with the placebo group (-0.53 ± 0.20 %, p= 0.166) at week 12. Compared with the Placebo group, Unani drug significantly decreased fasting plasma glucose levels (-26. 34 ± 11.73, p= 0.002 vs -4.82 ± 2.63 mg/dl, p= 0.089) and postprandial plasma glucose (-60.02 ± 10.18 mg/dl, p= 0.001 vs -18.70 ± 2.06 mg/dl, p= 0.039). Conclusion Unani polyherbal drug as an add on therapy to Metformin in patients with type 2 diabetes who were not at their goal for glycaemic control with Metformin monotherapy produced significant reduction in HbA1c, FPG and PPG levels. Unani drug (or Placebo) add on therapy with Metformin was well tolerated, and showed an overall safety profile similar to placebo.
Keywords
Type 2 Diabetes; Metformin; Adjuvant Therapy; Nigella sativa; Trigonella foenum; Cichorium intybus; Azadirachta indica
Type 2 Diabetes articles; Metformin articles; Adjuvant Therapy articles; Nigella sativa articles; Trigonella foenum articles; Cichorium intybus articles; Azadirachta indica articles
Article Details
1. Introduction
Diabetes mellitus (DM) is the most common metabolic disease and 90% of Diabetic patients have Type 2 diabetes mellitus [1]. Type 2 diabetes mellitus refers to chronic hyperglycaemia with carbohydrate, fat, and protein metabolic disorder caused due to insulin resistance and/ or inadequate insulin secretion [2, 3, and 4]. The worldwide prevalence of type 2 diabetes is showing an alarming rise to epidemic proportions due to sedentary lifestyles, unhealthy diets, and increased prevalence of obesity [5].
The rapid and alarming emergence of type 2 diabetes mellitus is a serious issue in developing countries [6]. The International Diabetes Federation (IDF) has reported that 578 million people will suffer from Diabetes by 2030, and 700 million by 2045[7]. India, the diabetes capital of the world, has taken lead due to the high prevalence of diabetic patients. The International Diabetes Federation (IDF) has estimated that in 2017 the prevalence of diabetic patients in India, was 72.9 million, and which may likely upsurge to 134.3 million by the year 2045 [8]. Moreover, around 193 million undiagnosed and untreated diabetics, worldwide, are at high risk to develop complications including retinopathy, nephropathy, neuropathy, and cardiovascular diseases [9],[10]. The risk of complications of diabetes is reduced significantly by strict upkeep of glycaemic control in diabetic patients [9],[11]. The management of patients with type 2 diabetes mellitus focuses on lifestyle modification which includes alteration in diet, increased physical activity, and exercise. Metformin is the first-line oral anti-hyperglycaemic drug in most patients with Type 2 diabetes mellitus when glycaemic control cannot be attained only by lifestyle interventions [12, 13].
Metformin monotherapy is effective in the average patient with T2DM, but still is not sufficient to maintain normoglycaemia due to various pathophysiological factors playing role in the disturbance of glucose metabolism in T2DM and the disease is a progressive disorder characterized by ongoing deterioration of glycaemic control [11]. It has been reported that 40-50% of patients fail to halt the progression of the disease and maintain normoglycaemia after 2 years, 70% after 3 years, and 90% after 9 years of metformin monotherapy [15]. When Metformin monotherapy fails to control hyperglycaemia, additional therapies are required to maintain appropriate glycaemic targets [11]. The drugs that are added to metformin therapy to extend the efficacy of oral diabetes treatment mainly include sulfonylureas, dipeptidyl-peptidase-4 (DPP-4) inhibitors, or thiazolidinediones (TZD). These often show reduced efficacy over time and are associated with many adverse reactions such as cardiovascular disease, liver, and kidney dysfunction [14]. However, many of the anti-diabetics used as second-line treatment have tolerability issues and are accompanied by adverse effects such as gastrointestinal side effects, hypoglycaemia, weight gain, pancreatitis and pancreatic cancer, heart failure, etc. [15, 16, 17]. Therefore, there is a need for new agents that are not only effective, well-tolerated, and safe but also improve the quality of life and can be added to metformin therapy when metformin alone fails to maintain glycaemic control.
Many medicinal plants have been used worldwide for antidiabetic effects and the possibility that these herbal components enhance or facilitate the action of antidiabetic conventional drugs through additive or synergetic actions besides neutralizing or protecting the body from the toxic effects of the conventional treatments in diabetes. This has led to the search for a new antidiabetic pharmaceutical agent from medicinal plants useful in the treatment of various diseases. Many medicinal plants and their formulations are used for treating diabetes in the Unani medical system. However, most of these drugs have not been evaluated clinically on scientific parameters. In the present double-blind, randomised, 12-week study, Unani drug ‘Diabeat ', was compared with the Placebo as adjuvant therapy in patients who had poor glycaemic control with metformin alone. The investigational product ‘Diabeat ' is a Unani polyherbal formulation that contains fine powder of dried Nigella sativa Linn. (Kalonji) seed, dried Trigonella foenum-graecum Linn. (Methi) seed, Cichorium intybus Linn. (Kasni) seed, and Azadirachta indica A. Juss.) (Neem) leaf.
2. Material and Methods
This phase 2, Randomized, Parallel Group, Placebo Controlled study was conducted at Clinical Research Unit, Majeedia Hospital, Jamia Hamdard, New Delhi. The institutional ethics committee, Jamia Hamdar, reviewed and approved the study protocol, patient information sheet and informed consent and the study was conducted in accordance with Good Clinical Practice guidelines, and the Declaration of Helsinki [18]. This study was registered at Clinical Trials Registry of India, vide CTRI No. CTRI/2009/091/000702.
2.1 Study population and eligibility criteria
Patients were assessed for study eligibility at the screening visit; all patients provided written informed consent before any study-related procedures were conducted.
2.1.1 Key inclusion criteria were
- Patients diagnosed with Type 2 diabetes mellitus as defined in the American Diabetes Association Standards of Medical Care in Diabetes 2011(HbA1c ≥6.5% or FPG ≥126 mg/dl or 2-h plasma glucose ≥200 mg/dl or a random plasma glucose ≥200 mg/dl in a patient with classic symptoms of hyperglycaemia or hyperglycaemic crisis)[19].
- Male or female of 30-60 years age, on stable (≥ 3 months) metformin mono therapy ≥ 1000 mg/day with uncontrolled hyperglycaemia (HbA1c between 7% to 10%) and Body Mass Index (BMI) between 25-29.9 kg/m2.
- Patients who were able and willing to follow anti-diabetic therapy under a stable life style and diabetic diet.
2.1.2 Key exclusion criteria were
- History of treatment with any medication for glycaemic control (except metformin) within 3 months
- History of treatment with systemic corticosteroids within 3 months
- Diabetes mellitus with complications
- Pregnant or Lactating women.
- History of any severe systemic disease.
- Patients with uncontrolled hypertension.
- History of drug or alcohol abuse.
2.2 Assessments
Screening was performed after an overnight fast for ≥10 hours. The usual Metformin dose was administered in the night before, but no Metformin was given on the morning when screening was performed. Demographic data, height and weight were recorded at baseline and assessment of vital signs, general physical examination, laboratory investigations (PFG,PPG, CBC, ESR, LFT, KFT, Urine R/M) were carried out on each scheduled visit during the 12-week treatment period (baseline, 4,8 and 12 weeks). HbA1c was assessed at baseline and at the end of the study (after 12 weeks). Fasting blood samples were also tested for Lipid Profile (total cholesterol, triglycerides, high density lipoprotein and low-density lipoproteins at baseline and week 12, as deranged blood lipid profile is usually associated with uncontrolled diabetes mellitus and an important risk factor for cardiovascular diseases.
The Primary efficacy end-point was change in HbA1c levels at 12 weeks as compared to the baseline levels in both the groups. The secondary outcome measures were change in FPG and PPG levels at 12 weeks. As compared to baseline levels and the percentage of patients with HbA1c £ 6.5% at 12 weeks. Safety assessment included adverse events reporting by the patients, clinical evaluation (physical examinations, examination of vital signs) and laboratory evaluation (CBC, ESR, LFT, KFT, Urine R/M).
2.3 Intervention
Unani polyherbal drug ‘Diabeat ' (500 mg ) and Placebo (500 mg ) were prepared as identical capsules by Hamdard Laboratories, India. Each ‘Diabeat ' capsule contains Tukhm-e-Kalonji (Nigella Sativa seed)- 240.0 mg, Tukhm-e-Methi (Trigonella foenum graecum seed)- 120.0 mg, Tukhm-e-Kasni (Cichorium intybus seed)- 120.0 mg and Barg-e-Neeb (Azadirachta Indica leaf)- 20.0 mg.
The patients were randomly assigned to receive either ‘Diabeat ' (02 capsules, 500 mg each twice daily) or Placebo 02 capsules 500 mg twice daily) 30 minutes before meals in addition to Metformin SR (500 mg twice daily) marketed by Franco Indian Pharmaceuticals Pvt Ltd (Glycephage SR). The patients were advised to continue with the lifestyle modifications including daily moderate exercise (around 150 minutes/week) and controlled diet 1600 calorie diabetic diet. Patient compliance was checked by counting remaining capsules/tablets on every follow up visit.Any missed visit that occurs during the double-blind treatment period was rescheduled within 1 week. Two or more consecutive missed visits were grounds for immediate subject discontinuation from the study.
2.4 Statistical Analyses
The results were expressed as mean ± SD, differences between normally distributed data were assessed using a two-tailed Student t test (paired within groups and unpaired between groups).
The results were expressed as mean ± SD.
3. Results
3.1 Enrollment of Patients
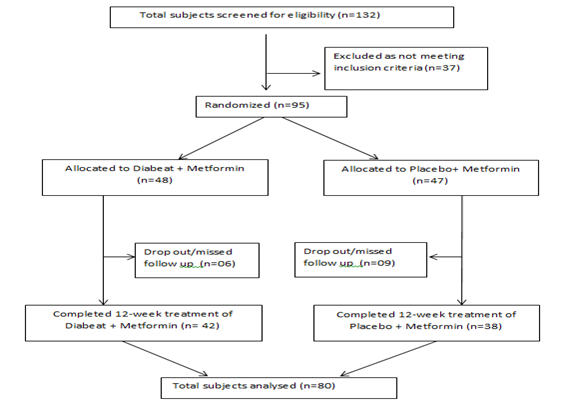
Total 132 patients of type 2 diabetes mellitus with a background of metformin therapy were screened for eligibility criteria, out of them 95 as per inclusion/exclusion criteria were enrolled and randomized to receive double-blind study medication (Diabeat +Metformin, n=48 or Placebo + Metformin, n=47). Six patients in ‘Diabeat ' group and 09 patients in Placebo group missed the subsequent follow up visits and considered as dropped out. Total 80 participants (‘Diabeat ', n=42; Placebo, n=38) completed all scheduled study visits and included in statistical analysis (Figure-1).
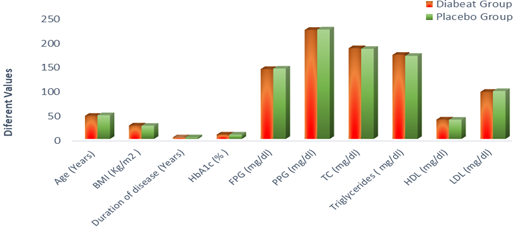
The baseline demographics of ‘Diabeat ' (+ Metformin) and Placebo (+ Metformin) treated patients were comparable (Table-1, Figure-2). In the ‘Diabeat 'and Placebo groups, mean ages were 47.18 (SD ± 12.21) and 48.38 (SD ± 10.46) years, body mass index (BMI) were 27.40 Kg/m2 (SD ±1.62) and 26.88 Kg/m2 (SD±1.28), and durations of T2DM were 3.34 years (SD ± 1.21) and 3.18 years (SD ± 1.61), respectively. The mean HbA1c levels were 8.95% (SD ± 0.84) and 8.89% (SD ± 0.79), FPG levels were 142.64 mg/dl (SD± 38.42) and 143.62 mg/dl (SD ± 39.20) respectively and PPG levels were 222.52 mg/dl (SD± 24.42) and 223.46mg/dl (SD ± 19.32) respectively. The levels of TC, Triglyceride, HDL and LDL were 185.11mg/dl (SD ± 32.09), 171.70mg/dl (SD ± 64.11), 39.55mg/dl (SD ± 8.32) and 96.15 mg/dl (SD ± 28.87) respectively in Diabeat group. The levels of TC, Triglyceride, HDL and LDL were 183.91 mg/dl (SD ± 36.82), 169.60 mg/dl (SD ± 74.19), 39.35 mg/dl (SD ± 8.71) and 96.65 mg/dl (SD ± 34.13) respectively in Placebo group.
Table-1: Demographic and Baseline characteristics of patients in each group (n=80)
|
Parameters |
Diabeat Plus Metformin (n=42) |
Placebo Plus Metformin (n=38) |
Tota3 (n=80) |
|
Gender: Male Female |
18 24 |
15 23 |
33 47 |
|
Age (Years) - Mean± SD |
47.18±12.21 |
48.38±10.46 |
47.78±11.38 |
|
BMI (Kg/m2 )-Mean ± SD |
27.40±1.62 |
26.88±1.28 |
27.14±1.49 |
|
Duration of disease (Years) |
3.34 ± 1.21 |
3.18 ± 1.61 |
3.27 ± 1.42 |
|
HbA1c (% )-Mean± SD |
8.95 ± 0.84 |
8.89 ± 0.79 |
8.94 ± 0.84 |
|
FPG (mg/dl)-Mean± SD |
142.64 ± 38.42 |
143.62 ± 39.20 |
143.13 ± 38.85 |
|
PPG (mg/dl)-Mean± SD |
222.52 ± 24.42 |
223.46 ± 19.32 |
143.13 ± 23.13 |
|
TC (mg/dl)-Mean± SD |
185.11 ± 32.09 |
183.91 ± 36.82 |
184.53 ± 34.46 |
|
Triglycerides ( mg/dl)-Mean± SD |
171.70 ± 64.11 |
169.60 ± 74.19 |
170.65 ± 69.17 |
|
HDL (mg/dl)-Mean± SD |
39.55 ± 8.32 |
39.35 ± 8.71 |
49.40 ± 8.54 |
|
LDL (mg/dl)-Mean± SD |
96.15 ± 28.87 |
97.65 ± 34.13 |
96.90 ± 31.51 |
BMI= Body Mass Index; HbA1c=Glycosylated Haemoglobin; FPG=Fasting Plasma Glucose; PPG=Postprandial Plasma Glucose; TC=Total Cholesterol; TG=Triglycerides; HDL=High Density Lipoproteins; LDL = Low Density Lipoproteins; SD=Standard Deviation
3.2 Primary outcome
3.2.1 Glycosylated hemoglobin (HbA1c) levels
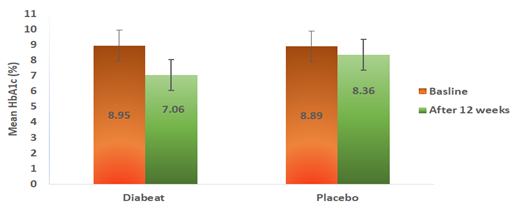
As shown in Table-2 and Figure-3, by the end of treatment (12 weeks), the add on Diabeat to ongoing Metformin therapy significantly (p =0.001) reduced HbA1c from a baseline of 8.95% (SD ± 0.84) to 7.06% (SD ± 0.79) compared with no significant change from a baseline of 8.89 % (SD± 0.79 ) to 8.36% (SD ± 0.99) in the Placebo group (p=0.166). On applying unpaired.‘t’ test The mean reduction in HbA1c in Diabeat group was also found significantly greater the that of Placebo group (p=0.005).
3.3 Secondary outcomes:
3.3.1 Fasting plasma glucose
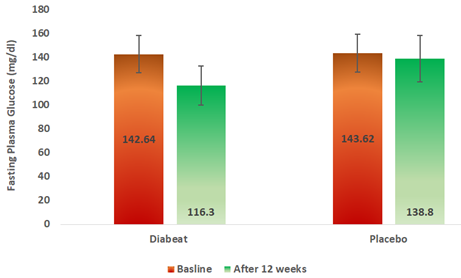
At the end of 12 weeks, Diabeat add on therapy significantly (p = 0.002) reduced fasting (116.30 ± 26.69mg/dl) plasma glucose levels as compared to baseline (142.64 ± 38.42 mg/dl) (Table-2, Figure-4).Whereas, in the Placebo add on to Metformin group there was an insignificant reduction (p = 0.089) in fasting blood sugar levels (138.80 ± 41.83 mg/dl) in 12 weeks as compared to the baseline (143.62 ± 39.20 mg/dl) (Table-2, Figure-4). The difference in pre-treatment and post-treatment FPG levels was significantly greater in Diabeat group than in Placebo group (p = 0.001)
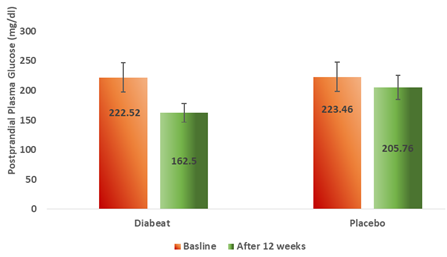
3.3.2 Postprandial plasma glucose
At the end of 12 weeks, Diabeat add on therapy significantly (p = 0.001) reduced 2 hour postprandial plasma glucose (162. 50 ± 14.24 mg/dl) levels as compared to baseline (222.52 ± 24.42mg/dl) (Table-2, Figure-5). Whereas, in the Placebo add on to Metformin group there was an insignificant reduction (p = 0.039) in postprandial plasma glucose levels (205.76 ± 21.38 mg/dl) as compared to the baseline (223.46 ± 19.32 mg/dl) (Table-2, Figure-5). The change in pre-treatment and post-treatment postprandial plasma glucose (PPG) levels was significantly greater in Diabeat group than in Placebo group (p = 0.001)
3.3.3 Fasting lipid profile
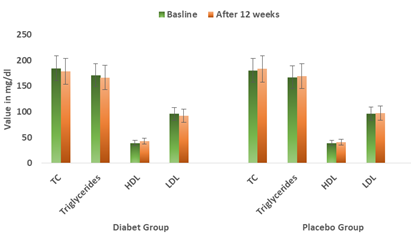
Treatment with Diabeat or Placebo ads on therapy for 12 weeks did not demonstrate a statistically significant change in any of the lipid profile parameters. At 12 weeks, the patients on Diabeat add on therapy showed statistically insignificant reduction in levels of total cholesterol (-6.41 ± 1.67mg/dl; p= 0.026), triglycerides (-4.7 ± 5.29 mg/dl; p =0.389), and low-density lipoproteins (Mean reduction 3.75 ± 4.77= mg/dl; p = 0.510). The levels of high density lipoprotein were insignificantly increased by +3.65 ± 0.80 mg/dl after 12-week Diabeat add on therapy (Table-2, Figure-6). In Placebo plus Metformin group, there was an insignificant increase in TC (+2.89 ± 2.68 mg/dl; p =0.862) and TG (+2.00 ± 4.73 mg/dl; p = 0.967), HDL (+1.45 ± 0.68 mg/dl; p =0. 885) and LDL (+1.15 ± 2.86 mg/dl; p = 0.794).
3.3.4 Safety and tolerability
The measured treatment compliance was over 98% in both Diabeat (+Metformin) and Placebo (+Metformin) treatment groups. Both treatments were very well-tolerated over 12 weeks. Occasional side effects observed in few patients were nausea, abdominal discomfort and/or diarrhea (the known side effects of Metformin). The intensity of these adverse effects was mild in nature. There were similar rates of reported adverse effects with no differences between groups at each time point of inquiry. There were no significant changes in clinical laboratory variables related to the haematology, hepatic function, renal function, lipid profile and electrolytes from baseline to Week 12 (end of treatment) in any of the treatment groups.
Table-2: Comparative effects of Diabeat and Placebo adjuvant Therapy on various parameters (baseline to week 12)
|
Parameters |
Group |
Diabeat plus Metformin |
Placebo plus Metformin |
P value# |
|
HbA1c (%))-Mean ± SD |
Baseline |
8.95 ± 0.84 |
8.89 ± 0.79 |
0.294 |
|
Week 12 |
7.06 ± 0.79 |
8.36 ± 0.99 |
0.005 |
|
|
Difference |
-1.89 ± 0.05 |
-0.53 ± 0.20 |
||
|
P value* |
0.0012 |
0.166 |
||
|
FPG (mg/dl)-Mean ± SD |
Baseline |
142.64 ± 38.42 |
143.62 ± 39.20 |
0.287 |
|
Week 12 |
116.30 ± 26.69 |
138.80 ± 41.83 |
0.001 |
|
|
Difference |
-26. 34 ± 11.73 |
-4.82 ± 2.63 |
||
|
P value* |
0.002 |
0.089 |
||
|
PPG (mg/dl)-Mean ± SD |
Baseline |
222.52 ± 24.42 |
223.46 ± 19.32 |
0.279 |
|
Week 12 |
162. 50 ± 14.24 |
205.76 ± 21.38 |
0.001 |
|
|
Difference |
-60.02 ± 10.18 |
-18.70 ± 2.06 |
||
|
P value* |
0.001 |
0.039 |
||
|
TC (mg/dl)-Mean ± SD |
Baseline |
185.11 ± 32.09 |
181.02 ± 36.82 |
0.267 |
|
Week 12 |
178.70 ± 33.76 |
183.91 ± 34.14 |
0.010 |
|
|
Difference |
-6.41 ± 1.67 |
+ 2.89 ± 2.68 |
||
|
P value* |
0.026 |
0.862 |
||
|
TG (mg/dl)-Mean± SD |
Baseline |
171.70 ± 64.11 |
167.60 ± 74.19 |
0.269 |
|
Week 12 |
167.00 ± 58.82 |
169.60 ± 69.46 |
0.052 |
|
|
Difference |
-4.7 ± 5.29 |
+2.00 ± 4.73 |
||
|
P value* |
0.389 |
0.967 |
||
|
HDL (mg/dl)-Mean± SD |
Baseline |
39.55 ± 8.32 |
39.35 ± 8.71 |
0.301 |
|
Week 12 |
43.20 ± 7.52 |
40.80 ± 8.03 |
0.053 |
|
|
Difference |
+3.65 ± 0.80 |
+1.45 ± 0.68 |
||
|
P value* |
0.3452 |
0.885 |
||
|
LDL (mg/dl)-Mean± SD |
Baseline |
96.15 ± 28.87 |
96.50 ± 34.13 |
0.299 |
|
Week 12 |
92.40 ± 24.10 |
97.65 ± 36.99 |
0.027 |
|
|
Difference |
-3.75 ± 4.77 |
+1.15 ± 2.86 |
||
|
P value* |
0.510 |
0.794 |
HbA1c=Glycosylated Haemoglobin; FPG=Fasting Plasma Glucose; PPG=Postprandial Plasma Glucose; TC=Total Cholesterol; TG=Triglycerides; HDL=High Density Lipoproteins; LDL = Low Density Lipoproteins; SD=Standard Deviation.
4. Discussion
Metformin is the first-line anti-diabetic treatment and the most recommended monotherapy for type 2 diabetes mellitus [20-23]. Metformin improves insulin resistance in peripheral tissue and hepatic cells, making it generally accepted as an oral anti-diabetic drug of choice, particularly for newly diagnosed patients with T2DM [24]. But, with the progress of the disease and the ongoing beta-cell failure, Metformin mono-therapy fails to control the hyperglycaemia and patients generally require the addition of a second line anti diabetic medication [25]. To maintain glucose control, a combination of antihyperglycemic medications like sulfonylureas, dipeptidyl peptidase 4 inhibitors, thiazolidinediones, GLP-1 receptor agonists, or sodium-glucose co-transporter-2 (SGLT2) inhibitors, as add-on therapy with metformin is usually recommended [14, 21, 26]. However, most of these drugs are associated with frequent episodes of hypoglycaemia and also have other widely varying side-effects [15-17].
This randomized placebo-controlled clinical trial aimed to assess the safety and efficacy of a polyherbal Unani drug ‘Diabeat’ as an add-on to metformin therapy in Type 2 diabetes mellitus patients with poor glycaemic control with Metformin. The addition of Diabeat to a stable dose of Metformin therapy provided significant reductions in HbA1c, FPG, and PPG levels in comparison to the placebo after 12-weeks. Reduction in FPG and PPG levels was observed as early as the fourth week of therapy and the significant effect persisted in the subsequent visits (8, 12 weeks) as compared to the placebo group. The mean HbA1c% recorded after 12 weeks of Diabeat with Metformin treatment, was 7.0 % with a mean standard deviation of ± 0.79%, which is close to the target level of HbA1c (£ 6.5%). In Diabeat plus Metformin group at the endpoint (12 wks), 14 patients achieved HbA1c between 7.0-7.5% ( as compared with Placebo plus Metformin, only 04 patients could attain this target); 22 patients HbA1c between 6.5-7.0% (compared to 02 patients reached this level with Placebo plus Metformin ) and HbA1c £ 6.5% was attained by 06 patients with Diabeat plus Metformin. In the Placebo plus metformin group, the HbA1c level remained above 7.5% with a mean value of 8.36% (SD ± 0.99%) in 32 of the total 38 patients, and an HbA1c level of £ 6.5% was not achieved by any of the patients in this group.
Treatment with Diabeat or Placebo with Metformin for 12 weeks did not demonstrate a statistically significant change in any of the lipid parameters. Mean total cholesterol and low-density lipoprotein cholesterol levels were numerically lower with Diabeat plus Metformin therapy after 12 weeks of treatment and on the other hand, there was a slight numerical increase in all lipid parameters with Placebo plus Metformin therapy.
Diabeat (or Placebo) add on therapy with Metformin was well tolerated and safe. The proportion of patients with side effects of nausea, abdominal discomfort, and diarrhea of mild intensity (the known side effects of Metformin) was almost similar in both groups during the 12-week double-blind treatment period. There was no significant change in laboratory safety parameters like hematology, hepatic function, renal function, lipid profile, and electrolytes from baseline to end of the treatment (Week 12) in both the treatment groups. No patient in any group of treatment-experienced hypoglycaemic episodes.
In summary, this study demonstrated that Diabeat as an add on therapy to Metformin in patients with type 2 diabetes who were not at their goal for glycaemic control with Metformin monotherapy resulted in a significant reduction in HbA1c level and a greater proportion of patients (approximately 53% ) achieving an HbA1 c level between 6.5-<7%, and £ 6.5% in approximately 14% of patients compared with those patients receiving Placebo add-on to Metformin therapy. Diabeat adjuvant therapy, also significantly improved glycaemic control (both FPG and PPG levels) without any risk of hypoglycaemia and was not associated with any pronounced side effects during or after 12-week therapy. A few patients reported nausea, abdominal discomfort, and/or diarrhea (the known side effects of Metformin). The intensity of these adverse effects was mild.
The anti-hyperglycaemic potential, of all the ingredients present in Diabeat capsule, have been reported through several scientific studies and the effects of this polyherbal Unani drug could be attributed to various mechanisms revealed regarding the anti-hyperglycemic activity of Neem (Azadirachta Indica), Methi (Trigonella foenum graecum), Kalonji (Nigella Sativa) and Kasni (Cichorium intybus). In a study conducted by Satyanarayana K et al., Azadirachta indica leaf extract has regularised the abnormal levels of blood glucose, serum insulin, lipid profile, and insulin signaling molecules as well as glucose transporter 4 (GLUT4) proteins, in rats who were treated with 400 mg/kg b.wt dose once daily for 30 daysafter developing diabetes (type-2) by giving high-fat diet and 25% fructose feeding through drinking water [27].
Methi (Trigonella foenum-graecum), is a Unani herbal drug well-known for its antidiabetic activity, and its seeds and leaf extracts are being widely used as source material for antidiabetic compounds. Several studies have proved its anti-hyperglycaemic, anti-hyperlipidaemic and anti-oxidant activities [28],[29],[30]. Trigonella foenum decreases fasting and postprandial blood glucose levels through its insulin-resistance reducing action [31]. The abundant dietary fibers of Trigonella foenum, which are mainly composed of the indigestible polysaccharide galactomannan, have revealed antidiabetic properties via reducing dietary carbohydrates and lipids absorption by inhibiting intestinal carbohydrate-hydrolyzing enzymes and lipases respectively [32].
Kasni (Cichorium intybus) is extensively used as a traditional treatment in India for diabetes mellitus as it has significant anti-hyperglycaemic and anti-hyperlipidaemic activities [33], [34],[35,44]. Cichorium intybus has additionally been shown to reduce insulin resistance and increase glucose uptake by muscle cells as well as by adipocytes [36],[37],[38] and also enhance pancreatic b-Cells [43]. Furthermore, it has been studied that the Cichorium intybus reduces glucose absorption in the intestine by inhibiting the breakdown of disaccharides in the gastrointestinal tract and it also prevents the release of glucose from the liver [38].
Kalonji (Nigella sativa ), commonly known as black seed, is a well-known Unani herb used for centuries for treating various illnesses including diabetes mellitus. Many studies have demonstrated the anti-diabetic and anti-hyperlipidaemic activities of Nigella sativa in diabetic animal models [39, 40]. Nigella sativa has been shown to increase hepatic glycogen content, thus inhibiting gluconeogenesis probably through its thymoquinone antioxidant potential [41, 42]. Nigella sativa has also been shown to increase glucose uptake in skeletal muscles, hepatocytes, and adipocytes [41] and decrease insulin resistance [42].
The anti-hyperglycaemic effects of Diabeat observed in the present study could have resulted from the above described combined activities of its herbal ingredients Neem (Azadirachta Indica), Methi (Trigonella foenum graecum), Kalonji (Nigella Sativa), and Kasni (Cichorium intybus). The findings of this study suggest that Diabeat could be used as an adjuvant drug for oral antidiabetic treatment in patients with diabetes type 2. The effect can be further validated on a larger population of diabetic patients.
5. Conclusion
Diabeat has therapeutic value as an anti-hyperglycaemic effect and significantly improves the glycaemic control in the treatment of diabetes type 2 when added to metformin in patients not efficiently controlled on metformin monotherapy, without any increased risk of adverse effects.
Limitations of the Study
Limitations of the study are the small sample size and the short course of treatment. Studies of longer duration of at least 6 months comparing Diabeat monotherapy with Metformin monotherapy and combination of both Diabeat and Metformin can also help us in further ascertaining the efficacy and safety of Diabeat.
Acknowledgements
The authors would like to acknowledge Hamdard National Foundation, New Delhi, India, for supporting this research and providing the study drugs. We are indebted to Late Dr. Arun Mukharjee, Formerly Consultant, Majeedia Hospital and Medical Director and Founder Trustee FSMHP-UDAAN, New Delhi, for his valuable suggestions and generous support throughout this study.
Conflicts of Interest
The author declares no conflict of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- Wang Y, Yan A, Li S, Liu B, Li H & Yan Y. Efficacy and safety of berberine in the treatment of type 2 diabetes with insulin resistance: Protocol for a systematic review. Medicine (Baltimore) 98 (2019): e1694.
- Wu Y, Ding Y, Tanaka Y & Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int J Med Sci 11 (2014): 1185-1200.
- Harsh Mohan. Diabetes Mellitus. In Textbook of Pathology 7th Edition (p. 808). New Delhi, India: Jaypee Health Science Publishers (2015).
- Classification of diabetes mellitus. Retrieved from www.who.int/health-topics/diabetes (2019).
- Khan MA, Hashimi MJ, King JK, Govender RD Mustafa H & Al Kaabi J. Epidemiology of Type 2 Diabetes - Global Burden of Disease and Forecasted Trends. Journal of Epidemilogy and Global Health 10 (2020): 107-111.
- Yeole UL, Jiandani MP, Kunjir SR, Bhat SM. Quality of life of patients with type 2 diabetes mellitus: A cross-sectional study. Med J DY Patil Vidyapeeth 13 (2020): 311-4.
- International Diabetes Federation. IDF Atlas 8th Edition (2019).
- ICMR Guidelines for Management of Type 2 Diabetes (2018).
- Ganie MA & Kotwal S. Recent Advances in Management of Diabetes Mellitus. JIMSA 25 (2012): 171-175.
- Chawla A, Chawla R & Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum?. Indian J Endocrinol Metab 20 (2016): 546-551.
- Cahn A & Cefalu W. Clinical Considerations for Use of Initial Combination Therapy in Type 2 Diabetes. Diabetes Care 39 (2016): S137-S145.
- McIntosh B, Cameron C, Singh S & et al. Second-line therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a systematic review and mixed-treatment comparison meta-analysis. Open Med 5 (2011): e35-e48.
- Lipsombe L, Booth G, Butalia S, Dasgupta K & et al. Diabetes Canada 2018-Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada Pharmacologic Glycemic Management of Type 2 Diabetes in Adults. Can J Diabetes 42 (2018): S88-S103.
- Khunti K, Seidu S, Kunutsor S & Davies M. Association between adherence to pharmacotherapy and outcomes in type 2 diabetes: a meta-analysis. Diabetes Care 40 (2017): 588-96.
- Hans-Ulrich H, Ludwig M, Elke S-B, Marc W, Thomas M, Uli C B & Hans JW. Empaglif lozin as Add-On to Metformin in Patients With Type 2 Diabetes: A 24-Week, Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes Care 37 (2014): 650-1659.
- Naimi M, Vlavcheski F, Shamshoum H & Tsiani E. Rosemary Extract as a Potential Anti-Hyperglycemic Agent: Current Evidence and Future Perspectives. Nutrients 9 (2017): 968.
- Shrestha H. Adverse Effects of Oral Hypoglycemic Agents and Adherence to them among Patients with Type 2 Diabetes Mellitus in Nepal. Journal of Lumbini Medical College 5 (2017): 34-40.
- World Medical Association. World medical association declaration of helsinki: ethical principles for medical research involving human subjects. JAMA 2013 (310): 2191-4.
- American Diabetes Association. Standards of Medical Care in Diabetes-2011. Diabetes Care 34 (2011): S11-S61.
- Zhu H, Zhu, Zhang X & et al. Comparative efficacy of glimepiride and metformin in monotherapy of type 2 diabetes mellitus: meta-analysis of randomized controlled trials. Diabetol Metab Syndr 5 (2013).
- Marín PJ, Martín TI, Sevillano CC & Del Cañizo GF. Update on the treatment of type 2 diabetes mellitus. World J Diabetes 7 (2016): 354-395.
- Yehia Ghanem M. Glimepiride as Add-on Therapy in Type 2 Diabetic Patients with Metformin Monotherapy: A Real-Life Study from Egypt. Med. J. Cairo Univ 86 (2018): 4699-4704.
- Melanie JD, David AD, Judith F, Walter NK, Chantal M, Geltrude M, John BB. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 41 (2018): 2669-2701.
- Chaudhury A, Duvoor C & Reddy Dendi V. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front Endocrinol (Lausanne) 8 (2017).
- Rojas L & Gomes M. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr 5 (2013): 6.
- Diabetes Care. Management of hyperglycemia in type 2 diabetes a patient-centered approach: update to a position statement of the American diabetes association and the European associationfor the study of diabetes. Diabetes Care (2015).
- Satyanarayana K, Sravanthi K, Shaker I & Ponnulakshmi R. Molecular approach to identify antidiabetic potential of Azadirachta indica. J Ayurveda Integr Med 6 (2015): 165-174.
- Sajad A, Mohammad Ali A, Fatemeh H, Ashril Y, Maghsoud P, Salar B, Firouzeh D. Evaluation of Trigonella foenum-graecum extract in combination with swimming exercise compared to glibenclamide consumption on type 2 Diabetic rodents, Food & Nutrition Research. Food & Nutrition Research 59 (2015).
- Gaddam A, Galla C, Thummisetti S, Marikanty R, Palanisamy U & Rao P. Role of Fenugreek in the prevention of type 2 diabetes mellitus in prediabetes. J Diabetes Metab Disord 14 (2015).
- Serairi Beji R, Bettaieb Rebey I, Jameleddine S & Ksouri R. Assessment of the antidiabetic, antihyperlipidemic and antioxidant properties of Trigonella foenum-graecum Linnaeus, 1753 (Fenugreek) in alloxan-induced diabetic rats. Journal of new Sciences, Agriculture and Biotechnology 28 (2016): 1602-1609.
- Mowla AA. Mgi. Antihyperglycemic effect of Trigonella foenum-graecum (fenugreek) seed extract in alloxan-induced diabetic rats and its use in diabetes mellitus: a brief qualitative phytochemical and acute toxicity test on the extract. Afr J Tradit Complement Altern Med 6 (2009): 255-261.
- Fuller S & Stephens J. Diosgenin, 4-hydroxyisoleucine, and fiber from fenugreek: mechanisms of actions and potential effects on metabolic syndrome. Adv Nutr 6 (2015): 189-197.
- Pushparaj P, Low H, Manikandan J & Tan BK. Anti-diabetic effects of Cichorium intybus in streptozotocin-induced diabetic rats. . Journal of ethnopharmacology 111 (2007): 430-4.
- Ali Esmail A-S. Medical importance of Cichorium intybus - A review. ournal Of Pharmacy 6 (2016): 41-56.
- Reza G, Jan-Mohamad M, Haibatollah S, Jamshid M & Fariba M. The Effects of Chicory Leaf Aqueous Extract on Body Weight, Serum Glucose and Lipid Levels in Streptozotocin Induced Diabetic Rats. Nutrition and Food 4 (2017): 1-8.
- Muthusamya V, Ananda Sangeethaa K, Sujathaa S, Balakrishnan A & Lakshmia B. Tannins present in Cichorium intybus enhance glucose uptake and inhibit adipogenesis in 3T3-L1 adipocytes through PTP1B inhibition. Chemico-Biological Interactions 174 (2008): 69-78.
- Ghamarian A, Abdollahi M, Su X, Amiri A, Ahadi A & Nowrouzi A. Effect of chicory seed extract on glucose tolerance test (GTT) and metabolic profile in early and late stage diabetic rats. Daru Journal of Pharmaceutical Sciences 20 (2012): 56.
- Peyman SN, Mohammad M, Lotfollah R & Shokofeh B. Mechanism and Clinical Aspects of the Effects of Chicory on Diabetes. Asian Journal of Research in Medical and Pharmaceutical Sciences 1 (2017): 1-11.
- Alimohammadi S, Hobbenaghi R, Javanbakht J & et al. Protective and antidiabetic effects of extract from Nigella sativa on blood glucose concentrations against streptozotocin (STZ)-induced diabetic in rats: an experimental study with histopathological evaluation. Diagn Pathol 11 (2016): 125.
- Balbaa M, El-Zeftawy M, Ghareeb D, Taha N & Mandour A. Nigella sativa Relieves the Altered Insulin Receptor Signaling in Streptozotocin-Induced Diabetic Rats Fed with a High-Fat Diet. . Oxid Med Cell Longev (2016): 2492107.
- Abdullah OB. A review on the hypoglycemic effect of nigella sativa and thymoquinone 3 (2015): 2-7.
- Abdelrazek H, Kilany O, Muhammad M, Tag H & Abdelazim A. Black Seed Thymoquinone Improved Insulin Secretion, Hepatic Glycogen Storage, and Oxidative Stress in Streptozotocin-Induced Diabetic Male Wistar Rats. Oxid Med Cell Longev (2018): 8104165.
- Dina K, Karim R, Abdalla E-L, Safaa B & Maha A-E. Phytochemical Compounds of Cichorium intybus by Exploring its Antioxidant and Antidiabetic Activities. Pharmacogn J 11 (2019): 248-257.
- Sharma M, Afaque A, Dwivedi S, Jairajpuri ZS, Shamsi Y, Khan MF, Khan MI & Ahmed D.Cichorium intybusattenuates streptozotocin induced diabetic cardiomyopathy via inhibition of oxidative stress and inflammatory response in rats.Interdisciplinary toxicology12 (2019): 111-119.


 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 