Genetic Variants Linked to HIV-1 Exposure in Cameroonian Children and Adolescents and Their Association to Non-Communicable Diseases
Béatrice Dambaya Ounzia*1,2, Georges Teto*1, Marcel Tongo3, Laure Lemee4, Elise Elong Lobe1, Charlotte Tangimpundu1, Guy Benoit Lekeufack5, Céline Nguefeu Nkenfou1, Flobert Njiokou5, Vittorio Colizzi6, Simon Eyongabane Ako3,7 and Alexis Ndjolo1
1Chantal Biya International Reference Center, Yaoundé, Cameroon
2National Advanced School of Engineering, Department of Hydraulics and Water Management, University of Maroua, Cameroon
3Center of Research for Emerging and Re-Emerging Diseases (CREMER), Institute of Medical Research and Study of Medicinal Plants (IMPM), Yaoundé, Cameroon
4Pasteur Institute, Paris Cité University, Biomics Technological Platform, Paris F-75015, France
5Faculty of Sciences, University of Yaounde I, Yaoundé, Cameroon
6University of Rome Tor Vergata, Rome, Italy
7Faculty of Health Science, University of Buea, Cameroon
*Corresponding author: Béatrice Dambaya Ounzia, National Advanced School of Engineering, Department of Hydraulics and Water Management, University of Maroua, Cameroon.
Georges Teto, Chantal Biya International Reference Center, Yaoundé, Cameroon.
Received: 06 August 2025; Accepted: 11 August 2025; Published: 08 September 2025
Article Information
Citation: Béatrice Dambaya, Georges Teto, Marcel Tongo, Laure Lemee, Elise Elong Lobe, Charlotte Tangimpundu, Guy Benoit Lekeufack, Céline Nguefeu Nkenfou, Flobert Njiokou, Vittorio Colizzi, Simon Eyongabane Ako and Alexis Ndjolo. Genetic Variants Linked to HIV-1 Exposure in Cameroonian Children and Adolescents and Their Association to Non- Communicable Diseases. Fortune Journal of Health Sciences. 8 (2025): 903-916.
View / Download Pdf Share at FacebookAbstract
Background: Children with HIV continue to be at a higher risk of non-communicable diseases (NCDs), even with improvements in the treatment of prenatal exposure to HIV-1 infection. This study aimed to identify genetic variations associated with HIV-1 and their potential influence on the risk of NCDs in offspring exposed during pregnancy.
Methods: We conduct a genome-wide association study (GWAS) on a cohort of 689 participants from Cameroon, employing a case-control design and a genomic epidemiology approach. Of these, 421 were HIV-infected and 268 were not. Single nucleotide polymorphisms (SNPs) were performed using Illumina BeadChips.
Results: Twenty SNPs in all were found, and the majority of the alterations were silent or synonymous. Those linked to HIV rapid progression include rs1422884 (GRIA1, Chr5) and exm665143 (TRPV6, Chr7) 7.9x10-6, with OR = 0.01, rs4915847 (C1orf87, Chr1), rs7578597, and exm189601 (THADA, Chr 2). The NCD-related SNPs rs1693687 (Chr10) 8.4x10-7 with OD=0.04 and rs7175885 (Chr15) showed significant associations with slow progression in HIV-infected individuals: THADA (rs7578597, exm189601, Chr2) was linked to type 2 diabetes and metabolic disorders. Both TENM2 (rs10057680, Chr5) and RBFOX1 (rs9302839, Chr16) have been associated with neurocognitive impairment. rs1693687 (Chr10) and rs10231785 (Chr7) were linked to cardiovascular diseases (CVDs). PHLPP1 (rs692916, Chr18) and ZNF208 (rs10426971, Chr19) have been connected to cancer risk and immune response.
Conclusion: The genetic variations linked to protective effects against HIV-1's rapid development as well as other variants with possible genetic resistance mechanisms are highlighted in this work. Additionally, several SNPs overlap with risk factors for NCDs, indicating that there may be common genetic processes. These results highlight the value of precision medicine strategies in HIV care.
Keywords
<p>HIV-1, GWAS, SNPs, metabolic disorders, cardiovascular diseases, non-communicable diseases (NCDs)</p>
Article Details
Introduction
Complex host-parasite interactions are influenced by both extrinsic and intrinsic factors that affect host adaptation and susceptibility. HIV-1 infection remains a significant global health burden despite global efforts to eradicate it, especially in sub-Saharan Africa, where environmental factors and genetic diversity contribute to the variability in treatment outcomes and disease progression [1]. Although antiretroviral therapy (ART) has greatly raised survival rates, persons living with HIV (PLWHIV) are still particularly concerned about long-term consequences including non-communicable diseases (NCDs) [2, 3]. Managing HIV/AIDS in low- and middle-income countries (LMICs) such as Cameroon is more difficult. According to a study, some regions/areas of the country have observed stable HIV prevalence over time at around 5% despite widespread ARV use [4]. While it is difficult to determine why the prevalence stays high within communities, several factors could be speculated, including host genetic variables known to be important in determining HIV susceptibility, disease progression, and ART response [5-7]. However, despite Africa's enormous genetic variety, the majority of genomic research has been done on people in North America and Europe, with very few studies being carried out in African locales [8,9]. The phenotypic heterogeneity seen in exposed people is one of the main obstacles to comprehending HIV-1 infection. Following infection, some people develop quickly, slowly, or do not progress for a long time [6], while others resist HIV-1 infection even after several exposures [10]. A complex interaction between viral, environmental, and host genetic variables influences this diversity in virus acquisition, disease development, and responsiveness to treatment [11]. Through candidate gene techniques and genome-wide association studies (GWAS), host genetic research has played a significant role in identifying genetic variations linked to HIV susceptibility and disease progression over the years [12]. The majority of this research, however, has concentrated on people with European ancestry, which limits their relevance to communities in Africa, which have the highest genetic variety in the world [8, 13, 14]. Due to population-specific genetic variants and structural variations, genome-wide association studies (GWAS) have found multiple SNPs (single nucleotide polymorphisms) linked to HIV-1 progression; however, these findings frequently do not replicate in African populations [13, 15]. Furthermore, while GWAS has mostly focused on HIV-related immune response genes, accumulating data suggests that many of these variations also overlap with genes connected to NCDs, including cancer, metabolic diseases, and neurocognitive deficits [16]. Given the increasing burden of NCDs among PLWHIV, it is crucial to examine genetic risk factors that may contribute to both HIV progression and NCD susceptibility.
Only candidate gene studies, which concentrate on genes linked to HIV progression, resistance, and mother-to-child transmission, have been conducted in Cameroon on children and adolescents exposed to HIV-1 [17-19]. Expanding research to find genetic variants linked to HIV infection as well as metabolic, cardiovascular, and neurodevelopmental disorders that may develop as a result of prolonged exposure to antiretroviral therapy (ART) and host genetic predisposition is imperative given the rising burden of NCDs among PLWHIV [2, 19]. Although important single nucleotide polymorphisms (SNPs) linked to diabetes, cancer, and other NCDs have been identified by studies conducted in developed countries, little is known about these findings in African contexts [16,20]. Non-communicable diseases (NCDs) are recognized as one of the primary causes of morbidity and mortality worldwide, particularly for people living with HIV (PLWHIV) [3]. By performing a genome-wide association study (GWAS) on Black African children and adolescents who were exposed to HIV-1 at birth and those who were not, this study seeks to close this gap by examining the genetic variability of the participants and finding correlations with variants linked to both HIV-1 susceptibility and NCD risk. This study is an important step in understanding host genetic factors that contribute to HIV-related health disparities and the increasing burden of non-communicable diseases in PLWHIV, especially considering the high genetic diversity of African populations and the limited funding available for large-scale genomic research in LMICs. In order to contribute to precision medicine techniques that are specifically designed for African populations, this research aims to offer new insights into the genetic drivers of disease susceptibility and progression by utilizing genome-wide methodologies [15, 21].
Materials and Methods
Study design, setting and population
This study was done at the Chantal Biya International Reference Center and the BiOmics unit of the Pasteur Institute of Paris. A total of 689 participants were recruited after obtaining a proxy consent form, signed by parents before enrollment. The classification was done according to their profile at the time of the inclusion, focusing on their records files and their biological parameters such as their CD4+ T cells count and viral load (VL) values, together with clinical parameters and their status regarding the treatment as described by Dambaya and colleagues (2019). Among the participants, 268 were HIV-1 negative and 421 were HIV-1 positive. We obtained ethical clearance from the Cameroon National Ethics Committee for research of Human health (CNERSH) under N°2013/11/374/L/CNERSH/SP/ and an administrative authorization from the Ministry of Public Health of Cameroon under the N0631-05.14/AAR/MINSANTE/SG/DROS/CRC/ZAMC. As well, this study was conducted following the Declaration of Helsinki.
Data sources, Analytical processes and measurement
Blood collection: five millilitres of blood were collected from each participant included in the study at each appointment. Part of whole blood was used for CD4 count and the rest was centrifuged at 1,000 g for 10 minutes, plasma was aliquoted and stored at -20oC for further analyses. Determination of CD4 counts: CD4 cell counts were quantified using a FACScalibur flow cytometer (Becton-Dickinson, Immunocytometry System, San Jose, CA, USA).
Determination of viral load: HIV-1 viral load was determined from plasma by Abbott m2000rt Real-Time HIV-1 assay (Abbott Molecular Diagnostics, Wiesbaden, Germany), using the 200 and 600 µl of plasma protocol with a detection limit of 160 copies/ml (1.8log) and 40 copies/ml (1.6log) respectively. DNA extraction: this was done according to the QIamp DNA Mini and the Blood Mini Handbook, from 200μl of whole human blood or from dried blood spot (DBS), three puncher-out circles from a DBS were used. Quality control and quantification of DNA samples: we used microarray-based genome-wide association studies (GWAS) to identify disease associations. The experiment was done at the time of sample collection and was undertaken at the Genotype Eukaryotes unit of the Pasteur Institute of Paris (France) actually named Biomic’s unit. This platform at the time of this analysis used SNP genotyping on Illumina beadchips. The genotyping chip technology was a standardized and automated (using kits and robots) protocol. From the 285 included subjects, we selected a total of 120 extracted DNA of good quality from the different involved groups of participants. Genotyping clustering was performed using the Illumina Genome studio program.
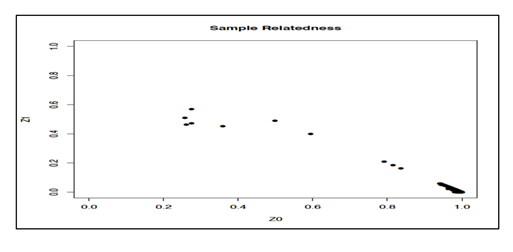
Quantification was done on the extracted DNA from the whole blood or from the DBS. The PicoGreen assay was useful for the quantification of the amount of double-stranded DNA (dsDNA) that was present in a given sample in an ultra-sensitive fluorescent nucleic acid stain because a specific amount of DNA (at least 75 ng/ul) was required for SNP identification. The measured amount of dsDNA was used as a marker of the success of the genotyping process. The samples were then separated on 1% agarose gel, for checking the DNAs’ integrity (no smearing) and to verify the requested amount of DNA needed. Quality control of the raw data was done using the software PLINK which requires a computer server Illumina BeadStudio v3.1 according to several parameters. First of all the genotypes were assigned using a classification provided by Illumina, generated on an African population. We performed a common measure of relatedness (or duplication) between pairs of samples, based on identity by descent (IBD). When a link between samples was observed, one of the two samples was excluded for further analysis. In IBD estimation coefficient greater than 0.20 may suggest relatedness, duplicates, or sample mixture. In the same way, gender identity was also checked at this stage to confirm that self-reported gender is consistent with the observed X and Y chromosomes. After classification, individuals with a call rate (percentage of genotyped SNPs per individual) of less than 97% were deleted. On the other hand, SNPs with a "call frequency" (percentage of individuals genotyped by SNP) of less than 99% were re-classified. Finally, SNPs with a call frequency of less than 98% (i.e. a missing data rate >2%) were excluded. All of these steps ensure reliable genotyping data with little missing data. Finally, only 96 DNA were genotyped using Illumina Human core exome analysis beadchips. The deviation of the Hardy-Weinberg equilibrium was evaluated for each SNP in each group by comparing the distribution of observed genotype frequencies and theoretical genotypic frequencies using an exact statistical test [21]. The realization of multiple tests has been taken into account by applying the corrections of Bonferroni, one of the simplest approaches to correct for multiple testing.
Genome-wide association analysis
We research the association between HIV status (positive infant vs negative participants) and HIV progression and, the association between the different groups of infected individuals with logistic regression, using PLINK.
Statistical methods
In this GWAS study, we used bead chips containing more than 240,000 SNPs, so the statistical significance of the SNP association has been set at 2.08 e-7. In order to identify additional signals while controlling the risk of false discoveries, we also calculated the FDR (False-Discovery Rate) q-value for each p-value: the q-value estimates the proportion of false positives below a threshold of p-value [22]. The local estimation FDR has been applied in this study with a threshold of 25%. For each SNP passing the statistical threshold of significance, the quality control was individually re-verified. Then, for each identified signal, we verified that the allelic frequency in an HIV-positive population was similar to that of the population control, to confirm that the observed association accurately reflects progression to AIDS and not HIV-1 infection. For the identification of population stratification, case and control genotypes were analyzed using the EIGENSTRAT method which allows detecting and correcting the stratification of our study population. This method, based on a principal component analysis, allows to modelling of the ancestral differences according to continuous axes of variation.
Results
Summary Participant enrolment, Classification, and Genomic Sample Processed in the CBIRC HIV Pediatric Cohort (2010–2015) involved in the GWAS analysis
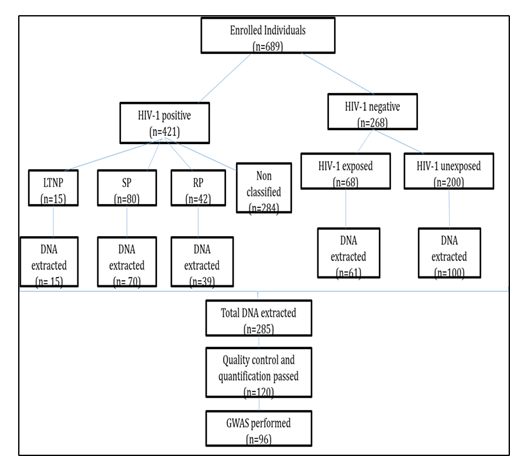
A total of 689 infants were recruited at the Chantal Biya International Reference Center (CBIRC), from October 2010 to April 2015. Among them, 268(38.9%) were HIV-negative cases, 68 (25.4%) were HIV-exposed uninfected (HEU), and 200 (74.6%) were HIV non-exposed uninfected (HNEU). The group of positive participants was composed of 15 enrolled LTNP (3.6%), 80 SP (19%) and 42 RP (9.9%). The rest, 284 (67.5%) participants were excluded because of not respect any criteria of classification of these three different groups. From the 285 extracted genomic materials to be genotyped in the genomic laboratory of the Pasteur Institute of Paris, only 120 passed the initial quality control filtering. Five individuals have been removed from the study for gender misclassification. Finally, principal component association (PCA) was performed on 96 samples to assess population stratification. After this correction, these participants were then considered for further analysis (figure 1).
Socio-demographic and immune-virologic characteristics of participants involved in the GWAS analysis
Socio-demographic and immune-virologic characteristics of participants involved in the GWAS analysis are resume on table 1. From the 15 enrolled LTNP, 9 past the last quality control analysis with 33 SP and 21 RP. Inside the group of HIV-1 negative the majority, 82% were HIV-1 exposed uninfected and the rest was non-exposed. Most of the participants were originated from the Center South and West regions of the country.
Table 1: Socio-demographic and immune-virologic characteristics of participants involved in the GWAS analysis
|
Groups (number; % male) |
Mean age |
Mean CD4 cells (%) |
Mean Viral load copies (Log) |
|
LTNP (9; 11%) |
13.44 |
477a (21) |
145097b (4.06) |
|
SP (33; 45%) |
11.33 |
608a (22) |
44048b (3.6) |
|
RP (21; 36%) |
3.04 |
1243a (23) |
38467b (4.2) |
|
HEU (27; 38%) |
5.53 |
/ |
/ |
|
HNEU (6; 50%) |
7.5 |
/ |
/ |
LTNP: long term non progressor; SP: slow progressors; RP: rapid progressors; HEU: HIV exposed uninfected; HNEU: HIV non-exposed uninfected; a:p-value for mean CD4 cells (p=0.02); b:p-value for mean Viral load copies (p =0.02)
Sample relatedness and principal component analysis (ancestry plot)
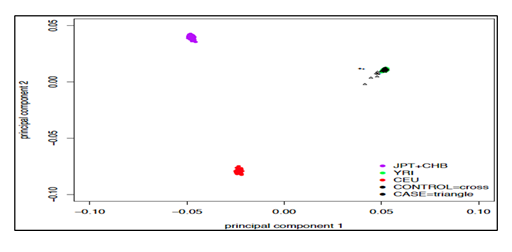
Principle component analysis plot was used to identify axes of genetic variation for each individual. The relatedness of samples is shown in Figure 2, each point represents an individual (figure 3), and no patients and no control were too different from others in terms of their genotypes (black crosses and triangles are our HIV positive and controls samples, next to their genotypic ancestry in green in the figure 3). The differences appear clearly when the genetic comparison is between our study population versus Asiatic (Japanese and Chinese) shown in purple and located on the upper left of Figure 3, with the European population in red located on the lower left part. All the samples (cases and controls) completely match with African ancestry population in green located on the upper right part of Figure 3.
SNPs with best p values obtained in the overall study population
We identified in overall 20 SNPs in our study population which have the best p values as shown in Table 2. Eleven genes have been identified with only three missense mutations in the overall study population. The majority of the SNPs were located on chromosome 2 (with 4 SNPs), followed by 20 and 6 with 2 SNPs respectively. Most of the significant SNPs (45% and 18%) were involved in cancer and type 2 diabetes respectively, the other SNPs were associated with only one disease. Table 3 summarizes the different SNPs identified in NCDs in our study group that were reported in other studies.
Table 2: List of the best SNP signal results and genes involved in the overall study population of infected and uninfected participants
|
SNPs name |
CHR |
Allele |
Gene symbol |
Characteristic |
Mutation |
|
rs1693687 |
10 |
[T/C] |
/ |
/ |
|
|
rs9302839 |
16 |
[A/G] |
RBFOX1 |
Silent |
|
|
rs7175885 |
15 |
[T/C] |
/ |
/ |
|
|
rs6140412 |
20 |
[T/C] |
/ |
/ |
|
|
rs6136995 |
20 |
[T/G] |
C20orf26 |
Silent |
|
|
rs10057680 |
5 |
[A/G] |
TENM2 |
Silent |
|
|
rs10804512 |
3 |
[A/G] |
/ |
/ |
|
|
rs6718327 |
2 |
[T/C] |
/ |
/ |
|
|
rs1422884 |
5 |
[T/C] |
GRIA1 |
Silent |
|
|
exm665143 |
7 |
[A/G] |
TRPV6 |
Exon variant |
Missense_M681T |
|
rs4915847 |
1 |
[T/G] |
C1orf87 |
Silent |
|
|
rs7578597 |
2 |
[T/C] |
THADA |
Exon variant |
Missense_T1187A |
|
exm189601 |
2 |
[T/C] |
THADA |
Exon variant |
Missense_T1187A |
|
rs12214839 |
6 |
[A/G] |
FUT9 |
Silent |
|
|
rs9270986 |
6 |
[A/C] |
/ |
/ |
|
|
rs5926263 |
X |
[A/G] |
LOC100873065 |
Silent |
|
|
rs10231785 |
7 |
[A/G] |
|||
|
rs40428 |
12 |
[T/C] |
/ |
/ |
|
|
rs692916 |
18 |
[T/C] |
PHLPP1 |
Silent |
|
|
rs10426971 |
19 |
[A/G] |
ZNF208 |
Silent |
Legend: CHR= chromosome number
Table 3: Identifier SNPs and their localization on genes involved in some NCDs
|
Gene symbol |
SNPs |
Role/Disease involved |
|
RBFOX1 (RNA Binding Fox-1 Homolog 1) |
rs9302839 |
Involved in Psychiatric disorders, Autism, Epilepsy and Colorectal Cancer. [23-25] |
|
C20orf26 (chromosome 20 open reading frame 26) |
rs6136995 |
Oxidoreductase activities are involved in innate and adaptative immunity [26] |
|
TENM2 (Teneurin Transmembrane Protein 2) |
rs10057680 |
involved in cancer tissue, and chronic kidney disease [27] |
|
GRIA1 (glutamate ionotropic receptor AMPA type subunit 1) |
rs1422884 |
Intellectual development disorder [28] |
|
TRPV6 (Transient Receptor Potential cation channel subfamily V member 6) |
exm665143 |
Hyperparathyroidism, Breast cancer [29-31] |
|
C1orf87 (chromosome 1 open reading frame 87) |
rs4915847 |
Involved in somatic mutations in cancer, spinal muscular atrophy [32] |
|
exm189601 |
Associated with type 2 diabetes and polycystic ovary syndrome [33-35] |
|
|
FUT9 (Fucosyltransferase 9) |
rs12214839 |
Associated with Susceptibility to placental malaria infection, Intellectual Developmental Disorder, and Colorectal cancer [36,37] |
|
LOC100873065 |
rs5926263 |
Cancer, autism and autoimmune disease [38,39] |
|
PHLPP1 (PH domain and Leucine-rich repeat Protein Phosphatase) |
rs692916 |
Involved in multiple types of cancer, obesity and type 2 diabetes [40] |
|
ZNF208 (Zinc finger protein) |
rs10426971 |
Bind DNA and regulate gene transcription [41,42] |
SNPs identified in perinatally HIV-1 infected participants: impact on disease progression
Comparison between long-term non-progressors (LTNP) versus Rapid Progressors (RP)
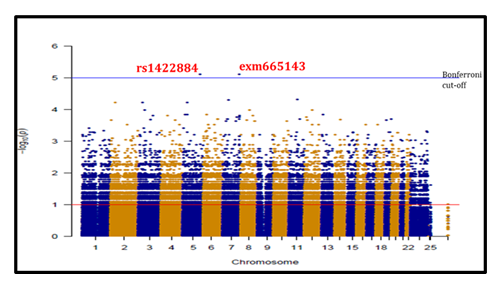
After accessing the genotype magnitude of SNPs between LTNP and RP using a Manhattan plot (Figure 4), showing many SNPs with log10 p values <10-4, only 2 SNPs (rs1422884 and exm665143) emerge with an unadjusted p-value of 7.9x10-6 (Table 4). The odd ratio of r=0.01[0.0001-0.2010] and the false discovery rate (FDR_BH) was 81%. Adjusted value with Bonferroni correction indicates a p-value of 4.5x10-5 (upper horizontal blue line) located in chromosomes 5 and 7 respectively.
Table 4: Association analysis results between LTNP and RP
|
CHR |
Gene |
SNP |
BP |
A1 |
F_A |
F_U |
A2 |
CHISQ |
p-value |
OR |
|
FDR-BH |
||||||||||
|
5 |
GRIA 1 |
rs1422884 |
153009489 |
T |
0.05 |
0.727 |
C |
19.95 |
7.90E-06 |
0.01 |
|
0.81 |
||||||||||
|
7 |
TPV6 |
exm665143 |
142569596 |
A |
0.05 |
0.727 |
G |
19.95 |
7.90E-06 |
0.01 |
|
0.81 |
CHR: chromosome; SNP: single nucleotide polymorphism; BP: base-pair location; A1 minor allele; A2 major allele; F_A & F_U frequency of the minor allele in LTNP and RP respectively; CHISQ: Chi-square probability value; Nominal P unadjusted asymptotic probability value; OR odds ratio; the level of significance is 10E-7. FDR_BH = False Discovery Rate Benjamin-Hochberg; G= Guanine; C= Cytosine; A=Adenine; T=Thymine
Comparison between long-term non-progressor (LTNP) and Slow Progressor (SP)
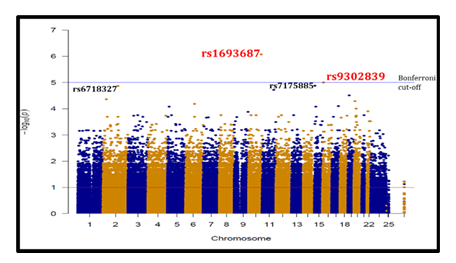
When we compared LTNP and SP as shown in red colour on Figure 5, 3 SNPs appeared above p<10-5; rs1693687, rs9302839 and rs7175885. The best statistical significances were observed with rs1693687 (p=8.4x10-7) and rs9302839 (p=9.8x10-6) located respectively on chromosomes 10 and 16. As shown in Table 5 the odds ratios were not significant. The FDR-BH of only rs1693687 was below the threshold of 25% and above for the 2 other SNPs (86%).
Table 5: Association analysis results for SNPs identified in LTNP and SP
|
CHR |
Gene |
SNP |
BP |
A1 |
F_A |
F_U |
A2 |
CHISQ |
p-value |
OR |
|
FDR-BH |
||||||||||
|
10 |
/ |
rs1693687 |
123438113 |
T |
0.105 |
0.727 |
C |
24.26 |
8.4x10-7 |
0.044 |
|
0.24 |
||||||||||
|
16 |
RBFOX1 |
rs9302839 |
7187784 |
A |
0.026 |
0.5 |
G |
19.54 |
9.8x10-6 |
0.027 |
|
0.86 |
||||||||||
|
15 |
/ |
rs7175885 |
25055302 |
C |
0.05 |
0.4 |
T |
13.46 |
1.3x10-5 |
0.08 |
|
0.86 |
CHR: chromosome; SNP: single nucleotide polymorphism; BP: base-pair location; A1 minor allele; A2 major allele; F_A & F_U frequency of the minor allele in RP and SP respectively; CHISQ: Chi-square probability value; Nominal P unadjusted asymptotic probability value; OR odds ratio; the level of significance is 10E-7. FDR_BH = False Discovery Rate Benjamin-Hochberg; G= Guanine; C= Cytosine; T=Thymine
Comparison between Rapid Progressor (RP) and Slow Progressor (SP)
By comparing RP and SP (Table 6) and considering their unadjusted p values, 8 SNPs showed a signal with a p-value between 0.00024 and 10-6, 3 of them located on chromosomes 1, and 2 and could be involved in rapid disease progression if we consider the odds ratio value as shown on table 6. The most significant SNPs are rs4915847 located on the C1orf87 gene, exm189601 and rs75785997 on the gene THADA. By calculating the FDR-BH of the 3 SNPs, all of them were above the threshold (36%).
Table 6: Association analysis results for SNPs identified in rapid progressors and slow progressors participants
|
CHR |
Gene |
SNP |
BP |
A1 |
F_A |
F_U |
A2 |
CHISQ |
p-value |
OR |
|
FDR-BH |
||||||||||
|
1 |
C1orf87 |
rs4915847 |
60461958 |
G |
0.65 |
0.078 |
T |
21.39 |
3.7x10-6 |
21.67 |
|
0.36 |
||||||||||
|
2 |
THADA |
exm189601 |
43732823 |
C |
0.6 |
0.052 |
T |
21.44 |
3.7x10-6 |
27 |
|
0.36 |
||||||||||
|
2 |
THADA |
rs7578597 |
43732823 |
C |
0.6 |
0.052 |
T |
21.44 |
3.7x10-6 |
27 |
|
0.36 |
CHR: chromosome; SNP: single nucleotide polymorphism; BP: base-pair location; A1 minor allele; A2 major allele; F_A & F_U frequency of the minor allele in RP and SP respectively; CHISQ: Chi-square probability value; Nominal P unadjusted asymptotic probability value; OR odds ratio; FDR_BH = False Discovery Rate Benjamin-Hochberg; the level of significance is 10E-7. G= Guanine; C= Cytosine; T=Thymine
SNPs identified in HIV-1 perinatally infected participants: impact on disease acquisition
We identified 7 genome-wide SNPs with unadjusted p-values not greater than 10-6 log when comparing HIV-1 infected participants (LTNP, SP, and RP) versus negative controls (HEU). Only 2 SNPs, rs40428 and rs692916 of the PHLPP1 gene located respectively on chromosomes 12 and 18 have a p-value of 10-6 log without significant odd ratio as shown in table 7. The unadjusted p-value gives a value of 6.6x10-6 and 9.6x10-6, respectively for rs40428 and rs692916. The FDR-BH was above the threshold for both of them.
Table 7: SNPs potentially associated with HIV-1 virus acquisition
|
CHR |
SNP |
A1 |
Frequency |
A2 |
Frequency |
CHISQ |
p-value |
OR |
|
12 |
rs40428 |
T |
0 |
C |
0.6154 |
20.31 |
6.6x10-6 |
0 |
|
0.84 |
||||||||
|
|
SP |
HEU |
||||||
|
18 |
rs692916 |
A |
0.2105 |
G |
0.7692 |
19.58 |
9.6 x10-6 |
0.08 |
|
0.86 |
Legend: CHR=chromosome; OR= odds ratio and frequency is given for the A1 allele where OR > 1 indicates a protective effect; FDR_BH = False Discovery Rate Benjamin-Hochberg; CHISQ= Chi-square; G= Guanine; C= Cytosine; A=Adenine; T=Thymine
Discussion
In this study, we identified in overall 20 SNPs with the best signals, with some potentially associated with some profiles and/or clinical status of participants. Limited studies have observed these SNPs with p-values between 0.0002 to 8.42x10-7, in a pediatric context related to genome-wide association studies (GWAS). Missense or non-synonymous mutations which are characterized by changes in protein sequence, were few represented compared to the majority of the identified SNPs who were synonymous. Those synonymous SNPs also termed silent mutations [43] occur at the non-coding region of the gene [44] and naturally do not affect the gene product. However, they could also be found in the coding region because each amino acid is coded by more than one codon. In most cases, they were previously not considered important. However, recent studies show that synonymous SNPs affect gene function and expression by changing the expression of neighbouring genes. Many studies have demonstrated silent mutation could also affect the function of the cell by altering the gene’s expression and regulation [45]. Some research reveals that they can have disease-driving effects, this impact of silent mutation has been demonstrated in some NCDs such as cancer development [46-48]. Thus, missense or silent SNPs observed in our study population need to be more investigated.
Concerning SNPs potentially involved in disease progression only 2 SNPs with p value of 7.9x10-6, emerged when comparing LTNP to RP. The same comparison done between RP versus SP brings out 3 other SNPs with p values not greater than 10-6 with a higher odd ratio. These five SNPs were more represented in the RP participants and therefore need to be more investigate in order to effectively characterize their role in rapid disease progression. To the best of our knowledge, these SNPs with the best signals have never been identified in GWAS studies regarding HIV disease progression. These new signals such as those found by other researchers will help to better understand host genetic variability in the progression of the disease [49-51]. On the contrary, only 2 SNPs also require further research related to slow disease progression as they have presented the best p values when comparing LTNP to SP. Nevertheless, this finding is controversial since those 2 SNPs were more distributed inside the SP group instead of LTNP. These results can be attributed to our poor sample size especially referring to the LTNP group of participants. The same explanation can be made referring to the poor number of SNPs with a very low odd ratio, observed when assessing the SNPs involved in HIV-1 disease acquisition. Overall, when considering the significant signal (p=2.08x10-7), needed to consider the significant impact of an SNP in this study, we observed that only one SNP has a p-value above this threshold. Moreover, by assessing the adjusted p values and using false discovery values, no SNP passed this significant signal. Referring to the fact that a wide significance threshold is usually set in the region between p< 10-7 and p<10-8 [52-55], we can realize that our GWAS results globally are not enough statistically significant to make a clear conclusion. This poor genome-wide significance can be justified by many reasons, among them, the low sample size mentioned above. It was difficult for us due to the lack of technical and financial reasons, to ascertain and analyze more samples in both cases and controls in order to obtain adequate power for a typical genome-wide association study. In case samples, the number of LTNP analyzed was not enough compared to the sample size usually involved in genome-wide association studies all over the world. Regarding the number of SNPs which did not pass the quality control, it could be attributed to the genotyping tools especially the beadchips used in our analysis, in addition to the high genetic variability of the African population. African populations are genetically more diverse than European and Asian populations [56-58], the fundamental reason has always been the challenge of how to develop an appropriate methodology for GWAS in Africa in order to search for significant associations for a better exploitation of the results [59,60]. This methodology was economically challenging for us, and our experience just explained one of the reasons why GWAS is very rare in African countries. If some GWAS have been already conducted in some African countries, they are still rare and this has to be improved because GWAS studies might provide important insights into the development of more effective therapeutics public health interventions, and even vaccines. This is by looking for the molecular mechanisms involved in the resistance and susceptibility to disease. To the best of our knowledge, no GWAS study has ever been conducted in Cameroon, especially in a pediatric setting of HIV infection and this explorative GWAS study can serve as an indicator.
Our poor sample size may also have limited our ability to detect statistically high significant associations in some regions of interest, in particular for small effects. However, this study provides some new insights into the genetics of HIV-MTCT and aims to facilitate future genetic studies for this phenotype. Apart from the SNP identified on FUT9 which is associated both with placental malaria infection [35] and colon cancer [36], the observed signals were mostly associated with non-communicable diseases, such as cancer, epilepsy, type 2 diabetes, hyperparathyroidism, intellectual development disorder, and spinal muscular atrophy. These findings corroborate those of Auslander et al. (2017) who predict a variant seen in FUT9, which catalyzes the biosynthesis of Ley glycolipids, as a driver of advanced-stage colon cancer. The variant rs9302839 of RBFOX1 found in this study like those previously reported by other authors, are causal factors of psychiatric disorders, autism, epilepsy and colorectal cancer [22-24]. Overall the majority of variants with the best signals found on TENM2, C1orf87, PHLPP1 and TRPV6 genes were associated with different types of cancer [26,28,29,31,39,]. In HIV-1 infection tat gene have been associated with neurogenerative disorder (HAND) by acting on brain cells and neurons, even in the absence of active HIV-1 replication [61-65]. These results bring out the importance of the exploration of the whole human genome in order to anticipate and ameliorate the follow-up of the patients.
Conclusions
In this study, we focused on children and adolescents who were exposed to HIV-1 by conducting a GWAS in a Cameroonian population. Our findings offer new insights into host genetic determinants impacting HIV disease progression and the potential risk of NCDs in PLWHIV. Twenty SNPs with the strongest associated signals were found by our analysis; many of these had not been previously documented in pediatric HIV investigations. The majority of SNPs were synonymous (silent) mutations. Five SNPs were also found to be strongly linked to the progression of HIV disease; rapid progressors (RP) had the largest proportion of these SNPs when compared to slow progressors (SP) and long-term non-progressors (LTNP). Furthermore, two SNPs were linked to slow illness development. Notably, several SNPs identified in our study, including variants in FUT9, RBFOX1, THADA, TRPV6, and TENM2 have been linked in the past to a number of NCDs, including epilepsy, hyperparathyroidism, type 2 diabetes, intellectual developmental abnormalities, and many cancers. These results highlight the significance of incorporating genomic insights into HIV care regimens by indicating that some genetic variations may have a dual function in HIV progression and NCD vulnerability.
Recommendation
This work emphasizes how urgently larger-scale genetic research in African populations is needed to further precision medicine strategies for HIV treatment. It will be essential to comprehend how genetic variants affect HIV progression and NCD risk in order to create tailored therapies and enhance the long-term health of people living with HIV in sub-Saharan Africa.
Acknowledgements
We would like to thank all the participants who gave their consent for the present study to take place. We would also like to thank Beatrice Regnault and Laure Lemee of the Pasteur Institute of Paris for their helpful technical support, comments and feedback. We are also grateful to the University of Yaounde I-Cameroon, for providing the administrative facilities needed for our internship at the Pasteur Institute of Paris.
Limitations of the Study
The limited financial facility received did not give rise to analysing more samples as a genetic association study required in order to draw reliable conclusions.
Funding
This study was supported by the HIV Trust Research Grant Program and the Chantal Biya International Reference Centre.
Conflict of interest
The authors declare no conflict of interest.
References
- Global HIV & AIDS statistics Fact sheet 2024 - Latest global and regional HIV statistics. Joint United Nations Programme on HIV/AIDS (2024).
- Deeks SG, Lewin SR, & Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 382 (2013): 1525–1533.
- Integration of noncommunicable diseases into HIV service packages: technical brief.| Technical document (2023).
- Ngoume YF, Teagho UC, Eselacha B, et al. Differences in HIV infection trends in two regions of Cameroon with a longstanding HIV epidemic: Insights from 2012 and 2022. Frontiers in Public Health 13 (2025): 1517213.
- Fellay J, Shianna KV, Ge D, et al. A whole-genome association study of major determinants for host control of HIV-1. Science. 317 (2007): 944–947.
- Fellay J, Ge D, Shianna KV, et al. Common genetic variation and the control of HIV-1 in humans. PLoS Genet. 5 (2009): e1000791.
- Dalmasso C, Carpentier W, Meyer L, et al. Distinct genetic loci control plasma HIV-RNA and cellular HIV-DNA levels in HIV-1 infection: the ANRS Genome Wide Association 01 study. PLoS One. 3 (2008): e3907.
- Le Clerc S, Limou S, Coulonges C, et al. Genomewide association study of a rapid progression cohort identifies new susceptibility alleles for AIDS (ANRS Genomewide Association Study 02). J Infect Dis. 200 (2009): 1194–1201.
- Limou S, Le Clerc S, Coulonges C, et al. Genomewide association study of an AIDS-nonprogression cohort emphasizes the role played by HLA genes (ANRS Genomewide Association Study 03). J Infect Dis. 199 (2009): 419–426.
- Pelak K, Goldstein DB, Walley NM, et al. Host determinants of HIV-1 control in African Americans. J Infect Dis. 201 (2010): 1141–1149.
- McLaren PJ, Ripke S, Pelak K, et al. Fine-mapping classical HLA variation associated with durable host control of HIV-1 infection in African Americans. Hum Mol Genet. 21 (2012): 4334–4347.
- International HIV Controllers Study. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 330 (2010): 1551–1557.
- Pereyra F, Jia X, McLaren PJ, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 330 (2010): 1551–1557.
- Jia X, Han B, Onengut-Gumuscu S, et al. Imputing amino acid polymorphisms in human leukocyte antigens. PLoS One. 8 (2013): e64683.
- McLaren PJ, Coulonges C, Ripke S, et al. Association study of common genetic variants and HIV-1 acquisition in 6,300 infected cases and 7,200 controls. PLoS Pathog. 9 (2013): e1003515.
- Carrington M, Alter G. Innate immune control of HIV. Cold Spring Harb Perspect Med. 2 (2012): a007070.
- Alter G, Martin MP, Teigen N, et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med. 204 (2007): 3027–3036.
- Martin MP, Qi Y, Gao X, et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet. 39 (2007): 733–740.
- Thomas R, Apps R, Qi Y, et al. HLA-C cell surface expression and control of HIV/AIDS correlate with a variant upstream of HLA-C. Nat Genet. 41 (2009): 1290–1294.
- Apps R, Qi Y, Carlson JM, et al. Influence of HLA-C expression level on HIV control. Science. 340 (2013): 87–91.
- Kulkarni S, Savan R, Qi Y, et al. Differential microRNA regulation of HLA-C expression and its association with HIV control. Nature. 472 (2011): 495–498.
- Pereyra F, Addo MM, Kaufmann DE, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 197 (2008): 563–571.
- O’Brien SJ, Gao X, Carrington M. HLA and AIDS: a cautionary tale. Trends Mol Med. 7 (2001): 379–381.
- Kiepiela P, Leslie AJ, Honeyborne I, et al. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature. 432 (2004): 769–775.
- Kiepiela P, Ngumbela K, Thobakgale C, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 13 (2007): 46–53.
- Leslie A, Matthews PC, Listgarten J, et al. Additive contribution of HLA class I alleles in the immune control of HIV-1 infection. J Virol. 84 (2010): 9879–9888.
- Kawashima Y, Pfafferott K, Frater J, et al. Adaptation of HIV-1 to human leukocyte antigen class I. Nature. 458 (2009): 641–645.
- Goulder PJ, Walker BD. HIV and HLA class I: an evolving relationship. Immunity. 37 (2012): 426–440.
- Brumme ZL, John M, Carlson JM, et al. HLA-associated immune escape pathways in HIV-1 subtype B Gag, Pol and Nef proteins. PLoS One. 4 (2009): e6687.
- Carlson JM, Brumme CJ, Martin E, et al. Correlates of protective cellular immunity revealed by analysis of population-level immune escape pathways in HIV-1. J Virol. 86 (2012): 13202–13216.
- Migueles SA, Sabbaghian MS, Shupert WL, et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci USA. 97 (2000): 2709–2714.
- Miura T, Brockman MA, Schneidewind A, et al. HLA-B57/B*5801 human immunodeficiency virus type 1 elite controllers select for rare gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphocyte recognition. J Virol. 83 (2009): 2743–2755.
- Schneidewind A, Brockman MA, Yang R, et al. Escape from the dominant HLA-B27-restricted cytotoxic T-lymphocyte response in Gag is associated with a dramatic reduction in human immunodeficiency virus type 1 replication. J Virol. 81 (2007): 12382–12393.
- Alter G, Heckerman D, Schneidewind A, et al. HIV-1 adaptation to NK-cell-mediated immune pressure. Nature. 476 (2011): 96–100.
- Walker BD, Yu XG. Unravelling the mechanisms of durable control of HIV-1. Nat Rev Immunol. 13 (2013): 487–498.
- Saez-Cirion A, Pancino G, Sinet M, et al. HIV controllers: how do they tame the virus? Trends Immunol. 28 (2007): 532–540.
- Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 27 (2007): 406–416.
- Walker BD, Burton DR. Toward an AIDS vaccine. Science. 320 (2008): 760–764.
- Fauci AS, Johnston MI, Dieffenbach CW, et al. HIV vaccine research: the way forward. Science. 321 (2008): 530–532.
- Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 366 (2012): 1275–1286.
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 361 (2009): 2209–2220.
- Barouch DH, Whitney JB, Moldt B, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 503 (2013): 224–228.
- Klein F, Mouquet H, Dosenovic P, et al. Antibodies in HIV-1 vaccine development and therapy. Science. 341 (2013): 1199–1204.
- Chun TW, Fauci AS. HIV reservoirs: pathogenesis and obstacles to viral eradication and cure. AIDS. 26 (2012): 1261–1268.
- Siliciano JD, Siliciano RF. HIV-1 eradication strategies: design and assessment. Curr Opin HIV AIDS. 8 (2013): 318–325.
- Archin NM, Margolis DM. Emerging strategies to deplete the HIV reservoir. Curr Opin Infect Dis. 27 (2014): 29–35.
- Richman DD, Margolis DM, Delaney M, et al. The challenge of finding a cure for HIV infection. Science. 323 (2009): 1304–1307.
- Rasmussen TA, Lewin SR. Shocking HIV out of hiding: where are we with clinical trials of latency reversing agents? Curr Opin HIV AIDS. 11 (2016): 394–401.
- Margolis DM, Garcia JV, Hazuda DJ, et al. Latency reversal and viral clearance to cure HIV-1. Science. 353 (2016): aaf6517.
- Deeks SG. HIV: Shock and kill. Nature. 487 (2012): 439–440.
- Hütter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 360 (2009): 692–698.
- Allers K, Hütter G, Hofmann J, et al. Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood. 117 (2011): 2791–2799.
- Gupta RK, Abdul-Jawad S, McCoy LE, et al. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature. 568 (2019): 244–248.
- Tebas P, Stein D, Tang WW, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 370 (2014): 901–910.
- Xu L, Yang H, Gao Y, et al. CRISPR/Cas9-mediated CCR5 ablation in human hematopoietic stem/progenitor cells confers HIV-1 resistance in vivo. Mol Ther. 25 (2017): 1782–1789.
- Das AT, Binda CS, Berkhout B. Elimination of infectious HIV DNA by CRISPR-Cas9. Curr Opin Virol. 38 (2019): 81–88.
- Kaminski R, Chen Y, Fischer T, et al. Elimination of HIV-1 genomes from human T-lymphoid cells by CRISPR/Cas9 gene editing. Sci Rep. 6 (2016): 22555.
- Yin C, Zhang T, Li F, et al. Functional screening of guide RNAs targeting the regulatory and structural HIV-1 viral genome for a cure of AIDS. AIDS. 30 (2016): 1163–1174.
- Wang Z, Pan Q, Gendron P, et al. CRISPR/Cas9-Derived Mutations Both Inhibit HIV-1 Replication and Accelerate Viral Escape. Cell Rep. 15 (2016): 481–489.
- Ueda S, Ebina H, Kanemura Y, et al. Anti-HIV-1 potency of the CRISPR/Cas9 system insufficient to fully inhibit viral replication. Microbiol Immunol. 60 (2016): 483–496.
- Lebbink RJ, de Jong DC, Wolters F, et al. A combinatorial CRISPR-Cas9 attack on HIV-1 DNA extinguishes all infectious provirus in infected T cell cultures. Cell Rep. 17 (2016): 2819–2826.
- Yoder KE, Bundschuh R. Host double strand break repair generates HIV-1 strains resistant to CRISPR/Cas9. Sci Rep. 6 (2016): 29530.
- Wang G, Zhao N, Berkhout B, et al. CRISPR-Cas based antiviral strategies against HIV-1. Virus Res. 244 (2018): 321–332.
- Ophinni Y, Inoue M, Kotaki T, et al. CRISPR/Cas9 system targeting regulatory genes of HIV-1 inhibits viral replication in infected T-cell cultures. Sci Rep. 8 (2018): 7784.
- Dampier W, Nonnemacher MR, Sullivan NT, et al. Targeted editing of the HIV-1 genome and provirus for cure strategies. Front Microbiol. 8 (2017): 1617.


 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 