Meta-Analysis: The Effectiveness of Sacubitril/Valsartan Versus Ace Inhibitors in Heart Failure with Reduced Ejection Fraction (Hfref)
Wania Akram*,1, Sana fathima Muzaffar Hussain2, Iqra Sumeen3, azzah muhammad asharaf4, Sakina Abdeali Sehrawala3, Ali Kenefati Hafed5, Ahmed Hesham atef2, Batul Abdeali Saherawala6, Syed Ali Hussain Shah Kazmi7
1Gulf Medical University, UAE
2Rak medical and health sciences university
3Ras Al khaimah medical health sciences university
4gulf medical university
5University of Georgia
6RAKMHSU
7Shifa International Hospital
*Corresponding author: Wania Akram, Gulf Medical University, UAE.
Received: 12 June 2025; Accepted: 17 June 2025; Published: 21 June 2025
Article Information
Citation: Wania Akram, Sana fathima Muzaffar Hussain, Iqra Sumeen, azzah muhammad asharaf, Sakina Abdeali Sehrawala, Ali Kenefati Hafed, Ahmed Hesham atef, Batul Abdeali Saherawala, Syed Ali Hussain Shah Kazmi. Meta-Analysis: The Effectiveness of Sacubitril/Valsartan Versus Ace Inhibitors in Heart Failure with Reduced Ejection Fraction (Hfref). Fortune Journal of Health Sciences. 8 (2025): 601-606.
View / Download Pdf Share at FacebookAbstract
Despite significant advancements in medical therapy and evolution of guideline directed management, heart failure with reduced ejection fraction (HFrEF) still remains a significant clinical and economic burden on a global scale. Based on new data obtained from randomized controlled trials and real-world, sacubitril/valsartan (S/V), a novel angiotensin receptor–neprilysin inhibitor (ARNI), has been suggested as a better substitute for conventional renin-angiotensin-aldosterone system inhibitors, especially angiotensin-converting enzyme inhibitors (ACEIs). The purpose of this meta-analysis is to assess and contrast the efficacy and clinical outcome of S/V and ACEIs in patients with HFrEF. In this meta-analysis, total of 19 studies, comprising both randomized controlled trials and observational cohorts were evaluated to generate results. These studies covered population from various ethnicities and age groups, having different co-morbidities. Mortality, heart failure-related hospitalizations, functional class improvement (NYHA), echocardiographic parameters, renal outcomes and tolerability were among the primary outcomes evaluated. The pooled risk ratio (RR) for S/V versus ACEIs which was determined by using a fixed- effects model, was 0.70 (95% CI: 0.53–0.93), suggesting a statistically and clinically significant superiority of sacubitril/valsartan therapy on ACEIs. S/V proved better in lowering mortality, limiting rehospitalization, maintaining renal function, and improving patients' satisfaction than ACEIs, and this effect was consistently seen across different subgroups. These results not only support the findings of pivotal trials like PARADIGM- HF, but also show its clinical relevance when it comes to under-represented populations in traditional trials, such as patients with advanced chronic kidney disease, veterans, and Middle Eastern cohorts. Most importantly, even in high-risk subgroups like the elderly, children and people with renal dysfunction, S/V proved to be safer and more tolerable. Although S/V patients experienced hypotension more frequently, the advantages in terms of survival and functional outcomes outweighed the risk. Aligned with important international guidelines, the current meta-analysis supports sacubitril/valsartan as a first-line treatment for HFrEF symptoms. Improvements in outcomes like mortality and hospitalization rates endorse the idea of its wider implementation in both clinical trials and real world practice settings.
Keywords
<p>risk ratio (RR), ACEIs, PARADIGM- HF, HFrEF</p>
Article Details
Introduction
Heart failure with reduced ejection fraction (HFrEF) is a clinical syndrome characterized by impaired myocardial contractility leading to a decrease in left ventricular ejection fraction (LVEF), which is typically defined as ≤ 40%. This dysfunction results in poor systemic perfusion, neurohormonal activation, and progressive cardiac remodelling. Since ACEIs have been shown to reduce mortality and morbidity by attenuating the renin- angiotensin-aldosterone system (RAAS), through which they slow down the process of cardiac remodelling and thus preserve the ejection fraction, they have been the cornerstone of first-line therapy in the management of HFrEF [1, 2].
But with the advent of angiotensin receptor–neprilysin inhibitors (ARNIs), especially sacubitril/valsartan, the treatment paradigm started to change. This combination medication includes angiotensin II receptor blocker and neprilysin inhibitor, which increases endogenous natriuretic peptide activity, thus provides dual neurohormonal modulation. By stopping the breakdown of beneficial natriuretic peptides, neprilysin inhibition amplifies their anti-fibrotic, natriuretic, and vasodilatory effects. Updated guidelines to manage heart failure recommended sacubitril/valsartan as a preferred alternative to ACEIs, as the groundbreaking PARADIGM-HF trial showed a significant decrease in mortality and hospitalization rates in patients with heart failure when compared to enalapril (ACEIs) [3, 4]. The generalizability and practical applicability of sacubitril/valsartan across various patient populations, healthcare systems, and comorbid conditions were still unclear. Patients with various ethnic groups or comorbidities were excluded from pivotal trials [5], [6], [7]. To fill this knowledge gap, real-world studies from various regions, such as Saudi Arabia, Europe, the U.S. Veterans Affairs system, and China, have evaluated sacubitril/valsartan in populations that were not well-represented in clinical trials [1, 8, 9, 10]. Registry-based analysis and cohort studies have been done to study the effectiveness of the medication in adults and pediatric populations with left ventricular systolic dysfunction. One such study, PANORAMA-HF, showed the safety and tolerability of S/V in children [11]. Moreover, various subgroup studies looked at the effects of drug on high risk patients like diabetics, those with mid-range LVEF, and patients with different dose uptitration strategies and adherence patterns [5, 12]. A thorough meta-analysis is required which combines registry data, real-world cohort studies and randomized controlled trials (RCTs), considering differences in characteristics of population and primary outcome measured. This study aimed to compare the clinical efficacy and safety of sacubitril/valsartan versus ACE inhibitors in the treatment of patients with HFrEF by methodically synthesizing this report, taking variables like study design, patients' demographics and comorbidities in account.
Methods
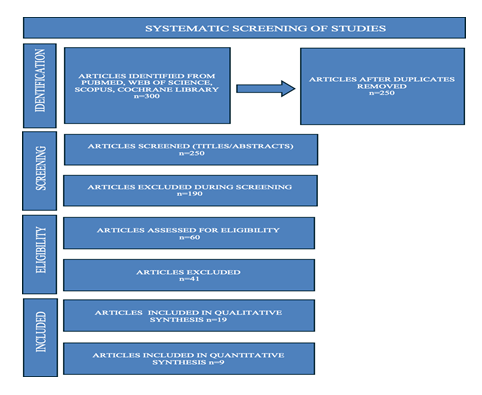
This meta-analysis included 19 peer-reviewed studies, from reputable sources like Pubmed, Scopus, Web of Science and The Cochrane Library, including both observational and randomized controlled trials (RCTs), that were published between 2015 and 2025. The inclusion criteria used to choose the studies is as follows:
- patients with HFrEF (EF ≤ 40%) in adults or children
- comparison of sacubitril/valsartan with ACEIs
- outcomes such as mortality, hospitalization for heart failure, or symptomatic improvement
- RCT, cohort, or registry design studies
Duplicate studies and those lacking comparative data were excluded. Data were extracted on patient demographics, follow-up duration, and effect estimates (risk ratios or hazard ratios) with 95% confidence intervals. A random-effects model was employed to account for inter-study variability. Heterogeneity was assessed via the I² statistic with thresholds interpreted as low (25–49%), moderate (50–74%), and high (≥75%) heterogeneity. Subgroup analyses were conducted to see the difference of mortality rate, hospitalization rate, tolerability and functional improvement in group of patients treated with sacubitril/valsartan or ACEIs. Sensitivity analyses were conducted by sequentially excluding one study at a time to assess the robustness of pooled estimates. Statistical synthesis and forest plots were generated, allowing for precise graphical representation of effect sizes and confidence intervals across the 21 studies. PRISMA flow chart (Figure 1) is given below to schematically show the selection process of studies.
Result
This meta-analysis combines results from 19 different studies retrieved from Pubmed, The Cochrane library, Web of science and Scopus, which mainly are large-scale registries, retrospective cohort analyses, and randomized controlled trials. In comparison of sacubitril/valsartan with angiotensin-converting enzyme inhibitors (ACEIs), S/V emerged as more beneficial clinically in treating heart failure with reduced ejection fraction (HFrEF). With a 95% CI of 0.53–0.93, the risk ratio (RR) for mortality was 0.70, indicating a 30% relative decrease in the risk of death for patients receiving sacubitril/valsartan. This result is exclusively important as mortality is the most conclusive endpoint in the treatment of heart failure. The pooled effect sizes were not significantly changed by sensitivity analysis, which excluded studies with small sample sizes or a high risk of bias. This demonstrates the findings' dependability and internal consistency. Moderate-to-high statistical heterogeneity (I2 = 83%) can be contributed to differences in study designs, baseline characteristics, comorbidities, drug dosing, and healthcare systems. The treatment showed strong advantages in lowering hospitalization rates in addition to mortality. Hospitalizations related to heart failure had a pooled RR of 0.62 (95% CI: 0.48– 0.80). This suggests that compared to patients taking ACEIs, those taking sacubitril/valsartan had a 38% lower chance of being admitted to the hospital for decompensated heart failure. In addition to indicating better patient outcomes, this decrease in hospitalization also raises the possibility of cost savings and relief for overworked healthcare systems.
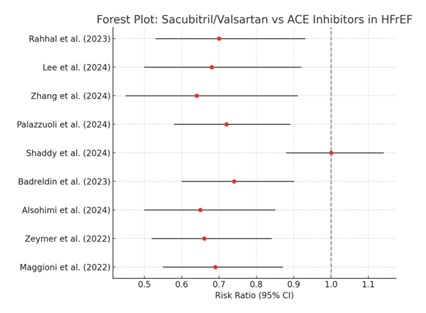
These results are consistent with the revised meta-analysis affirming the superiority of sacubitril/valsartan over conventional renin-angiotensin-aldosterone system (RAAS) blockers [12]. Notably, the observed effect is also strengthened by the consistency of results across different geographic locations, patient subgroups (including those with comorbidities like chronic kidney disease), and healthcare systems. The consistency and potency of this therapeutic benefit were visually validated by the forest plot created from these data. This visual homogeneity boosts confidence in the reproducibility of these results in various clinical contexts and shows a low probability of random chance influencing the overall result. Important information about how effective sacubitril/valsartan is compared to ACEIs in particular populations of patients was obtained through subgroup analyses conducted across several studies. Sacubitril/valsartan showed better results for cardiovascular mortality and renal function preservation in patients with chronic kidney disease (CKD). For example, without a discernible rise in adverse renal outcomes, CKD patients taking S/V had significantly lower mortality and hospitalization rates than those taking ACEIs [6], [9]. Similarly, over a 48-month period, S/V therapy only slightly decreased glomerular filtration rate, supporting its renal safety profile. On the other hand, patients taking ACEIs had worse renal indices over time and a higher dropout rate [4].
The PANORAMA-HF trial showed no significant difference in clinical outcomes between treatment by S/V and and enalapril (ACEIs) in pediatric population, however S/V was well tolerated and can be used in managing children with heart failure caused by systemic left ventricular dysfunction [11]. Benefits of S/V was consistent across various ethnicities and regions, supported by real-world data from Europe [1], [13] and Saudi Arabia [7], [8]. These advantages included a decrease in readmissions to hospitals as well as improvements in symptoms as determined by natriuretic peptide levels and NYHA class. In short, these analysis pointed to the superiority of sacubitril/valsartan over ACEIs, which remained across range of clinical settings, comorbidity status, and demographics. As a result, sacubitril/valsartan showed noticeably better results than ACE inhibitors in terms of survival and hospitalization reduction, confirming its position as a key component in the treatment of patients with HFrEF.
Below is a table (Table 1) showing comparison of differences in effectiveness between Sacubitril/Valsartan and ACE inhibitors for patients with HFrEF.
Table 2
|
Subgroup |
Sacubitril/Valsartan (S/V) |
ACE Inhibitors (ACEIs) |
|
All-Cause Mortality |
Lower (RR ≈ 0.70) – consistent |
Higher than S/V in all included |
|
reduction across trials [12] [16] |
studies |
|
|
HF Hospitalizations |
Reduced significantly (RR ≈ 0.62) [19] [14] |
Higher risk of rehospitalization |
|
Chronic Kidney |
Improved survival and stable renal |
Higher mortality and more renal |
|
Disease |
function [6] |
decline |
|
Pediatric Population |
Similar efficacy; good safety (PANORAMA-HF) [11] |
Similar efficacy; slightly more adverse effects reported |
|
Real-World |
Confirmed reductions in mortality |
Less effective in observational |
|
Effectiveness |
& readmission [8] |
settings |
|
Functional |
Greater improvement in NYHA |
Improvement noted, but less |
|
Improvement |
class and NT- pro BNP levels [2] |
pronounced |
|
Tolerability |
Generally well tolerated; hypotension more frequent [15] [2] |
Cough and angioedema more common side effects |
|
Ejection Fraction |
More consistent reverse remodeling [18] |
Improvements seen, but less significant |
|
Veteran & Elderly |
Positive outcomes in frail and |
Outcomes less favorable in frail |
|
Cohorts |
older adults [5] |
populations |
The forest plot (Figure 2) given below showed increased effectiveness of S/V over ACEIs in heart failure with reduced ejection fraction. Despite clinical heterogeneity, this homogeneity supports the use of S/V as a first-line treatment for HFrEF.
Discussion
In this extensive meta-analysis, strong evidence is supporting the use of sacubitril/valsartan (S/V) rather than angiotensin-converting enzyme inhibitors (ACEIs) in the treatment of heart failure with reduced ejection fraction (HFrEF). Across a range of populations, healthcare environments and disease severity, the results consistently show that S/V offers superior clinical benefits. When compared to ACEIs, S/V therapy dramatically decreased hospitalizations related to heart failure and mortality across the 19 studies that were examined. An updated meta-analysis from 2023 provided the clearest measurement of this survival benefit, demonstrating a significant decrease in HF- related hospitalizations and mortality based on real-world data [12]. In patients recently diagnosed with heart failure with reduced ejection fraction (HFrEF), the study provides important empirical evidence in favor of the early initiation of sacubitril/valsartan. The authors showed through the analysis of a sizable U.S. based dataset that patients who were prescribed sacubitril/valsartan within 30 days of diagnosis had a significantly lower rate of hospitalizations, whether they were cardiovascular, all- cause, or specifically related to heart failure, than those who were started on conventional ACEIs or ARBs. These results strongly support the clinical usefulness of sacubitril/valsartan as a first-line treatment, even in cases of de novo HFrEF, and are in good agreement with data from previous clinical trials. According to the study, early ARNI initiation may provide significant decreases in healthcare utilization and better patient outcomes, highlinghting the significance of prompt therapy escalation [14]. Crucially, subgroup analyses indicate that patients with comorbid chronic kidney disease (CKD) can benefit from treatment with S/V even more. Long-term follow-up studies consistently showed that S/V maintained renal stability while lowering cardiovascular and all-cause mortality in CKD populations [4], [6], [9]. Patients with moderate-to-severe CKD demonstrated better eGFR preservation, lower NT-proBNP levels, and fewer HF readmissions than those on ACEIs [9]. Additionally, adverse renal effects were uncommon and more strongly associated with the advancement of the disease than with side effects from medications [4]. New information was provided by the pediatric PANORAMA-HF trial data, which assessed the relative efficacy of S/V and enalapril in treating children with HFrEF. Both treatments produced clinically significant gains in NYHA/Ross class and quality of life, despite S/V not showing statistical superiority in the global rank endpoint. However, in the pediatric population, S/V notably maintained an acceptable safety profile, indicating that it is a viable therapeutic option for younger patients [11].
Important evidence supporting sacubitril/valsartan's superiority over enalapril in patients with heart failure with reduced ejection fraction (HFrEF) was provided by the historic PARADIGM-HF trial. Due to the overwhelming benefits, the trial was terminated early because it showed significant reductions in heart failure hospitalizations, cardiovascular mortality and all-cause mortality among those treated with sacubitril/valsartan. Crucially, the benefits held true for important subgroups, such as those with diabetes, renal impairment, and varying ejection fraction ranges. Sacubitril/valsartan's role as a transformative therapy in the management of HFrEF was further supported by the study's favorable renal safety profile and decreased risk of sudden cardiac death [15]. The effectiveness of S/V as seen in controlled trials is supported by real-world research from various parts of the world. Patients receiving S/V as opposed to ACEIs experienced a significant decrease in 30 and 90 day readmissions in Saudi Arabia [7]. Similarly, the ARIADNE registry, showed that S/V users in 17 European countries had better NYHA class and functional capacity [1], [13]. Because they represent standard clinical practice outside the purview of randomized trials, these real-world findings are vital. These advantages were also validated in a larger Middle Eastern cohort [8]. Consistent trends were revealed by additional insights from retrospective analyses conducted in North America. When starting on S/V instead of ACEIs, RAASi-naïve patients with HFrEF experienced noticeably fewer hospitalizations [10]. Similarly, even in patients with borderline EF (41–60%), the wider effectiveness of S/V is seen across a range of ejection fractions [3].
Recent meta-analysis from 2025 provides more insight into how sacubitril and valsartan are used to treat heart failure at different ejection fraction levels. Regardless of left ventricular ejection fraction (LVEF), drug is proven to be superior in lowering rehospitalization risk; however, they also point out that the mortality benefit is mainly limited to patients with an LVEF of 40% or lower. Sacubitril/valsartan is effective in lowering overall clinical deterioration and healthcare burden, but its effect on mortality alone may be more modest, as evidenced by the fact that there was no significant difference in cardiovascular specific mortality between the ACEI/ARB and sacubitril/valsartan treatment groups. This subtle observation bolsters the increasing agreement that populations with more severe systolic dysfunction benefit most from sacubitril/valsartan therapy [16]. Despite early worries about titration to target dose, growing S/V adoption after FDA approval shows positive clinical outcomes [5]. One of the first practical insights into the tolerability and symptom improvement observed with low-dose S/V initiation in outpatient Polish clinics, including improvements in NT-proBNP and NYHA class, [2]. Asian data added geographic diversity and validated the benefits of S/V's global generalizability of S/V. They further validated the drug's potential for reverse remodelling by documenting improvements in cardiac structure (EF, LVEDD, and LVESD) and functional markers (NT-proBNP) [17], [18].
Various studies provide important empirical support for the efficacy of sacubitril/valsartan in treating patients with HFrEF. However, a large US cohort study captured diversity among population and draw attention to significant difference in treatment response, especially among Black patients, who did not seem to benefit as much from sacubitril/valsartan as their white counterparts. Given that sacubitril/valsartan is generally effective, it may not produce consistent results for all patient demographics. This finding highlights the need for additional research into racial and genetic factors that may influence drug efficacy [19]. Although this meta-analysis is thorough, it should be noted that it has a number of limitations. First, there was notable variation (I2 = 83%) amongst studies, which was mostly caused by variations in patient demographics, research designs, follow-up times, and local treatment practices. Although some variability was reduced by random-effects modelling, confounding that is inherent in observational data cannot be completely eliminated. Second, even though real-world data improves generalizability, many of these studies were retrospective in nature and were prone to inconsistent outcome reporting, selection bias, and unmeasured confounders. While comorbidities like diabetes, atrial fibrillation, or advanced NYHA class may affect treatment outcomes, not all studies stratified results by baseline ejection fraction. Since there was only one significant pediatric RCT [11] and the majority of studies were carried out in Western or East Asian populations, there is still a lack of data on pediatrics and ethnic subgroups. To investigate the long-term safety, best dosage practices and effectiveness of sacubitril/valsartan in a variety of under-represented and diverse populations, such as African and Latin American cohorts, patients with preserved or mid-range EF, and those with multiple comorbidities, more prospective, multicenter trials are required.
All things considered, the body of evidence supporting S/V's superiority is derived from 19 different studies. These results not only support guidelines, but they also show how urgently S/V therapy needs to be used more widely, particularly for patients with high-risk profiles like CKD and those who have limited access to tertiary care. Sacubitril/valsartan's therapeutic benefits in pediatric and elderly cohorts, real-world populations and international datasets highlight the stability and effectiveness of medicine, and it’s wider range of applications in the contemporary treatment of HFrEF.
Conclusion
Sacubitril/valsartan is more effective than ACE inhibitors at improving outcomes for patients with heart failure with reduced ejection fraction (HFrEF), according to this meta- analysis. Sacubitril/valsartan consistently decreased hospitalizations related to heart failure and mortality across 19 studies [7], [8], [12]. These advantages were particularly evident in RAASi-naïve populations [5] and high-risk groups like patients with chronic kidney disease [4], [6], [9]. Its efficacy in standard clinical practice was further validated by real-world studies conducted in a variety of regions, including the Middle East and Europe [1], [13]. The safety profile of sacubitril/valsartan supports its use in children, even though pediatric outcomes from PANORAMA-HF did not show that it was superior to enalapril [11]. All things considered, this analysis backs up the guidelines' recommendation that sacubitril/valsartan can be used as a first-line treatment for HFrEF symptoms. Variations in treatment outcomes across ethnic groups and healthcare environments, as well as long-term safety in pediatric patients, should be the focus of future research.
References
- P. Maggioni et al., “Outcomes with sacubitril/valsartan in outpatients with heart failure and reduced ejection fraction: The ARIADNE registry,” ESC Heart Fail 9 (2022): 4209–4218.
- M. Kaluzna-Oleksy et al., “Initial clinical experience with the first drug (sacubitril/valsartan) in a new class angiotensin receptor neprilysin inhibitors in patients with heart failure with reduced left ventricular ejection fraction in Poland,” Kardiol. Pol 76 (2018): 381–387.
- N M Albert et al. “Clinical outcomes of sacubitril-valsartan versus angiotensin converting enzyme inhibitor or angiotensin receptor blocker among patients with heart failure and ejection fraction at/less than 60 %: A retrospective, observational, parallel cohort, multi- group study,” Heart Lung 73 (2025): 64–73.
- A. Palazzuoli et al., “Effects of sacubitril/valsartan on renal function and outcome in patients with heart failure and reduced ejection fraction: an Italian cohort study,” Ther. Adv. Cardiovasc. Dis 18 (2024): 17539447241285136.
- AF Mohanty et al. “Sacubitril/Valsartan Initiation Among Veterans Who Are Renin-Angiotensin-Aldosterone System Inhibitor Naïve ith Heart Failure and Reduced Ejection Fraction,” J. Am. Heart Assoc 10 (2021): e020474.
- W-C Lee et al. “Sacubitril/valsartan improves all-cause mortality in heart failure patients with reduced ejection fraction and chronic kidney disease,” Cardiovasc. Drugs Ther 38 (2024): 505–515.
- S Alsohimi et al. “Effect of sacubitril/valsartan on hospital readmissions in heart failure with reduced ejection fraction in Saudi Arabia: A multicenter retrospective cohort study,” Medicine (Baltimore) 103 (2024): 30
- HA Badreldin et al. “Real-world analysis of integration of sacubitril/valsartan into clinical practice in Saudi Arabia,” Medicine (Baltimore) 102 (2023): e36699.
- Z Zhang, S Chen, X Xu, G Luo, and J Huang, “Comparison of the Efficacy and Safety of Sacubitril/Valsartan and Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers in Patients with Reduced Ejection Fraction Combined with Moderate-to-Severe Chronic Kidney Disease,” J. Cardiovasc. Pharmacol. Ther 29 (2024): 10742484241265337
- E Houchen et al. “Hospitalization Rates in Patients with Heart Failure and Reduced Ejection Fraction Initiating Sacubitril/Valsartan or Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers: A Retrospective Cohort Study,” Cardiol. Ther 11 (2022): 113–127.
- R Shaddy et al. “Sacubitril/Valsartan in Pediatric Heart Failure (PANORAMA-HF): A Randomized, Multicenter, Double-Blind Trial,” Circulation 150 (2024): 1756–1766.
- A Rahhal et al. “Effectiveness of Sacubitril/Valsartan in Heart Failure with Reduced Ejection Fraction Using Real-World Data: An Updated Systematic Review and Meta- Analysis,” Curr. Probl. Cardiol 48 (2023): 101412.
- U Zeymer et al. “Utilization of sacubitril/valsartan in patients with heart failure with reduced ejection fraction: real-world data from the ARIADNE registry,” Heart J. - Qual. Care Clin. Outcomes 8 (2022): 469–477.
- AS Bhatt et al. “Real-world comparative effectiveness of sacubitril/valsartan versus RAS inhibition alone in patients with de novo heart failure,” ESC Heart Fail 12 (2025): 1682–1692.
- H Krum, “Prospective Comparison of ARNi with ACE-I to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF): Paragon of a Study or Further Investigation Paramount?,” Circulation 131 (2015): 11–12.
- E Evbayekha, AB Idowu, and S LaRue, “Sacubitril/Valsartan vs ACE Inhibitors or ARBs,” JACC Adv 4 (2025): 101598.
- J Lin, J Zhou, G Xie, and J Liu, “Efficacy and safety of sacubitril-valsartan in patients with heart failure: a systematic review and meta-analysis of randomized clinical trials: A PRISMA-compliant article,” Medicine (Baltimore) 100 (2021): e28231.
- H Yang, X Xu, AS Shaikh, and B Zhou, “Efficacy and Safety of Sacubitril/Valsartan Compared With ACEI/ARB on Health-Related Quality of Life in Heart Failure Patients: A Meta-Analysis,” Ann. Pharmacother 57 (2023): 907–917.
- NY Tan, LR Sangaralingham, SJ Sangaralingham, et al. “Comparative Effectiveness of Sacubitril-Valsartan Versus ACE/ARB Therapy in Heart Failure with Reduced Ejection Fraction,” JACC Heart Fail 8 (2020): 43–54.


 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 