Modification of Sarcolemmal Ca2+- transport and Na+- K+ ATPase Defects by Propionyl L-Carnitine during the Development of Diabetic Cardiomyopathy
Vijayan Elimban1, Khushman Kaur1, Roberto Ferrari2, Naranjan S. Dhalla*,1
1Institute of Cardiovascular Sciences, St. Boniface Hospital Albrechtsen Research Centre, Department of Physiology and Pathophysiology, Max Rady College of Medicine, University of Manitoba, Winnipeg, Canada
2Department of Cardiology, University of Ferrara, 44121 Ferrara, Italy
*Corresponding author: Naranjan S. Dhalla, Institute of Cardiovascular Sciences, St. Boniface Hospital Albrechtsen Research Centre, Department of Physiology and Pathophysiology, Max Rady College of Medicine, University of Manitoba, Winnipeg, Canada
Received: July 17, 2025; Accepted: July 24, 2025; Published: August 07, 2025
Article Information
Citation: Vijayan Elimban, Khushman Kaur, Roberto Ferrari, Naranjan S. Dhalla. Modification of Sarcolemmal Ca2+- transport and Na+- K+ ATPase Defects by Propionyl L-Carnitine during the Development of Diabetic Cardiomyopathy. Fortune Journal of Health Sciences. 8 (2025): 756-767.
View / Download Pdf Share at FacebookAbstract
Introduction: Since diabetic cardiomyopathy is associated with metabolic abnormalites and subcellular Ca2+- handling defects, this study examined the effects of metabolic therapy with propionyl L-carnitine (PPLC) in modifying diabetes induced changes in cardiac sarcolemma (SL) Ca2+- transport.
Methods: Three days after inducing diabetes by injecting 65 mg/kg streptozotocin, rats were treated with or without PPLC (100 mg/kg; daily) for 8 weeks. These animals were assessed hemodynamically for cardiac function and the activities of some Ca2+-transport systems were determined in cardiac SL preparations.
Results: Improvement of cardiac function in diabetic animals by PPLC was associated with lowering of plasma lipids. Depressions in SL Na+- K+ ATPase and Na+- Ca2+ exchange activities in diabetic herats were partially attenuated by PPLC treatment. Maximal enzyme activities (Vmax values) for control, diabetes and PPLC- treated preparations for Na+-K+ ATPase were 33.61, 18.46 and 27.64 (μmol Pi/mg/hr) whereas maximal Ca2+- accumulation activities (Bmax values) for Na+- Ca2+ exchange were 9.60, 5.09 and 7.65 μΜ Ca2+, respectively. Although both ATP- dependent Ca2+- uptake and Ca2+- stimulated ATPase were depressed in diabetic SL, treatment with PPLC did not show any improvement in these activities. Alterations in different biomarkers of oxidative stress in diabetic heart were attenuated by PPLC treatment.
Conclusions: These results indicate that alterations in SL Na+- K+ ATPase and Na+- Ca2+ exchange activities in diabetic heart may be attenuated by reduction in the development of oxidative stress by PPLC. It is suggested that modifictaion of SL defects by metabolic therapy with PPLC may contribute in reducing the occurence of intracellular Ca2+-overload and improving cardiac function in diabetic cardiomyopathy.
Keywords
diabteic cardiomyoptahy; cardiac sarcolemma; Na+-K+ ATPase; Na+- Ca2+exchange; Ca2+-pump activity
diabteic cardiomyoptahy articles; cardiac sarcolemma articles; Na+-K+ ATPase articles; Na+- Ca2+exchange articles; Ca2+-pump activity articles.
Article Details
Introduction
It is now well known that chronic diabetes as a consequence of insulin deficiency or insulin resistance is associated with the development of several cardiovascular complications including diabetic cardiomyoptahy as well as metabolic abnormailities, Ca2+- handling defects and alterations in cardiac ultrastructure [1- 7]. Cardiac dysfunction and metabolic defects in diabetic cardiomyoptahy have been shown to be induced by different pathogenic mechanisms such as oxidative stress, inflammation, intracellular Ca2+-overload and endothelial dysfunction [8- 15]. Several lipid lowering metabolic interventions and antioxidants have been reported to attenuate Ca2+- handling abnormalities, produce a shift in the oxidation of glucose and free fatty acids, and improve cardiac function in diabetic heart [16-21] . It is thus believed that metabolic changes, Ca2+-handling defects and oxidative stress are intimately involved in the pathogenesis of cardiovascular complications in chronic diabetes. Several studies have reported that metabolic therapay with propionyl L- carnitine (PPLC) has been shown to exert beneficial effects in a wide variety of cardiovascular diseases. In this regard, administration of carnitine or PPLC was observed to improve cardiac function and metabolic status in ischemia-reperfusion injury, myocardial infarction, cardiac hypertrophy and congestive heart failure [22-31]. Furthermore, carnitine or PPLC have been shown to protect different cardiac abnormalities in erucic acid-induced cardiomyoptahy, catecholamine- induced cardiomyopathy and genetically linked cardiomyopathy [32-36]. It should be noted that PPLC is a short-chain acyl-derivative of L-carnitine, which is required for the transport and oxidation of long-chain fatty acids for the production of ATP in mitochondria. It is thus evident that the protective effects of PPLC or L-carnitine in diverse cardiovascular diseases are associated with the maintenance of energy stores in the myocardium.
Treatment of chronic diabetes with PPLC has also been observed to produce several cardioprotective effects in diabetic cardiomyopathy [13, 37,38]. By employing a streptozotocin- induced rat model of diabetic cardiomyopathy, it was shown that PPLC not only improved ventricular function and prevented depression in high energy phosphate stores but also lowered plasma lipids without any changes in plasma insulin or glucose levels [13, 37, 38]. Ferrari et al. [37] have observed that depressions in cardiac sarcoplasmic reticulum Ca2+- uptake, Ca2+-pump ATPase and Mg2+- ATPase activities, unlike depression in myofibrillar Ca2+- stimulated ATPase activity, were prevented by the treatment of diabetic animals with PPLC. Preliminary studies have also shown that depressed sarcolemma (SL) Na+-K+ ATPase and Na+-Ca2+ exchange activities, unlike depressed Na+-H+ exchange activity, in diabetic cardiomyopathy were partially prevented by PPLC treatment [13, 39]. Although cardiac SL phosphatidylethanolamine N-methylation was depressed in chronic diabetes, this change was also unaltered by PPLC treatment [40]. These observations indicate that PPLC treatment may produce beneficial effects only on some specific targets in the SL membrane in diabetic cardiomyopathy. Thus, in order to gain further information as well as to establish the modification of diabetes- induced changes in cardiac SL by PPLC treatment, we have investigated the effects of PPLC treatment on the SL Na+-K+ ATPase, Ca2+-pump and Na+-Ca2+ exchange activities under various experimental conditions in chronic diabetes. Experiments were also carried out to rule out cross contamination problems or difference in the orientation of SL vesicles in the preparations. Furthermore, attempts were made to study the mechanisms as well as relationship of changes in the SL Ca2+- transport systems with cardiac dysfunction in diabetic cardiomyopathy.
Methods
All experimental protocols were approved by the Animal Care Committee of the University of Manitoba according to the guidelines of the Canadian Council for Animal Care and the Guide to the Care and Use of Labratory Animals (Protocol #07-048).
Experimental model. Male Sprague-Dawley rats weighing about 200g each were injected with either streptozotocin (65mg/ kg body wt) or the buffered vehicle (0.1 M citrate, pH 4.5) into the tail vein; these animals formed the diabetic and control groups, respectively. Both control and diabetic animals were further subdivided according to the administration of PPLC, which was injected intraperitoneally at a dosage of 100 mg/kg body wt. Drug treatment was started two days after stretozotocin injection and continued daily for the duration of the experimental period. The animals were killed by decapitation 8 weeks after injection of streptozotocin or the buffered vehicle. This duration of diabetes has been reported to produce diabetic cardiomyopathy associated with structural and functional changes in the myocardium [2, 21, 39]. Hearts were immediately removed, and the ventricular tissue was isolated from the atria, connective tissue, and major blood vessels, and then processed for the isolation of SL membrane vesicles. Blood was collected at the time of death in heparinized tubes. Plasma was prepared from the blood samples and stored at -20°C for the analysis of glucose, insulin, triiodothyronine, angiotensin II, norepinephrine, triglycerides, free fatty acids, and cholesterol [16, 41, 42].
SL membrane preparation. SL membranes were isolated by the sucrose density gradient method described by Pitts [43] with modification [34]. Briefly, ventricles from 3 animals were combined, washed, minced, and then homogenized in 0.6 M sucrose, 10 mM imidazole-HCl, pH 7.0 (3.5 mg/g tissue) with a polytron PT-20 (5 x 20 s, setting 5). The resulting homogenate was centrifuged at 12,000 g for 30 min, and the pellet was discarded. After diluting (5 ml/g tissue) with 140 mM KC1-10 mM 3-(N-morpholino) -propanesulphonic acid (MOPS), pH 7.4 (KC1-MOPS), the supernatant was centrifuged at 95,000 g for 60 min. The resulting pellet was suspended in the KC1-MOPS buffer and layered over a 30% sucrose solution containing 0.3 M KC1-50 mM Na4P207, and 0.1 M tris(hydroxymethyl) aminomethane (Tris)-HC1, pH 8.3. After centrifugation at 95,000 g for 90 min (utilizing a Beckman swinging bucket rotor), the band at the sucrose-buffer interface was taken and diluted with 3 vol of KCl-MOPS solution. A final centrifugation at 95,000 g for 30 min resulted in a pellet rich in SL. For the determination of Na+-Ca2+ exchange activity the pellet was suspended in 140 mM KCl-10 mM MOPS pH 7.4, at a concentration of 3.5 mg/ ml and quickly frozen in liquid N2. Samples were then stored in liquid N2 and tested 3-4 weeks later. When the purified vesicles were used for determining Ca2+-stimulated ATPase and ATP- dependent Ca2+-accumulation (sarcolemmal Ca2+- pump activity) or Na+-K+ ATPase activities, the pellet was suspended in 0.25 M sucrose histidine, pH 7.2 (3.5 mg/ml), and was stored as described above. The protein concentration was estimated by the method of Lowry et al [44].
Biochemical assay procedures. The purity of the membrane preparation was examined by measuring the activities of marker enzymes such as total Na+-K+ ATPase, K+-pNPPase, cytochrome c oxidase, and rotenone-insensitive NADPH cytochrome c reductase in both homogenate and SL fractions according to the procedures used previously [34, 39]. In brief, SL total ATPase activity was measured in a medium containing: 50 mM Tris-HC1, 120 mM NaC1, 3.5 mM MgC12, 1 mM EGTA, 5 mM NaN3, 20 mM KC1, pH 7.0 at 37° C. Na+-K+ ATPase activity was calculated as the difference of activities in the presence and abs ence of 3.5 mM MgC12. Total ATPase activities were also measured in the presence and absence of 1 mM ouabain or 10 uM digitoxigenin, and the differences were taken to reflect ouabin-sensitive and digitoxigenin- sensitive Na+-K+ ATPase activities, respectively. Unless otherwise mentioned in the text, the Na+-K+ ATPase reaction was started by 4 mM ATP and the activity was measured after 10 min incubation, and the results were presented per hour. In all experiments, Tris- ATP was used as the substrate for determination of ATPase activities.
Na+- dependent Ca2+ uptake measurements were carried out by a method described in detail elsewhere [34] with some modifications. Briefly, 5μl of SL vesicles (1.5mg/ml: 7.5 ug protein/ tube) preloaded with NaCl/MOPS buffer at 37° C for 30 min were rapidly diluted 50 times with Ca2+ uptake medium containing 140 mM KCl, 20 mM MOPS, 0.4 uM valinomycin, 0.3 μCi 45Ca2+ and various Ca2+ concentrations (5-80 μM), pH 7.4. After the appropriate time span, the reaction was stopped by the addition of ice cold 0.03 ml stopping solution containing 140 mM KCl, 1 mM LaCl3, 20 mM MOPS, pH 7.4. Samples (0.25 ml from 0.28 ml of the total reaction mixture) were filtered through Millipore filters (pore size= 0.45 µm) and washed twice with 2.5 ml of ice cold washing solution containing 140 mM KCl, 0.1 mM LaCl3, 20 mM MOPS, pH 7.4. Radioactivity of the filters was measured using a Beckman LS 1701 scintillation counter and Na+-dependent Ca2+ uptake activity was corrected by subtraction of the nonspecific Ca2+ uptake values.
For the determination of Mg2+-ATPase and Ca2+-stimulated ATPase activities, experimental conditions were the same as reported elsewhere [34]. SL vesicles (20-40 µg protein) were preincubated at 37° C for 5 min in 0.5 ml of medium containing 140 mM KC1-10 mM MOPS-Tris pH 7.4, 2 mM MgC12, 5 mM sodium azide, and 0.2 mM ethylene glycol-bis (β-aminoethyl ether) - N,N,N', N' -tetraacetic acid (EGTA). The reaction was started by the addition of 4 mM Tris-ATP, pH 7.4, and terminated 5 min later with 0.5 ml of ice cold 12% trichloroacetic acid; the liberated phosphate was measured by the method of Tausky and Shorr (1945). Estimation of total (Ca2+ and Mg2+)- ATPase was made in medium containing 140 mM KC1-10 mM MOPS-Tris pH 7.4, 2 mM MgC12, and 5 mM sodium azide, and 10-5M free Ca2+. The concentration of free Ca2+ in the medium was adjusted by using EGTA [46]. The Ca2+stimulated ATPase activity was the difference between the total ATPase and Mg2+-ATPase activities.
In order to measure ATP-dependent Ca2+ accumulation, sarcolemmal vesicles (100 μg protein) were preincubated at 37° C for 5 min in 0.5 ml of medium containing 140 mM KC1, 10 mM MOPS-Tris pH 7.4, 2 mM MgC12, and 45CaCl -EGTA, which contained 10-5M free Ca2+ [46]. Ca2+ accumulation was initiated by adding 4 mM Tris-ATP, pH 7.4. After 5 min of incubation at 37° C, 250 ul aliquots were immediately filtered through Millipore filters (pore size = 0.45 µm), washed twice with 2.5 ml ice cold KC1-MOPS and 1 mM LaCl3, pH 7.4, dried, and radioactivity determined for calculating the total Ca2+ accumulation. Nonspecific Ca2+ binding was measured in the absence of ATP for each set of experiments. The ATP- dependent Ca2+ accumulation was calculated by subtracting nonspecific Ca2+ binding from the total Ca2+ accumulation. Several biomarkers of oxidative stress such as conjugated dienes, malondialdehyde, oxidized glutathione, reduced glutathione, glutathione peroxidase, superoxide dismutase and catalase were measured in the heart to determine the mechanisms associated with observed effects of diabetes and treatment with PPLC [21].
Results
The data in Table 1 show that the diabetic animals exhibited reduced body and ventricle weights; these changes were not affected by treatment with PPLC. The diabetic animals also showed elevated level of systolic blood pressure and depressed heart rate. Treatment of diabetic animals with PPLC decreased the elevated blood pressure but did not increase the heart rate significantly. The left ventricular developed pressure (LVDP) as well as values for +dP/dT and -dP/dT were markedly depressed without any changes in the left ventricular end-diastolic pressure (LVEDP) in diabetic animals; these alterations in cardiac contractile parameters were significantly attenuated by treatment with PPLC. The data in Table 2 indicate that plasma glucose, triglycerides, free fatty acids and cholesterol levels were elevated in the diabetic animals. Treatment of diabetic animals with PPLC reduced the increased levels of plasma triglycerides and free fatty acids significantly without any changes in plasma glucose and cholesterol levels. While the plasma insulin and triiodothyronine levels were depressed, the plasma norepinephrine level was increased and plasma angiotensin II level was unaltered in the diabetic animals; these alterations in plasma hormonal levels due to diabetes were not affected significantly by treatment with PPLC (Table 2).
Table 1: Hemodynamic and cardiac function in diabetic rats with or without propionyl L-carnitine (PPLC) treatment
|
Control |
Diabetic |
PPLC-treated Diabetic |
|
|
Body Weight (g) |
440 ± 11 |
275 ± 8* |
286 ± 10 |
|
Ventricular weight (g) |
1.2± 0.03 |
0.8 ± 0.04* |
0.8 ± 0.03 |
|
Blood pressure (mm Hg) |
110 ± 8.6 |
138 ± 6.4* |
118 ± 4.5† |
|
Heart rate (beats/min) |
416 ± 20.6 |
312± 12.7* |
340 ± 14.5 |
|
LVDP (mm Hg) |
135 ± 9.8 |
84 ± 6.9* |
115 ± 6.6† |
|
LVEDP (mm Hg) |
5.0 ± 0.3 |
5.8 ± 0.3 |
4.7 ± 0.2 |
|
+dP/dt (mm Hg/sec) |
8780 ± 756 |
5022 ± 708* |
7086 ± 65.4† |
|
-dP/dt (mm Hg/sec) |
7640 ± 814 |
4464 ± 784* |
7182 ± 620† |
In order to examine the effect of PPLC treatment on cardiac Na+-K+ ATPase activity in diabetic animals, SL membrane preparations were isolated from the control, diabetic and PPLC- diabetic treated hearts. It can be seen from Table 3 that Na+-K+ ATPase activity, when measured at different times of incubation, in the diabetic preparations was significantly depressed in comparison to the control and this change was attenuated by PPLC treatment. As seen from the values for Na+-K+ ATPase and K+-pNPPase activities, the SL preparations were about 16 fold purified with respect to heart homogenate activities in control, diabetic and PPLC- treated diabetic hearts (Table 3). Furthermore, the SL protein yields for the control and experimental preparations were not different from each other. Measurement of cytochrome C oxidase (mitochondrial marker) and NADPH cytochrome C reductase (sarcoplasmic reticulum marker) activities showed minimal, but equal, cross-contamination in the SL preparations (Table 3). It should be mentioned that the observed depression of Na+-K+ ATPase activity due to diabetes is in agreement with previous report [34].
The SL Na+-K+ ATPase activities, when determined at different pH of the incubation medium, were also depressed in diabetic hearts and were attenuated in PPLC- treated diabetic hearts (Table 4). The sensitivities of the SL Na+-K+ ATPase preparations to ouabain (which is impermeable
Table 2: Plasma glucose, lipids and hormone levels of diabetic rats with or without propionyl L-carnitine (PPLC) treatment
|
Control |
Diabetic |
PPLC-treated |
|
|
Plasma glucose (mg/100 ml) |
143 ± 12 |
658 ± 25* |
623 ± 17 |
|
Plasma triglycerides (nmol/L) |
2.76 ± 0.59 |
7.10 ± 0.44* |
3.87 ± 0.45† |
|
Plasma free fatty acids (meq/L) |
0.25 ± 0.03 |
0.38 ± 0.04* |
0.24 ± 0.02† |
|
Plasma cholesterol (μmol/L) |
1.63 ± 0.36 |
3.12 ± 0.29* |
2.88 ± 0.34 |
|
Plasma insulin (μU/ml) |
26.9 ± 1.3 |
11.2 ± 1.1* |
12.3 ± 1.0 |
|
Plasma triiodo- thyronine (ng/100ml) |
92 ± 2.5 |
47 ± 3.1* |
51 ± 4.2 |
|
Plasma angiotensin II (fmol/ml) |
6.9 ± 0.9 |
7.3 ± 0.5 |
7.5 ± 0.4 |
|
Plasma norepinephrine (ng/ml) |
1.6 ± 0.13 |
4.2 ± 0.14* |
3.9 ± 0.17 |
Values are mean ± SE of 6 animals in each group. * P<0.05 vs control; † P<0.05 vs diabetic.
Table 3: Characteristics of the heart sarcolemmal preparations containing Na+- K+ ATPase obtained from diabetic rats with or without propionyl L- carnitine (PPLC) treatment.
|
Control |
Diabetic |
PPLC-treated diabetic |
|
|
A. Time of incubation for determination of |
|||
|
Na+-K+ ATPase activity (μmol Pi/ mg): |
|||
|
5 min |
2.3 ± 0.3 |
1.2 ± 0.1* |
1.8 ± 0.2† |
|
10 min |
4.4 ± 0.6 |
2.5 ± 0.3* |
3.7 ± 0.3† |
|
20 min |
9.1 ± 1.0 |
5.3 ± 0.7* |
7.9 ± 0.5† |
|
B. Sarcolemma characteristics: |
|||
|
Membrane yield (mg/g heart) |
1.3 ± 0.4 |
1.2 ± 0.3 |
1.3 ± 0.3 |
|
Na+- K+ ATPase (μmol Pi/mg/ hr) |
25.6 ± 1.72 (16.4) |
14.5 ± 1.36* (15.8) |
21.4 ± 1.51† (16) |
|
K+-pNPPase (μmol phenolate/mg/hr) |
6.5 ± 0.23 (15.6) |
4.1 ± 0.17* (16) |
4.8 ± 0.24† (15.8) |
|
Cytochrome C oxidase (nmol cytochrome C/mg/min) |
46.2 ± 3.5 (0.23) |
51.0 ± 2.9 (0.21) |
48.7 ± 3.1 (0.26) |
|
NADPH cytochrome C reductase (nmol cytochrome C/mg/min) |
3.7 ± 0.2 (0.34) |
4.0 ± 0.3 (0.31) |
4.0 ± 0.2 (0.31) |
Values are means ± SE of 4 experiments. Figures in the paranthesis represent the ratio of the enzyme activity in the sarcolemmal membrane and that in the respective heart homogenate .
*-P<0.05 vs control; †- P<0.05 vs diabetic .
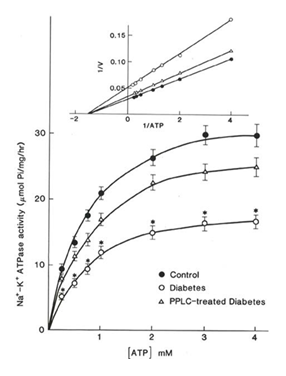
in the vesicular preparation) and digitoxigenin (which is freely permeable in the membrane) were also tested in this study. From the data in Table 4, it is evident that ouabain inhibited the Na+-K+ ATPase activities by 13 to 15% whereas depressed Na+-K+ ATPase activities in diabetic preparations, when determined in the presence of various concentrations of ATP, were observed to be attenuated in PPLC-treated preparations (Figure 1). The double reciprocal plots of the data digitoxigenin depressed the enzyme activities by 93 to 95% revealed that maximal velocities (V) of Na+-K+ ATPase in in control, diabetic and PPLC- treated diabetic preparations. These results show that the SL preparations for the control, diabetic and PPLC- treated hearts contained predominantly equal proportion of inside out vesicles. Furthermore, the control, diabetic and PPLC- treated SL preparations were 33.61, 18.46 and 27.64 (μmol Pi/mg protein/ hr) without any changes in the Km values (0.67 to 0.68 μM ATP), respectively (Figure 1).
Table 4: Na+-K+ ATPase activity at different pH as well as orientation of sarcolemmal preparations obtained from diabetic rats with or without propionyl L-carnitine (PPLC) treatment.
|
Control |
Diabetic |
PPLC-treated diabetic |
|
|
A. Na+-K+ ATPase activity (μmol Pi/mg/hr) at different pH: |
|||
|
pH 6.4 |
10.4 ± 0.84 |
0.2 ± 0.54* |
8.6 ± 0.44† |
|
pH 7.0 |
27.2 ± 1.46 |
14.9 ± 1.87* |
20.4 ± 2.05† |
|
pH 8.0 |
20.7 ± 1.23 |
11.8 ± 1.21* |
16.7 ± 0.84† |
|
B. Ouabain and digitoxigenin sensitive Na+-K+ ATPase activity (μmol Pi/mg/hr): |
|||
|
Untreated |
25.9 ± 1.47 |
14.2 ± 1.32* |
19.8 ± 0.74† |
|
Ouabain- sensitive |
3.4 ± 0.20 (13%) |
2.1 ± 0.16* (14%) |
2.9 ± 0.14† (15%) |
|
Digitoxigenin- sensitive |
24.6 ± 1.34 (95%) |
13.3 ± 1.21* (93%) |
18.6 ± 0.55† (94%) |
Values are means ± SE of 3 experiments. The concentrations of ouabain and digitoxigenin were 1mM and 10 μM, respectively. Figures in
parenthesis represent sensitivity of Na+-K+ ATPase to ouabain or digitoxigenin with respective untreated preparations. *- P<0.05 versus control; †- P<0.05 vs diabetic.
Figure 1: Na+- K+ ATPase activity as a function of ATP concentration [ATP] in sarcolemmal vesicles from control (closed circles), diabetic (open circles), and propionyl L- carnitine (PPLC) treated diabetic (open triangles) rat hearts. Time of incubation for Na+- K+ ATPase activity was 10 min. Each value is a mean SE of 6 experiments. *- P<0.05. Inset: The Lineweaver- Burke plot of data (inset) shows Vmax values for control, diabetic and PPL- treated diabetic preparations were 33.61, 18.46 and 27.64 (μmol Pi/mg/hr), whereas Km values were 0.6806, 0.6675 and 0.6720 (μMATP), respectively.
Table 5: Heart sarcolemmal Ca2+- pump activities in diabetic rats with or without propionyl L-carnitine (PPLC) treatment.
|
Control |
Diabetic |
PPLC- treated Diabetic |
|
|
Nonspecific Ca2+ binding (nmol/mg/5 min) |
4.3 ± 0.33 |
4.2 ± 0.54 |
4.3 ± 0.45 |
|
ATP dependent Ca2+uptake (nmol/mg/5 min) |
26.8 ± 1.24 |
10.2 ± 0.52* |
11.6 ± 0.47 |
|
Mg2+- ATPase (μmol Pi/mg /5 min) |
15.4 ± 0.75 |
13.9 ± 0.66 |
14.1 ± 0.71 |
|
Ca2+- stimulated ATPase (μmol Pi/mg/5 min) |
1.4 ± 0.04 |
0.52 ± 0.03* |
0.61 ± 0.05 |
Values are means ± SE of 5 experiments. The concentration of Ca2+ was 10 μM. *- P<0.05 vs control.
Table 6: ATP- dependent Ca2+ uptake in heart sarcolemma obtained from diabetic rats with or without propionyl L-carnitine (PPLC) treatment.
|
Concentration of Ca2+ |
Ca2+- uptake (nmol/mg/min) |
||
|
Control |
Diabetic |
PPLC- treated diabetic |
|
|
0.1 μM |
6.0 ± 0.22 |
2.6 ± 0.12* |
2.9 ± 0.21 |
|
0.5 μM |
13.6 ± 0.54 |
5.9 ± 0.31* |
6.3 ± 0.44 |
|
1.0 μM |
18.7 ± 0.82 |
9.1 ± 0.67* |
11.2 ± 0.57 |
|
5.0 μM |
21.6 ± 0.94 |
10.2 ± 0.55* |
12.0 ± 0.59 |
|
10.0 μM |
23.2 ± 0.75 |
10.8 ± 0.71* |
12.5 ± 0.61 |
Values are means ± SE of 6 experiments. *P<0.05 vs control.
In another set of experiments, SL Ca2+-pump activities in control, diabetic and PPLC- treated diabetic preparations were determined and that data are shown in Table 5. ATP- dependent Ca2+- uptake and Ca2+-stimulated ATPase activities, unlike nonspecific Ca2+-binding or Mg2+-ATPase activities, in diabetic SL preparations were significantly depressed. However, these changes were not altered in PPLC- treated hearts. It is pointed out that the diabetes- induced depressions in ATP- dependent Ca2+-uptake and Ca2+- stimulated ATPase activities are similar to those reported earlier [34]. The depressed Ca2+- uptake activities in the diabetic preparations, when determined at different concentrations of Ca2+ in the incubation media, were also not affected upon the treatment with PPLC (Table 6). It should be noted that the magnitude of the inhibitory effects on SL Ca2+-stimulated ATPase activity at different low concentrations of vanadate was similar in control, diabetic and PPLC- treated diabetic preparations (Table 7).
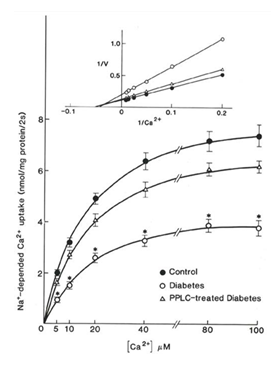
The Na+-dependent Ca2+-uptake in the control, diabetic and PPLC- treated diabetic sarcolemmal preparations was studied at different times of incubation as well as in the presence of different concentrations of Ca2+ and the results are shown in Table 8 and Figure 2, respectively. The Na+- dependent Ca2+-uptake in diabetic preparations was not only depressed in the diabetic preparations but this change was also attenuated upon treatment with PPLC. The values for Na+- dependent uptake in control and diabetic SL preparations are similar to those reported by Makino et al [34]. Treatment of diabetic animals with PPLC also showed beneficial effects on Na+- dependent Ca2+- uptake when measured at different incubation times (Table 8) or at different concentrations of Ca2+ (Figure 2). The double reciprocal plot of data revealed that the maximal Ca2+-accumulation (B ) values were 9.6, 5.09 and 7.65 nmol Ca2+/mg/ 2 sec) without much alterations in Kα values in control, diabetic and PPLC- treated diabetic preparations, respectively. In another experiment, Na+- dependent Ca2+- efflux was determined in Ca2+-loaded SL vesicle upon initiating Ca2+- efflux by the addition of 80 mM Na2+. The time-course of changes in SL vesicle Ca2+-content in control, diabetic and PPLC- treated diabetic preparations are shown in Table 9. The depression in Ca2+- efflux in diabetic preparations at initial times of incubation was not affected by PPLC treatment.
In order to gain some information regarding mechanisms of the observed alterations, some biomarkers for oxidative stress were also monitored in control, diabetic and PPLC- treated diabetic hearts. In this regard, it should be noted that oxidative stress has been reported to play a critical role in the development of metabolic defects, subcellular abnormalities and cardiac dysfunction in diabetic cardiomyopathy [13]. The results in Table 10 show that myocardial content for conjugated dienes, malondialdehyde and oxidized glutathione were increased whereas that for reduced glutathione as well as glutathione peroxidase and superoxide dismutase were decreased without any changes in catalase activity in the diabetic hearts. These alterations were partially prevented by treatment with PPLC.
Figure 2: Na+- dependent Ca2+ uptake as a function of Ca2+ concentration in sarcolemmal vesicles from control (closed circles), diabetic (open circles), and propionyl L- carnitine (PPLC) treated diabetic (open triangles) rat hearts. Na+- dependent Ca2+ uptake was measured at 2 sec at all points. Each values is a the mean SE of 6 experiments. *-P<0.05. Inset: The Lineweaver- Burke plot of data (inset) shows Bmax values for control, diabetic and PPL- treated diabetic preparations were 9.06, 5.09 and 7.65 (nmol Ca2+/ mg/ 2 sec) whereas K values were 17.72, 23.18 and 18.78 (μMCa2+), respectively.
Table 7: Influence of low concentrations of vanadate on the heart sarcolemmal Ca2+- stimulated ATPase activity in diabetic rats with or without propionyl L-carnitine (PPLC) treatment.
|
Inhibition of the Ca2+- stimulated ATPase activity (%) |
|||
|
Concentration of vanadate |
Control |
Diabetic |
PPLC- treated Diabetic |
|
0.5 μM |
23.9 ± 0.7 |
25.0 ± 0.9 |
21.8 ± 0.7 |
|
1.0 μM |
40.1 ± 1.6 |
42.7 ± 1.4 |
43.0 ± 1.8 |
|
2.0 μM |
62.5 ± 3.4 |
60.6 ± 2.8 |
65.0 ± 3.3 |
|
4.0 μM |
86.9 ± 4.1 |
84.0 ± 3.6 |
80.5 ± 4.0 |
Values are mean ± SE of 4 experiments.
Table 8: Na+- dependent Ca2+ accumulation in heart sarcolemma obtained from diabetic rats with or without propionyl L-carnitine (PPLC)
treatment.
|
Time of incubation |
Ca2+- accumulation (nmol/mg) |
||
|
Control |
Diabetic |
PPLC- treated Diabetic |
|
|
5 sec |
10.0 ± 0.3 |
5.8 ± 0.4* |
7.6 ± 0.3† |
|
10 sec |
16.8 ± 0.5 |
9.5 ± 0.7* |
12.8 ± 0.4† |
|
30 sec |
27.0 ± 0.8 |
13.2 ± 0.8* |
21.9 ± 0.7† |
|
60 sec |
38.2 ± 1.9 |
20.4 ± 1.4* |
29.5 ± 1.0† |
|
120 sec |
41.6 ± 2.3 |
20.8 ± 1.3* |
31.2 ± 1.2† |
Values are mean ± SE of 6 experiments. The concentration of Ca2+ was 40 μM. *- P<0.05 vs control; †- P<0.05 vs diabetic
Table 9: Na+- dependent Ca2+ efflux in heart sarcolemmal vesicles obtained from diabetic rats with or without propionyl L-carnitine (PPLP)
treatment.
|
Time of incubation |
Ca2+ content (nmol/mg) |
||
|
Control |
Diabetic |
PPLC- treated diabetic |
|
|
0 sec |
22.6 ± 0.6 |
12.3 ± 0.8* |
12.7 ± 0.7 |
|
2 sec |
11.0 ± 0.4 |
7.1 ± 0.2* |
6.8 ± 0.2 |
|
5 sec |
5.9 ± 0.2 |
4.1 ± 0.3* |
4.2 ± 0.3 |
|
10 sec |
3.1 ± 0.3 |
3.0 ± 0.2 |
3.4 ± 0.1 |
|
20 sec |
1.5 ± 0.2 |
1.4 ± 0.2 |
1.7 ± 0.2 |
Values are mean ± SE of 4 experiments. Vesicles were loaded with Ca2+ by ATP- dependent mechanism by using 10 μM Ca2+ and the efflux was induced by 80 mM Na+. *-P<0.05 vs control.
Discussion
In this study, we have observed that sarcolemmal Na+-K+ ATPase (determined in the presence of various concentrations of ATP) and Na+- dependent Ca2+-uptake (determined in the presence of various concentrations of Ca2+) were not only depressed in the diabetic hearts but these alterations were also attenuated upon treatment of diabetic animals with PPLC (100 mg/kg; daily). These observations confirm the beneficial effects of PPLC (250 mg/ kg; daily) treatment on SL Na+- K+ ATPase (determined in the presence of one concentration of ATP) and Na+- dependent Ca2+-uptake (determined in the presence of one concentration of Ca2+) activities reported earlier [38,39] . It should also be pointed out that the beneficial effects of PPLC on Na+-K+ ATPase and Na+- dependent Ca2+ uptake activities in the present study were observed at different times of incubation of the membrane preparation whereas that on Na+-K+ ATPase were also seen at different pH values of the incubation medium. Furthermore, these changes in diabetic and PPLC- treated diabetic Na+-K+ ATPase and Na+-dependent Ca2+- uptake activities in the SL preparations were not confounded by cross contamination as the presence of cytochrome C oxidase (mitochondria biomarker) and NADPH cytochrome C reductase (sarcoplasmic reticulum biomarker) was minimal. In addition, K+-pNPPase (SL biomarker) showed high but equal purification of the membrane preparations in control, diabetic and PPLC- treated hearts. The proportion of inside- out orientation of vesicles in the SL preparations was also similar in control and experimental groups. The observed Na+- dependent Ca2+- uptake activities in diabetic and PPLC- treated diabetic preparations cannot also be explained on the basis of membrane- leakness as values for the Na+- induced Ca2+- efflux at initial stages of incubation were similar in both groups.
Experiments carried out for studying the status of SL Ca2+- pump activity have revealed that both ATP-dependent Ca2+- uptake and Ca2+-stimulated ATPase activities were depressed in the diabetic heart but these changes were not improved in
Table 10: Some biomarkers of oxidative stress in hearts from diabetic rats with or without propionyl L- carnitine (PPLC) treatment
|
Control |
Diabetic |
PPLC- treated diabetic |
|
|
Conjugated dienes (nmol/mg tissue lipids) |
42.5 ± 3.6 |
65.6 ± 7.2* |
51.4 ± 3.6† |
|
Malondialdehyde (nmol/mg tissue lipids) |
5.1 ± 0.2 |
7.4 ± 0.3* |
6.2 ± 0.3† |
|
Oxidized glutathione (ng/mg protein) |
66.4 ± 4.9 |
131.6 ± 9.2* |
103 ± 8.6† |
|
Reduced glutathione (ng/mg protein) |
236 ± 8.9 |
156 ± 7.5* |
188 ± 6.8† |
|
Glutathione peroxidase (nmol/mg protein/ min) |
76 ± 4.5 |
58 ± 4.1* |
69 ± 3.6† |
|
Superoxide dismutase (Units mg protein) |
9.6 ± 0.8 |
4.2 ± 0.5* |
7.2 ± 0.9† |
|
Catalase (Units/mg protein) |
25.5 ± 1.4 |
24.9 ± 1.7 |
24.2 ± 1.5 |
Values are mean ± S.E. of 4 experiments. *-P<0.05 vs control;
†- P<0.05 vs diabetic.
the PPLC- treated diabetic preparations. These alterations in Ca2+-pump activities may be of specific nature as neither non-specific Ca2+- binding nor Mg2+- ATPase activities were altered in control, diabetic and PPLC-treated diabetic groups. Furthermore, SL ATP-dependent Ca2+- uptake was depressed in diabetic preparations and unaltered in PPLC- treated diabetic heart. Such observations cannot be explained on the basis of alterations in membrane permeability as no difference was observed in non-specific Ca2+-binding in SL preparations from any group. Since the magnitude of inhibitory effect of low concentrations of vanadate on Ca2+- stimulated ATPase in all preparations was similar, it is evident that the observations regarding depression of Ca2+- pump activity in diabetic heart and ineffectiveness of PPLC treatment to improve this diabetes-induced change were entirely related to SL membranes. However, the exact reason for the ineffectiveness of PPLC- treatment of diabetic animals in improving Ca2+- pump activities cannot be described at this time except to indicate that higher doses of PPLC were required for the beneficial effect.
Not only the development of diabetic cardiomyopathy was associated with depressions in SL Na+-K+ ATPase, Na+- Ca2+ exchange, and ATP- dependent Ca2+-pump activities as well as impaired cardiac function, prolonged treatment of diabetic animals with PPLC has been shown to attenuate SL alterations in Na+-K+ ATPase, Na+-Ca2+ exchange activities as well to improve cardiac function. In view of the direct or indirect roles of SL Na+-K+ ATPase, Na+-Ca2+ exchange and Ca2+- pump activities in Ca2+-entry and subsequent level of cytoplasmic Ca2+in cardiomyocytes [3,13], it has been suggested depressions in their activities would increase the cytoplasmic level of Ca2+ in the myocardium and impair contractile function of diabetic heart (13, 21). On the other hand, attenuation of the diabetes- induced depressions in SL Na+-K+ ATPase and Na+- Ca2+ exchange activities by PPLC treatment can be seen to prevent the elevation of the cytoplasmic levels of Ca2+ and improve cardiac function. It should also be noted that the sarcoplasmic Ca2+- pump (both ATP-dependent Ca2+-uptake and Ca2+- stimulated ATPase) activities as well as ventricular function were reported to be depressed in diabetic cardiomyopathy, and were prevented upon treatment with PPLC [13, 37]. Thus, it appears that defects in the SL Ca2+- transport system contribute to Ca2+- pump abnormalities in the sarcoplasmic reticulum and raise the cytoplasmic concentration of Ca2+ in the diabetic heart for the development of cardiac dysfunction and diabetic- cardiomyopathy. This view is consistent with the observations concerning the involvement of SL Ca2+- transport defects in impairing cardiac function due to myocardial infarction as well as the beneficial effects of therapy with PPLC in this experimental model [30, 31]. In this regard, it should also be noted that Ca2+- content have been reported to be increased in the diabetic heart [47-49] and the intracellular Ca2+-overload is known to produce marked alterations in cardiac structure, metabolism and function [50, 51].
It was interesting to observe that the development of cardiac dysfunction was associated with increased levels of plasma free fatty acids and triglycerides in diabetic cardiomyopathy, which changes were partially or fully prevented by metabolic therapy with PPLC. Since myocardial metabolism is markedly impaired in diabetes, and PPLC has been shown to promote the transport and oxidation of free fatty acids in mitochondria as well as increase the energy status of the diabetic heart [37, 38], it is likely that the observed alterations in ventricular function and SL Ca2+- transport systems in diabetic cardiomyopathy with or without PPLC therapy are the consequence of corresponding changes in lipid metabolism and energy status of the myocardium. Particularly, prolonged elevation of the cytoplasmic Ca2+ due to excessive entry of Ca2+ through SL membrane as well as Ca2+- handling defect in the sarcoplasmic reticulum can be seen to result in mitochondrial Ca2+- overload, impaired energy production and cardiac dysfunction during the development of diabetic cardiomyopathy. Since several biomarkers for the development and reduction of oxidative stress in the diabetic heart were observed to be associated with corresponding alterations in cardiac function and SL Ca2+- transport system with and without PPLC therapy, it is apparent that the status of oxidative stress may play a critical role in determining the deleterious effects of chronic diabetes as well as the beneficial effects of PPLC therapy. It is noteworthy that several investigators have demonstrated the involvement of oxidative stress in the development of diabetic cardiomyopathy [13, 52-54].
Conclusion
This study has provided evidence that SL Na+-K+ ATPase, Na+-Ca2+ exchange and ATP- dependent Ca2+- pump activities are depressed during the development of diabetic cardiomyopathy. Furthermore, alterations in the Na+-K+ ATPase and Na+-Ca2+ exchange activities in the diabetic heart were attenuated by metabolic therapy with PPLC. Since cardiac function was impaired and plasma levels of free fatty acids as well as triglycerides were elevated in diabetic cardiomyopathy and these alterations were attenuated by treatment with PPLC, it appears that the observed changes in cardiac function and lipid metabolism are intimately associated with alterations in the SL Ca2+- transport system. Such a relationship may involve the contribution of SL defects for the elevation of cytoplasmic level of Ca2+, occurrence of mitochondrial Ca2+- overload and impairment of energy production. In view of the observations that several biomarkers of oxidative stress show increase in diabetic heart and decrease upon PPLC therapy, it is suggested that oxidative stress may play an important role in determining the status of cardiac function and SL Ca2+- transport systems.
Acknowledgements
The authors wish to thank Dr. Tomoji Hata and Mr. Donald Chapman for their assistance in some of the experiments described in this study. We are also grateful to the St. Boniface Hospital Albrechtsen Research Centre for providing the infrastructure support for this project. Thanks are also due to Fondazione Anna Maria Sechi per il Cuore (FASC), Ferrara, Italy for their support.
Transparency declaration
The lead author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Author Contributions
Conceptualization, NSD, RF; methodology and data curation, VE, NSD; data analysis and writing of original draft, KK, VE; data interpretation, VE, NSD, RF; review and editing of final draft; NSD, RF, KK.
Funding information
This project was not funded by any agency.
References
- Dhalla NS, Pierce GN, Innes IR, et al. Pathogenesis of cardiac dysfunction in diabetes mellitus. Can J Cardiol 1 (1985): 263-281.
- Ganguly PK, Pierce GN, Dhalla NS. Diabetic cardiomyopathy: membrane dysfunction and therapeutic strategies. J Appl Cardiol 2 (1987): 323–338.
- Dhalla NS, Liu X, Panagia V, et Subcellular remodeling and heart dysfunction in chronic diabetes. Cardiovasc Res 40 (1998): 239–247.
- Candido R, Srivastava P, Cooper ME, et al. Diabetes mellitus: a cardiovascular disease. Curr Opin Investig Drugs 4 (2003): 1088–1094.
- Sharma V, McNeill Diabetic cardiomyopathy: where are we 40 years later? Can J Cardiol 22 (2006): 305–308.
- Boudina S, Abel Diabetic cardiomyopathy revisited. Circulation 115 (2007): 3213–3223.
- Asghar O, Al-Sunni A, Khavandi K, et al. Diabetic cardiomyopathy. Clin Sci 116 (2009): 741–760.
- Watanabe K, Thandavarayan RA, Harima M, et al. Role of differential signaling pathways and oxidative stress in diabetic cardiomyopathy. Curr Cardiol Rev 6 (2010): 280–290.
- Roul D, Recchia FA. Metabolic alterations induce oxidative stress in diabetic and failing hearts: different pathways, same outcome. Antioxid Redox Signal 22 (2015): 1502–1514.
- Varga ZV, Giricz Z, Liaudet L, et Interplay of oxidative, nitrosative/nitrative stress, inflammation, cell death and autophagy in diabetic cardiomyopathy. Biochim Biophys Acta 1852 (2015): 232–242.
- Verma SK, Garikipati VNS, Kishore R. Mitochondrial dysfunction and its impact on diabetic heart. Biochim Biophys Acta Mol Basis Dis 1863 (2017): 1098–1105.
- Sharma A, Tate M, Mathew G, et al. Oxidative stress and NLRP3-inflammasome activity as significant drivers of diabetic cardiovascular complications: therapeutic implications. Front Physiol 9 (2018): 114.
- Dhalla NS, Shah AK, Tappia Role of oxidative stress in metabolic and subcellular abnormalities in diabetic cardiomyopathy. Int J Mol Sci 21 (2020): 2413.
- Karan A, Bhakkiyalakshmi E, Jayasuriya R, et al. The pivotal role of nuclear factor erythroid 2-related factor 2 in diabetes-induced endothelial dysfunction. Pharmacol Res 153 (2020): 104601.
- Packer Differential pathophysiological mechanisms in heart failure with a reduced or preserved ejection fraction in diabetes. JACC Heart Fail 9 (2021): 535–549.
- Rupp H, Elimban E, Dhalla NS. Modification of myosin isozymes and SR Ca2+-pump ATPase of the diabetic rat heart by lipid lowering interventions. Mol Cell Biochem 132 (1994): 69–80.
- Kato K, Chapman DC, Rupp H, et Alterations of heart function and Na+-K+-ATPase activity by etomoxir in diabetic rats. J Appl Physiol 86 (1999): 812–818.
- Wang GG, Li W, Lu XH, et al. Taurine attenuates oxidative stress and alleviates cardiac failure in type I diabetic rats. Croat Med J 54 (2013):171–179.
- De Blasio MJ, Huynh K, Qin C, et Therapeutic targeting of oxidative stress with coenzyme Q10 counteracts exaggerated diabetic cardiomyopathy in a mouse model of diabetes with diminished PI3K(p110α) signaling. Free Radic Biol Med 87 (2015): 137–147.
- Zhang N, Yang Z, Xiang SZ, et al. Nobiletin attenuates cardiac dysfunction, oxidative stress, and inflammatory in streptozotocin: induced diabetic cardiomyopathy. Mol Cell Biochem 417 (2016): 87–96.
- Tappia PS, Elimban V, Shah AK, et al. Improvement of cardiac function and subcellular defects due to chronic diabetes upon treatment with sarpogrelate. J Cardiovasc Dev Dis 11 (2024): 215.
- Liedtke AJ, Nellis SH, Whitesell LF. Effects of carnitine isomers on fatty acid metabolism in ischemic swine hearts. Circ Res 48 (1981): 859–866.
- Paulson DJ, Schmidt MJ, Romens J, et al. Metabolic and physiological differences between zero-flow and low- flow myocardial ischemia: effects of L-acetylcarnitine. Basic Res Cardiol 79 (1984): 551–561.
- Hülsmann WC, Dubelaar ML, Lamers JM, et Protection by acyl-carnitines and phenylmethylsulfonyl fluoride of rat heart subjected to ischemia and reperfusion. Biochim Biophys Acta 847 (1985): 62–66.
- Siliprandi N, Di Lisa F, Pivetta A, et Transport and function of L-carnitine and L-propionylcarnitine: relevance to some cardiomyopathies and cardiac ischemia. Z Kardiol 76 (1987): 34–40.
- Yang XP, Samaja M, English E, et al. Hemodynamic and metabolic activities of propionyl-L-carnitine in rats with pressure-overload cardiac J Cardiovasc Pharmacol 20 (1992): 88–98.
- Micheletti R, Di Paola ED, Schiavone A, et Propionyl- L-carnitine limits chronic ventricular dilation after myocardial infarction in rats. Am J Physiol 264 (1993): H1111–H1117.
- Micheletti R, Giacalone G, Canepari M, et al. Propionyl- L-carnitine prevents myocardial mechanical alterations due to pressure overload in Am J Physiol 266 (1994): H2190–H2197.
- Li P, Park C, Micheletti R, et al. Myocyte performance during evolution of myocardial infarction in rats: effects of propionyl-L-carnitine. Am J Physiol 268 (1995): H1702–H1713.
- Sethi R, Dhalla KS, Ganguly PK, et Beneficial effects of propionyl L-carnitine on sarcolemmal changes in congestive heart failure due to myocardial infarction. Cardiovasc Res 42 (1998): 607–615.
- Sethi R, Wang X, Ferrari R, et al. Improvement of cardiac function and beta-adrenergic signal transduction by propionyl L-carnitine in congestive heart failure due to myocardial infarction. Coron Artery Dis 15 (2004): 65–71.
- Mathew S, Menon PV, Kuruo Effect of administration of carnitine on the severity of myocardial infarction induced by isoproterenol in rats. Aust J Exp Biol Med Sci 64 (1986): 79–87.
- Tahiliani AG, McNeill Effects of triiodothyronine and carnitine therapy on myocardial dysfunction in diabetic rats. Can J Physiol Pharmacol 64 (1986): 669–672.
- Makino N, Dhalla KS, Elimban V, Dhalla NS. Sarcolemmal Ca2+ transport in streptozotocin-induced diabetic cardiomyopathy in Am J Physiol 253 (1987): E202–E207.
- Whitmer JT. L-carnitine treatment improves cardiac performance and restores high-energy phosphate pools in cardiomyopathic Syrian hamster. Circ Res 61 (1987): 396–408.
- Pasini E, Comini L, Ferrari R, et al. Effect of propionyl- L-carnitine on experimental induced cardiomyopathy in rats. Am J Cardiovasc Pathol 4 (1992): 216–222.
- Ferrari R, Shah KR, Hata T, et al. Subcellular defects in diabetic myocardium: Influence of propionyl L-carnitine on Ca2+-transport. In: The Diabetic Heart; Eds: M. Nagano, N.S. Dhalla, Raven Press Ltd. New York, (1991): 167–181.
- Dhalla NS, Dixon IMC, Shah KR, et Beneficial effects of L-carnitine and derivatives on heart membranes in experimental diabetes. In: L-Carnitine and its Role in Medicine: From Function to Therapy; Eds: R. Ferrari, S. Di Mauro, G. Sherwood, Academic Press, London, UK, (1992): 411–426.
- Pierce GN, Ramjiawan B, Dhalla NS, et al. Na+-H+ exchange in cardiac sarcolemmal vesicles isolated from diabetic rats. Am J Physiol 258 (1990): H255–H261.
- Ou C, Majumder S, Dai J, et al. Cardiac phosphatidylethanolamine N-methylation in normal and diabetic rats treated with L-propionyl carnitine. In: Subcellular Basis of Contractile Ed: B. Kosecky, N.S. Dhalla. Kluwer Academic Publishers, Boston (1990): 219–234.
- Ganguly PK, Beamish RE, Dhalla KS, et al. Norepinephrine storage, distribution, and release in diabetic cardiomyopathy. Am J Physiol 252 (1987): E734–E739.
- Liu X, Suzuki H, Sethi R, et Blockade of the renin-angiotensin system attenuates sarcolemma and sarcoplasmic reticulum remodeling in chronic diabetes. Ann N Y Acad Sci 1084 (2006): 141–154.
- Pitts BJ. Stoichiometry of sodium-calcium exchange in cardiac sarcolemmal vesicles. Coupling to the sodium pump. J Biol Chem 254 (1979): 6232–6235.
- Lowry OH, Rosebrough AL, Farr AL, Randall Protein measurement with the Folin phenol reagent. J Biol Chem 193 (1951): 265–275.
- Tausky H, Shorr E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem 202 (1953): 675–685.
- Ganguly PK, Pierce GN, Dhalla KS, et al. Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. Am J Physiol 244 (1983): E528–E535.
- Regan TJ, Wu CF, Yeh CK, et Myocardial composition and function in diabetes. The effects of chronic insulin use. Circ Res 49 (1981): 1268–1277.
- Nagase N, Tamura Y, Kobayashi S, et al. Myocardial disorders of hereditary diabetic KK mice. J Mol Cell Cardiol 13 (1981):70.
- Pettit GW, Vick RL. Contribution of pancreatic insulin to extrarenal potassium homeostasis: a two-compartment model. Am J Physiol 226 (1974): 319–324.
- Dhalla NS, Das PK, Sharma GP. Subcellular basis of cardiac contractile failure. J Mol Cell Cardiol 10 (1978): 363–385.
- Dhalla NS, Pierce GN, Panagia V, et al. Calcium movements in relation to heart function. Basic Res Cardiol 77 (1982): 117–139.
- Ansley DM, Wang B. Oxidative stress and myocardial injury in the diabetic J Pathol 229 (2013): 232–241.
- Wilson AJ, Gill EK, Abudalo RA, et Reactive oxygen species signalling in the diabetic heart: emerging prospect for therapeutic targeting. Heart 104 (2018): 293–299.
- Pickering RJ, Rosado CJ, Sharma A, et al. Recent novel approaches to limit oxidative stress and inflammation in diabetic Clin Trans Immunology 7 (2018): e1016.
- Wu X, Huang L, Liu J. Relationship between oxidative stress and nuclear factor-erythroid-2-related factor 2 signaling in diabetic cardiomyopathy (Review). Exp Ther Med 22 (2021): 678.


 Impact Factor: * 6.124
Impact Factor: * 6.124 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 