Outcomes of Sacubitril–Valsartan Versus ACEIs/ARBs Post-PCI in HFrEF Following AMI: Evidence from a Bangladeshi Perspective
SM Ear-E-Mahabub*,1, Fakhrul Islam Khaled1, Immam Hossin2, Sayeed Ahmed3, Abdus Salam3, Mukhlesur Rrahman4, Nasir Uddin Patwary5, Zillur Rahman6, Rasel Ahmad7
1Associate Professor, Department of Cardiology, Bangladesh Medical University (BMU), Dhaka, Bangladesh
2PhD Fellow (Medical Education), Bangladesh Medical University (BMU), Dhaka, Bangladesh
3Assistant Professor, Department of Cardiology, Bangladesh Medical University (BMU), Dhaka, Bangladesh
4Professor, Department of Cardiology, Bangladesh Medical University (BMU), Dhaka, Bangladesh
5Research Assistant, Department of Cardiology, Bangladesh Medical University (BMU), Dhaka, Bangladesh
6Research Assistant, Professor, Department of Cardiology, Bangladesh Medical University (BMU), Dhaka, Bangladesh
7Assistant Professor, Medical Education, Department of Public Health and Informatics, Bangladesh Medical University (BMU), Dhaka, Bangladesh
*Corresponding author: SM Ear-E-Mahabub, Associate Professor, Department of Cardiology, Bangladesh Medical University (BMU), Dhaka, Bangladesh.
Received: 01 June 2025; Accepted: 04 June 2025; Published: 17 June 2025
Article Information
Citation: SM Ear-E-Mahabub, Fakhrul Islam Khaled, Immam Hossin, Sayeed Ahmed, Abdus Salam, Mukhlesur Rrahman, Nasir Uddin Patwary, Zillur Rahman, Rasel Ahmad. Outcomes of Sacubitril–Valsartan Versus ACEIs/ARBs Post- PCI in HFrEF Following AMI: Evidence from a Bangladeshi Perspective. Fortune Journal of Health Sciences 8 (2025): 533-538.
View / Download Pdf Share at FacebookAbstract
Background: After an acute myocardial infarction (AMI), heart failure with reduced ejection fraction (HFrEF) is still a leading cause of morbidity and death, especially in environments with limited resources. This study compared post-PCI patients with AMI and HFrEF in a Bangladeshi population for clinical efficacy between Sacubitril-Valsartan and ACEI/ARB therapy.
Methods: From July 2023 to June 2024, 80 AMI patients with LVEF <40% who had successful percutaneous coronary intervention (PCI) were enrolled in this prospective, comparative study at Bangabandhu Sheikh Mujib Medical University (BSMMU). Participants were split equally between two groups: ACEI/ARB (n = 40) and Sacubitril-Valsartan (n = 40). The baseline characteristics were similar. Patients were monitored for one and six months to evaluate changes in LVEF, NT-proBNP levels, cardiovascular mortality, heart failure-related hospitalisations, and treatment expenses.
Results: The Sacubitril-Valsartan group demonstrated a more marked decrease in NT-proBNP levels (from 3550 ± 1150 to 1250 ± 580 pg/mL vs 3400 ± 1100 to 2150 ± 890 pg/mL, p = 0.001) and a significantly larger improvement in LVEF at the 6-month follow-up (mean change 8.6% ± 3.3 vs 4.4% ± 2.7, p = 0.001). The Sacubitril-Valsartan group had significantly lower rates of heart failure-related hospitalisations (7.5% vs. 20%, p = 0.03) and cardiovascular mortality (5% vs. 15%, p = 0.04). The cost of treatment, however, was higher (USD 1550 ± 210 vs 850 ± 160, p = 0.04).
Conclusion: Despite higher treatment costs, sacubitril-valsartan significantly improved outcomes in post-PCI AMI patients with HFrEF by outperforming ACEI/ARB therapy.
Keywords
<p>Acute myocardial infarction, Sacubitril-Valsartan, ACEI, ARB, PCI, HFrEF, cardiovascular outcomes</p>
Article Details
Introduction
Acute myocardial infarction (AMI) remains one of the leading causes of morbidity and mortality worldwide, with a substantial burden on healthcare systems, particularly in low- and middle-income countries like Bangladesh [1]. Despite advances in early reperfusion strategies, including percutaneous coronary intervention (PCI), a significant proportion of patients develop heart failure with reduced ejection fraction (HFrEF) as a complication of AMI [2]. HFrEF after AMI is associated with poor long-term prognosis, increased hospitalizations, and higher mortality rates. Thus, optimizing medical therapy in this population is critical to improving clinical outcomes [3]. Current guideline-directed medical therapy for HFrEF post-AMI traditionally includes the use of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), alongside beta-blockers and mineralocorticoid receptor antagonists [4]. These agents have been proven to reduce morbidity and mortality by attenuating the maladaptive neurohormonal activation that characterizes heart failure [5]. However, despite these advances, residual risk remains high, and many patients continue to experience progressive left ventricular dysfunction and adverse clinical events [6].
Sacubitril-Valsartan, a first-in-class angiotensin receptor neprilysin inhibitor (ARNI), has emerged as a superior alternative to ACEI/ARB therapy in chronic HFrEF [7]. The PARADIGM-HF trial demonstrated that Sacubitril-Valsartan significantly reduced cardiovascular mortality and heart failure hospitalizations compared to enalapril in stable chronic HFrEF patients [8]. This breakthrough has led to updated heart failure guidelines recommending ARNI as a preferred therapy for patients with symptomatic HFrEF [9]. Nevertheless, evidence regarding its effectiveness specifically in patients immediately post-AMI who have undergone PCI remains limited, especially in resource-constrained settings. Following PCI, patients with AMI and HFrEF represent a distinct and high-risk group that may particularly benefit from optimized neurohormonal blockade. Early initiation of Sacubitril-Valsartan in this setting could potentially enhance left ventricular remodeling, reduce biomarker evidence of heart failure severity such as NT-proBNP, and improve clinical outcomes beyond those achievable with conventional ACEI/ARB therapy [10]. However, the safety, efficacy, and cost implications of Sacubitril-Valsartan versus ACEI/ARB in this acute post-PCI context have not been extensively studied, especially in the South Asian population, where genetic, socioeconomic, and healthcare factors may influence treatment response and accessibility [11]. Bangladesh, with a rising prevalence of ischemic heart disease and limited healthcare resources, presents a critical need for locally relevant data to guide clinical decisions. This study aims to compare the effectiveness and safety of Sacubitril-Valsartan versus ACEI/ARB in patients with AMI complicated by HFrEF after successful PCI. By assessing changes in left ventricular ejection fraction (LVEF), NT-proBNP levels, cardiovascular mortality, heart failure hospitalizations, adverse events, and cost-effectiveness over a six-month follow-up period, this prospective study will provide valuable evidence to inform optimal management strategies in this vulnerable population.
Methodology & Materials
This prospective, comparative study was conducted over 12 months from July 2023 to June 2024 at the Department of Cardiology, Bangabandhu Sheikh Mujib Medical University (BSMMU), a tertiary care center in Bangladesh. The study enrolled 80 patients who experienced acute myocardial infarction (AMI) and underwent successful percutaneous coronary intervention (PCI). Patients were allocated into two equal groups: one receiving sacubitril-valsartan (n=40) and the other receiving conventional ACEI/ARB therapy (n=40). Inclusion criteria included age between 18 and 80 years, confirmed AMI diagnosis based on clinical presentation, electrocardiographic findings, and cardiac biomarkers, left ventricular ejection fraction (LVEF) less than 40% as measured by echocardiography post-PCI, clinical stability, and provision of informed consent. Patients with contraindications to the study medications, major comorbidities, or inability to provide consent were excluded.
Sacubitril-valsartan was initiated at 24/26 mg twice daily and titrated up to a maximum of 97/103 mg twice daily based on patient tolerance. The ACEI/ARB group received either enalapril (2.5–20 mg daily) or losartan (25–100 mg daily) as determined by the treating physician. Patients were followed up at 1 month and 6 months post-PCI to assess clinical and biochemical outcomes. Primary endpoints included changes in LVEF, reduction in NT-proBNP levels, cardiovascular mortality, and heart failure-related hospitalizations. Secondary outcomes comprised adverse events such as renal dysfunction, hyperkalemia, hypotension, and cost-effectiveness analysis. Data collection involved standardized case report forms, echocardiographic evaluations, biomarker measurements, and monitoring for adverse events. Statistical analyses were performed using SPSS version 25. Continuous variables were analyzed using independent t-tests, categorical variables with chi-square tests, and survival analysis was conducted using Kaplan-Meier curves with log-rank tests. A p-value <0.05 was considered statistically significant. The study was approved by the Institutional Review Board of BSMMU, and all participants provided written informed consent.
Results
Table 1: Baseline Characteristics of the Study Population
|
Characteristics |
Group A (Sacubitril-Valsartan, n=40) |
Group B (ACEI/ARB, n=40) |
p-value |
|
Age (Mean ± SD) |
58.0 ± 9.5 |
59.2 ± 10.1 |
0.56 |
|
Sex (Male/Female) |
28/12 |
27 / 13 |
0.8 |
|
Hypertension (%) |
82% |
85% |
0.73 |
|
Diabetes Mellitus (%) |
40% |
42% |
0.84 |
|
Smoking History (%) |
52% |
50% |
0.83 |
|
Baseline LVEF (%) |
32.5 ± 5.5 |
33.0 ± 6.2 |
0.67 |
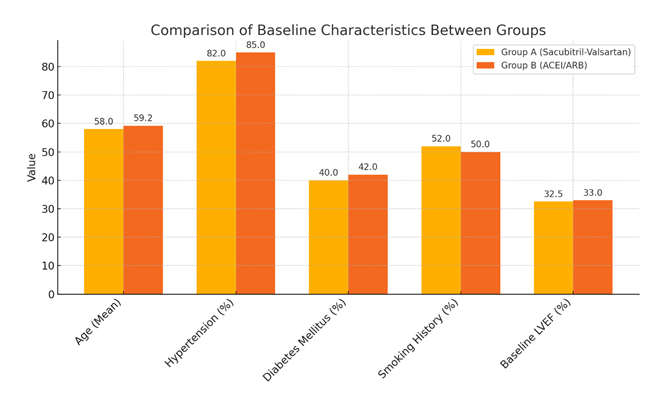
Table 1 outlines the baseline characteristics of patients in both treatment arms. The mean age was 58.0 ± 9.5 years in the Sacubitril-Valsartan group and 59.2 ± 10.1 years in the ACEI/ARB group (p=0.56). The gender distribution was similar (28 males and 12 females in Group A vs. 27 males and 13 females in Group B; p=0.80). Comorbidities such as hypertension (82% vs. 85%, p=0.73), diabetes mellitus (40% vs. 42%, p=0.84), and smoking history (52% vs. 50%, p=0.83) showed no significant difference between groups. Baseline LVEF was also comparable, with a mean of 32.5 ± 5.5% in the Sacubitril-Valsartan group and 33.0 ± 6.2% in the ACEI/ARB group (p=0.67), confirming that the study groups were well matched at baseline.
The study compared baseline characteristics between the Sacubitril-Valsartan group (Group A) and the ACEI/ARB group (Group B), each consisting of 40 patients. Both groups had shown similar values for age, hypertension, diabetes, smoking history, and baseline LVEF. No statistically significant differences had been observed (all p > 0.05), indicating that the two groups had been well matched at baseline.
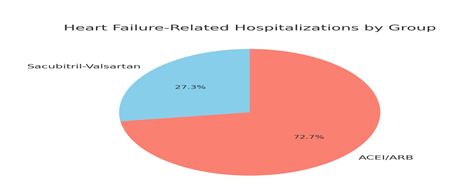
Figure 2: The distribution of heart failure-related hospitalizations between the two treatment groups was presented. Out of a total of 11 hospitalizations, 3 occurred in the Sacubitril-Valsartan group (27.3%) and 8 in the ACEI/ARB group (72.7%). The chart visually emphasized that the ACEI/ARB group had a higher proportion of hospitalizations compared to the Sacubitril-Valsartan group. This difference was statistically significant, with a p-value of 0.03, indicating that patients treated with Sacubitril-Valsartan experienced fewer heart failure-related hospitalizations during the study period.
Table 2: Change in LVEF at 6 Months
|
Group |
Baseline LVEF (%) |
6-Month LVEF (%) |
LVEF Change (Mean ± SD) |
p-value |
|
Sacubitril-Valsartan |
32.5 ± 5.5 |
41.1 ± 6.2 |
8.6 ± 3.3 |
0.001 |
|
ACEI/ARB |
33.0 ± 6.2 |
37.4 ± 5.8 |
4.4 ± 2.7 |
Table 2 presents the changes in LVEF over a 6-month follow-up period. Patients in the Sacubitril-Valsartan group demonstrated a significantly greater improvement in LVEF, with an increase of 8.6 ± 3.3%, compared to 4.4 ± 2.7% in the ACEI/ARB group (p=0.001).
Table 3: Change in NT-proBNP Levels from Baseline to 6 Months
|
Group |
Baseline NT-proBNP (pg/mL) |
6-Month NT-proBNP (pg/mL) |
p-value |
|
Sacubitril-Valsartan |
3550 ± 1150 |
1250 ± 580 |
0.001 |
|
ACEI/ARB |
3400 ± 1100 |
2150 ± 890 |
Table 3 shows the reduction in NT-proBNP levels over 6 months of follow-up. The Sacubitril-Valsartan group had a significantly greater reduction in NT-proBNP (from 3550 ± 1150 to 1250 ± 580 pg/mL) compared to the ACEI/ARB group (from 3400 ± 1100 to 2150 ± 890 pg/mL), with a statistically significant difference (p=0.001).
Table 4: Cardiovascular Mortality over 6 Months
|
Group |
Deaths (n) |
Mortality Rate (%) |
p-value |
|
Sacubitril-Valsartan |
2 |
5% |
0.04 |
|
ACEI/ARB |
6 |
15% |
Table 4 highlights cardiovascular mortality within 6 months post-PCI. The Sacubitril-Valsartan group experienced significantly fewer deaths (2 patients, 5%) compared to the ACEI/ARB group (6 patients, 15%). The difference in mortality was statistically significant (p=0.04), suggesting a survival benefit with Sacubitril-Valsartan therapy in this post-AMI HFrEF population.
Table 5: Heart Failure-Related Hospitalizations
|
Group |
Hospitalizations (n) |
Hospitalization Rate (%) |
p-value |
|
Sacubitril-Valsartan |
3 |
7.50% |
0.03 |
|
ACEI/ARB |
8 |
20% |
Table 5 compares heart failure-related hospitalizations within 6 months post-PCI. The Sacubitril-Valsartan group had significantly fewer hospitalizations (3 cases, 7.5%) compared to the ACEI/ARB group (8 cases, 20%), with a statistically significant difference (p=0.03).
Table 6: Cost-Effectiveness Analysis (Post-PCI)
|
Group |
Total Treatment Cost (Mean ± SD, USD) |
p-value |
|
Group A (Sacubitril-Valsartan) |
1550 ± 210 |
0.04 |
|
Group B (ACEI/ARB) |
850 ± 160 |
Table 6 presents the mean total treatment cost per patient over 6 months post-PCI. The Sacubitril-Valsartan group incurred significantly higher costs (USD 1550 ± 210) compared to the ACEI/ARB group (USD 850 ± 160), with the difference being statistically significant (p=0.04).
Discussion
This prospective, comparative study investigated the post-PCI effectiveness of sacubitril/valsartan versus conventional ACEI/ARB therapy in patients with acute myocardial infarction (AMI) and reduced ejection fraction (HFrEF). The findings suggest that sacubitril/valsartan significantly improved cardiac function, reduced NT-proBNP levels, and lowered cardiovascular mortality and heart failure-related hospitalizations compared to ACEI/ARB therapy, albeit at a higher treatment cost. Our results showed a significantly greater improvement in LVEF at 6 months in the sacubitril/valsartan group compared to the ACEI/ARB group (mean change 8.6% vs 4.4%, p = 0.001). This is consistent with prior findings by Fan et al., who reported enhanced LVEF recovery with early sacubitril/valsartan use following PCI in AMI patients [12]. Similarly, Yin et al., demonstrated the efficacy of sacubitril/valsartan in improving ventricular function and reducing mitral regurgitation post-revascularization [13]. The significant reduction in NT-proBNP in the sacubitril/valsartan group (from 3550 ± 1150 to 1250 ± 580 pg/mL, p = 0.001) is in line with the biomarker suppression effects observed by Murphy et al., reinforcing the drug’s potent neurohormonal modulation [14]. Zhao et al., also reported synergistic benefits when sacubitril/valsartan was combined with cardiac rehabilitation in post-AMI heart failure patients, particularly in terms of natriuretic peptide reduction and symptom improvement [15].
Cardiovascular mortality was notably lower in the sacubitril/valsartan group (5% vs 15%, p = 0.04), corroborating evidence from meta-analyses and RCTs. Rashid et al., concluded that early administration of sacubitril/valsartan post-MI significantly reduces all-cause and cardiovascular mortality, a finding echoed in our cohort [16]. Likewise, Zhang et al., in their meta-analysis found early sacubitril/valsartan use to be associated with lower mortality and better remodeling outcomes in patients with AMI [17]. Heart failure-related hospitalizations were also reduced in the sacubitril/valsartan group (7.5% vs 20%, p = 0.03), aligning with data from Pierce et al., who highlighted improved rehospitalization rates in patients initiated on sacubitril/valsartan post-discharge [18]. This supports its early initiation post-PCI, as emphasized by Gu et al., who reported favorable outcomes with ultra-early introduction in PCI-treated MI patients [19]. Despite clinical superiority, sacubitril/valsartan was significantly more costly (mean treatment cost USD 1550 vs 850, p = 0.04). While this presents a challenge in resource-limited settings like Bangladesh, the cost-effectiveness may be justified by reductions in hospitalizations and long-term complications. Jain et al., and Vaduganathan et al., have both underscored the favorable cost-benefit profile of sacubitril/valsartan when considering its effect on long-term outcomes [20, 21].
Our findings also mirror those of Liu et al., who reported improved cardiac remodeling and reduced adverse events in ACS patients with HFrEF on sacubitril/valsartan [22]. Although we did not observe statistically significant differences in baseline characteristics, the post-treatment improvements suggest a clear pharmacological advantage. These improvements are likely driven by the dual mechanism of neprilysin inhibition and angiotensin receptor blockade, enhancing natriuretic peptide activity while counteracting RAAS, as supported by the mechanistic insights from Solomon et al [23].
Limitations of the study
However, this study has limitations. The relatively small sample size (n=80) may reduce generalizability. Additionally, cost-effectiveness analysis was based on short-term costs and may not reflect long-term economic outcomes. Moreover, while adverse events were monitored, a detailed safety comparison was beyond this report's scope.
Conclusion
In conclusion, in patients with AMI and reduced LVEF post-PCI, sacubitril/valsartan therapy resulted in superior improvements in LVEF and NT-proBNP, and reduced mortality and heart failure hospitalizations compared to ACEI/ARB, albeit at a higher cost. These findings support the early introduction of sacubitril/valsartan in similar patient populations, especially when long-term clinical benefits and reduced healthcare utilization are considered.
Financial support and sponsorship
No funding sources.
Conflicts of interest
There are no conflicts of interest.
References
- Tan NY, Sangaralingham LR, Sangaralingham SJ, Yao X, Shah ND, Dunlay SM. Comparative effectiveness of sacubitril-valsartan versus ACE/ARB therapy in heart failure with reduced ejection fraction. JACC: Heart Failure 8 (2020): 43-54.
- Gao J, Zhang X, Xu M, Deng S, Chen X. The efficacy and safety of sacubitril/valsartan compared with ACEI/ARB in the treatment of heart failure following acute myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Frontiers in Pharmacology 14 (2023): 1237210.
- Dong Y, Xu Y, Ding C, Yu Z, Yu Z, Xia X, Chen Y, Jiang X. Comparing the efficacy of angiotensin receptor-neprilysin inhibitor and enalapril in acute anterior STEMI patients after primary percutaneous coronary intervention: a prospective randomized trial. Cardiovascular Diagnosis and Therapy 12 (2022): 42.
- Yang P, Han Y, Lian C, Wu X. Efficacy and safety of sacubitril/valsartan vs. valsartan in patients with acute myocardial infarction: A meta-analysis. Frontiers in Cardiovascular Medicine. 2022 Aug 24;9:988117.
- Wang G, Zhang R, Li X, Zuo S, Zhang B, Zhao Y, Sun S, Zhang J, Liu X. Efficacy of sacubitril/valsartan on improving clinical symptoms in patients with acute myocardial infarction complicated with heart failure: a retrospective study. PeerJ. 2025 Feb 11;13:e18873.
- She J, Lou B, Liu H, Zhou B, Jiang GT, Luo Y, Wu H, Wang C, Yuan Z. ARNI versus ACEI/ARB in reducing cardiovascular outcomes after myocardial infarction. ESC heart failure. 2021 Dec;8(6):4607-16.
- Xiong B, Nie D, Qian J, Yao Y, Yang G, Rong S, Zhu Q, Du Y, Jiang Y, Huang J. The benefits of sacubitril–valsartan in patients with acute myocardial infarction: a systematic review and meta-analysis. ESC heart failure. 2021 Dec;8(6):4852-62.
- Di Pietro G, Improta R, Severino P, D'Amato A, Birtolo LI, De Filippo O, Lattanzio A, De Cristofaro R, Galardo G, D'Ascenzo F, Badagliacca R. The in-hospital administration of sacubitril/valsartan in acute myocardial infarction: A meta-analysis. ESC Heart Failure. 2025 Apr;12(2):998-1012.
- Liu Y, Sun Y, Dai W. Effect of sacubitril–valsartan on left ventricular remodeling in patients with acute myocardial infarction after primary percutaneous coronary intervention: a systematic review and meta-analysis. Frontiers in Pharmacology. 2024 May 28;15:1366035.
- Yang P, Li X, Wang L, Wu X, Wang C, Li T, Wang H. Effects of sacubitril/valsartan on cardiac reverse remodeling and cardiac resynchronization in patients with acute myocardial infarction. Frontiers in Cardiovascular Medicine. 2023 Jan 13;9:1059420.
- Wang F, Li C, Zhang X. Sacubitril/valsartan improves the prognosis of acute myocardial infarction: a meta-analysis. Coronary Artery Disease. 2024 May 1;35(3):231-8.
- Fan H, Wang Y, Wang X, Dong X, Shao X, Yang F. Effect of emergency percutaneous coronary intervention combined with sacubitril and valsartan on the cardiac prognosis in patients with acute myocardial infarction. International Journal of General Medicine. 2023 Dec 31:499-505.
- Yin H, Ma L, Zhou Y, Tang X, Li R, Zhou Y, Shi J, Zhang J. Efficacy of early administration of sacubitril/valsartan after coronary artery revascularization in patients with acute myocardial infarction complicated by moderate-to-severe mitral regurgitation: a randomized controlled trial. Heart and Vessels. 2024 Aug;39(8):673-86.
- Murphy SP, Prescott MF, Camacho A, Iyer SR, Maisel AS, Felker GM, Butler J, Piña IL, Ibrahim NE, Abbas C, Burnett Jr JC. Atrial natriuretic peptide and treatment with sacubitril/valsartan in heart failure with reduced ejection fraction. Heart Failure. 2021 Feb 1;9(2):127-36.
- Zhao YM, Luo JT, Pang KF, Feng Y, Tan JP, Liu M, Lin ZH. Clinical efficacy of sacubitril/valsartan combined with cardiac rehabilitation in patients with heart failure after acute myocardial infarction: a single-center randomized trial. BMC Cardiovascular Disorders. 2025 Apr 2;25(1):246.
- Rashid M, Soto CJ, Virk GS, Mekowulu FC, Chaudhari SS, Batool S, Usama M. The Safety and Efficacy of the Early Use of Sacubitril/Valsartan After Acute Myocardial Infarction: A Meta-Analysis of Randomized Controlled Trials. Cureus. 2024 Feb 7;16(2).
- Zhang L, Yan K, Zhao H, Shou Y, Chen T, Chen J. Therapeutic effects and safety of early use of sacubitril/valsartan after acute myocardial infarction: a systematic review and meta-analysis. Annals of palliative medicine. 2022 Mar;11(3):1017027-1027.
- Pierce JB, Blumer V, Choi S, Hardy NC, Greiner MA, Carnicelli AP, Shen X, Lippmann SJ, Peterson PN, Allen LA, Fonarow GC. Comparative Outcomes of Sacubitril/Valsartan Use After Hospitalization for Heart Failure Among Medicare Beneficiaries Naïve to Renin-Angiotensin System Inhibitors. The American Journal of Cardiology. 2023 Oct 1;204:151-8.
- Gu J, Wang Y, Wang CQ, Zhang JF. The initial timing and dosage pattern of sacubitril/valsartan in patients with acute myocardial infarction undergoing percutaneous coronary intervention. European Journal of Internal Medicine. 2023 Jun 1;112:62-9.
- Jain A, Meyur S, Wadhwa L, Singh K, Sharma R, Panchal I, Varrassi G. Effects of Angiotensin Receptor-Neprilysin Inhibitors Versus Enalapril or Valsartan on Patients With Heart Failure: A Systematic Review and Meta-Analysis. Cureus. 2023 Jul 8;15(7).
- Vaduganathan M, Jhund PS, Claggett BL, Packer M, Widimský J, Seferovic P, Rizkala A, Lefkowitz M, Shi V, McMurray JJ, Solomon SD. A putative placebo analysis of the effects of sacubitril/valsartan in heart failure across the full range of ejection fraction. European Heart Journal. 2020 Jul 1;41(25):2356-62.
- Liu H, Su Y, Shen J, Jiao Y, Li Y, Liu B, Hou X, Jin Q, Chen Y, Sun Z, Xi Q. Improved heart function and cardiac remodelling following sacubitril/valsartan in acute coronary syndrome with HF. ESC Heart Failure. 2024 Apr;11(2):937-49.
- Solomon SD, Rizkala AR, Gong J, Wang W, Anand IS, Ge J, Lam CS, Maggioni AP, Martinez F, Packer M, Pfeffer MA. Angiotensin receptor neprilysin inhibition in heart failure with preserved ejection fraction: rationale and design of the PARAGON-HF trial. JACC: Heart Failure. 2017 Jul;5(7):471-82.


 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 