Systematic Review: Efficacy and Safety of Direct Oral Anticoagulants (Doacs) Versus Warfarin in Atrial Fibrillation (Af)
Sara El Moussa*,1, Ali Al Yasari2, Aya Abdelfattah3, Thuvan Dharvis Fathima Shasna4, Ahmed Ibrahim haji Ahmed5, Ala’a Thabet Abdel-Karim AL-Jarrad6, Lakshmi Teja Pemmasani7, Mohsin Abid8, Saif Khalid9
1Khalifa University, Abu Dhabi, United Arab Emirates
2Ras Al Khaimah Medical and Health Sciences University
3University of Sharjah
4Ryazan State I. P. Pavlov medical university
5Ras Al Khaima Medical & Health Science university
6Canadian Hospital, Dubai
7Ras Al Khaimah Medical and Health Science University
8Nishtar Medical University
9Royal College of Surgeons Ireland
*Corresponding author: Sara El Moussa, Khalifa University, Abu Dhabi, United Arab Emirates.
Received: 18 August 2025; Accepted: 22 August 2025; Published: 15 September 2025
Article Information
Citation: Sara El Moussa, Ali Al Yasari, Aya Abdelfattah, Thuvan Dharvis Fathima Shasna, Ahmed Ibrahim haji Ahmed, Ala’a Thabet Abdel- Karim AL-Jarrad, Lakshmi Teja Pemmasani, Mohsin Abid, Saif Khalid. Systematic Review: Efficacy and Safety of Direct Oral Anticoagulants (Doacs) Versus Warfarin in Atrial Fibrillation (Af). Fortune Journal of Health Sciences. 8 (2025): 880-887.
View / Download Pdf Share at FacebookAbstract
Background: The risk of thromboembolic event is greatly increased by atrial fibrillation (AF), the most common sustained arrhythmia in the world. The key to prevent stroke in AF is by anticoagulation. Direct oral anticoagulants (DOACs), which offer fixed dosage and fewer monitoring requirements, have become an alternative to warfarin, which has historically been the standard treatment.
Objective: Use real-world data, randomized controlled trials (RCTs), meta-analysis and guideline recommendations to systematically assess and compare the safety and effectiveness of DOACs versus warfarin in patients with non-valvular atrial fibrillation.
Methods: Studies published between 2008 and 2025 were systematically reviewed, including randomized controlled trials (RCTs), cohort studies, meta-analysis, guideline publications and observational registries. Outputs like major bleeding, intracranial hemorrhage (ICH), gastrointestinal bleeding, stroke prevention and treatment persistence were analyzed.
Results: Compared to warfarin, direct oral anticoagulants (DOACs) significantly decreased rates of intracranial hemorrhage (ICH) in the majority of populations and showed either same or superior efficacy in preventing stroke and systemic embolism. The best safety profile was consistently displayed by Apixaban. DOACs performed better than warfarin, especially for patients new to anticoagulants, high-risk elderly patients and those with inadequate international normalized ratio (INR) control. DOAC use was most beneficial for subgroups like Asians, the frail and patients with renal impairment.
Conclusion: DOACs are just as effective as warfarin and generally safer in preventing serious bleeding complications, especially intracranial hemorrhage. In terms of adherence, safety and efficacy, apixaban appears to be the best option. In certain subgroups, warfarin is still useful, but in general clinical settings, DOACs are becoming more effective.
Keywords
<p style="text-align:justify">Atrial Fibrillation (AF), Anticoagulation, Direct oral anticoagulants (DOACs), Warfarin, Stroke, Systemic embolism, Major bleeding, Intracranial hemorrhage (ICH), Apixaban, Rivaroxaban, Dabigatran, Edoxaban, CHAâ‚‚DSâ‚‚-VASc score, HAS-BLED score, Randomized controlled trials (RCTs), Subgroup analysis, International Normalized Ratio (INR)</p>
Article Details
Introduction
The most prevalent cardiac arrhythmia, atrial fibrillation (AF), affects more than 30 million people globally and is a major contributor of morbidity and mortality because of complications like ischemic stroke, heart failure and systemic embolism [1]. Oral anticoagulation is the best way to reduce thromboembolic risk. Stroke prevention is still a top priority in the long-term care of patients with AF. Vitamin K antagonists (VKAs) like warfarin have long been the cornerstone of anticoagulation treatment. Despite its demonstrated effectiveness, warfarin has several limitations, including a narrow therapeutic window, a high number of drug-drug and food- drug interactions and the need for regular international normalized ratio (INR) monitoring to preserve therapeutic efficacy while preventing complications [2], [3]. Furthermore, in older and comorbid populations, suboptimal time in therapeutic range (TTR) has been linked to a higher risk of stroke [4]. The landscape of stroke prevention in non-valvular AF (NVAF) has changed since the advent of direct oral anticoagulants (DOACs), such as dabigatran, rivaroxaban, apixaban and edoxaban. These medications provide fast onset of action, fixed dosage, predictable pharmacokinetics and above all, the elimination of need for regular INR monitoring. For the prevention of stroke and systemic embolism in patients with NVAF, large-scale randomized controlled trials (RCTs) have repeatedly shown that DOACs are at least non- inferior, and in many cases superior to warfarin. In addition to their similar or improved effectiveness, DOACs have demonstrated a markedly reduced risk of intracranial hemorrhage, one of the most dangerous side effects of anticoagulation treatment. Additionally, some DOACs, apixaban in particular have been linked to lower rates of severe and fatal bleeding, which has helped explain why doctors around the world are increasingly choosing them. Therefore, unless there are contraindications like severe renal impairment or the presence of mechanical heart valves, DOACs are increasingly considered the first-line anticoagulant therapy for the majority of NVAF patients [5, 6]. Meta-analysis and real-world research have confirmed these results for larger patient groups. DOACs have proven to be effective in patients who are frail, elders, new to anticoagulants or have poor INR control [7], [8]. On the other hand, use of warfarin is linked with optimal management of INR. This becomes very difficult to achieve in routine clinical practice, particularly in community-based or low-resource settings [3]. Despite these benefits, warfarin is still frequently prescribed, especially for settings where DOACs are contraindicated due to severe renal impairment, mechanical heart valves or valvular AF. Concerns about GI bleeding, especially with rivaroxaban, lack of familiarity among providers and high cost are additional obstacles to the use of DOACs [9], [10]. Furthermore, improper off-label dose reduction of DOACs are still a concern, because they may reduce efficacy without providing appreciable safety benefit [11].
According to 2020 European Society of Cardiology (ESC) guidelines, DOACs are now recommended as first-line therapy for stroke prevention in NVAF, with the exception of certain contraindicated cases [1]. These guidelines are backed by pharmacological data, practical experiences and high-quality evidence. More research needs to be done to compare the effects of DOACs and warfarin across various subgroups, like in different age groups, renal function, bleeding risk, regional anticoagulation practices and comorbidities. Moreover, instruments like the CHA2DS2- VASc score for stroke risk and the HAS-BLED score for bleeding risk has to be utilized, especially, when choosing a therapy for patient and stratifying them for risk [2, 12]. This systematic review attempts to thoroughly assess the safety and effectiveness of DOACs in comparison to warfarin in patients with atrial fibrillation. By combining the available data and identifying areas that need further study or clinical advice, this review seeks to assist clinicians in making well-informed decisions regarding the best way to select anticoagulants.
Methods
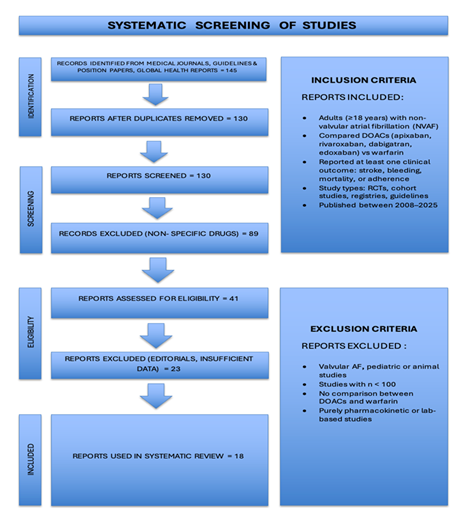
The main goal of this review was to compare the safety and effectiveness of warfarin and direct oral anticoagulants (DOACs) in patients with non-valvular atrial fibrillation (NVAF). Data from registry-based cohort studies, real-world observational studies, meta-analysis, clinical guideline documents and randomized controlled trials were included in the analysis. From articles obtained from peer-reviewed journals and cardiovascular societies, a total of 18 articles published between 2008 and 2025 were chosen. PubMed, JAMA Network, European Heart Journal, BMC Cardiovascular Disorders, Journal of the American Heart Association, Therapeutics and Clinical Risk Management and position papers or guidelines from national and international organizations like the Associazione Nazionale Medici Cardiologi Ospedalieri (ANMCO) and the European Society of Cardiology (ESC) were among the data sources. Studies comparing DOAC therapy (apixaban, rivaroxaban, dabigatran, or edoxaban) with warfarin were included if they reported adult patients (≥18 years) with non-valvular atrial fibrillation. At least one clinically significant outcome, such as stroke or systemic embolism (SEE), major bleeding (including intracranial haemorrhage), gastrointestinal bleeding, all-cause mortality or treatment adherence and discontinuation rates, had to be reported by eligible studies. Randomized controlled trials (RCTs), observational cohorts, national registry analysis and meta-analysis were all considered acceptable study designs. Studies that only looked at pharmacokinetic/pharmacodynamic data, pediatric populations, case reports or small case series (n < 100), non-human studies or those that looked at valvular atrial fibrillation (e.g mechanical valves or moderate-to-severe mitral stenosis) were excluded. Relevant statistical findings, such as hazard ratios (HRs), confidence intervals (CIs), p- values, as well as study design, population characteristics, intervention and comparison details, primary and secondary outcomes were extracted from each study. Stroke or systemic embolism, ischemic stroke and all-cause mortality were the main efficacy outcomes taken into account. Major bleeding like intracranial hemorrhage (ICH), and gastrointestinal bleeding (GI bleed) were among the safety outcomes. Additionally assessed were adherence metrics like persistence, treatment discontinuation and INR control. A formal meta-analysis was not conducted because of the heterogeneity in study design, populations and outcome reporting. Rather, a narrative synthesis was carried out, emphasizing absolute risk differences and hazard ratios where they were available. Subgroup analysis is given particular attention in the review in order to assess the differences in outcomes between specific populations of patients. These include elderly patients who are 75 years of age or older, as they are more likely to experience bleeding complications as well as thromboembolic events. Since renal function has a major impact on the pharmacokinetics and safety profiles of oral anticoagulants, especially DOACs, individuals with renal impairment are also evaluated. Patients with poor INR control represent another crucial subgroup. Effects in patients who have never taken warfarin before are also evaluated. To enable a deeper interpretation of comparative effectiveness and safety, outcomes are stratified based on established clinical risk scores, such as SAMe-TT2R2 for predicting warfarin control quality, HAS-BLED for bleeding risk and CHA2DS2-VASc for thromboembolic risk. Following is a prisma flowchart (Figure 1) which shows the screening process of studies included in this article.
Results
Efficacy Results: Prevention of thromboembolic events
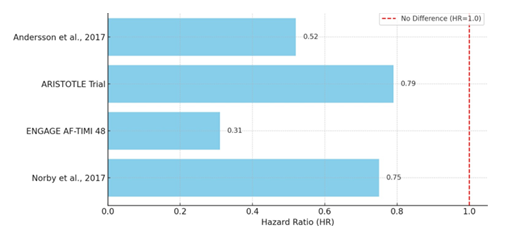
Warfarin therapy significantly decreased the risk of cerebral infarction when compared to no anticoagulation, according to a nationwide Swedish registry study that included over 48,000 AF patients. This protective effect was especially noticeable in women ≥75 years with a CHA2DS2-VASc ≥3 (HR 0.52) and men 65–74 years with a CHA2DS2-VASc score of 1 (HR 0.46) [13]. But when it’s compared with DOACs, randomized controlled trials (RCTs), observational cohort studies and real-world database analysis have consistently shown that DOACs are more effective than warfarin at preventing thromboembolic event, mainly ischemic stroke and systemic embolism. When apixaban was used instead of warfarin, patients in the ARISTOTLE trial experienced a statistically significant decrease in stroke or systemic embolism (HR 0.79; 95% CI, 0.66–0.95). Crucially, this benefit held true for all age groups, including those 80 years of age and older, who are generally more susceptible to thromboembolic events and bleeding complications [5]. Additional evidence was provided by the ENGAGE AF-TIMI 48 sub-analysis, particularly for East Asian patients with suboptimal INR control (TTR <65%). High-dose edoxaban significantly outperformed warfarin in this subgroup, lowering the risk of stroke/systemic embolism by 69% (HR 0.31; p=0.004). This suggests that DOAC efficacy may be particularly stronger in patients who are challenging to keep within the limited therapeutic window of warfarin [14].
These results were also supported by real-world data. Medicare claims data from a cohort of patients aged ≥65 who switched from warfarin to DOACs showed that apixaban was superior to both dabigatran and rivaroxaban, with lower systemic embolism rates (HRs 0.83 and 0.91 respectively), supporting apixaban’s high efficacy in the elderly [7]. In a large retrospective study involving over 44,000 new users, rivaroxaban was linked to a 25% relative risk reduction in ischemic stroke compared to warfarin among patients new to anticoagulant (HR 0.75; 95% CI, 0.62–0.91) [10]. All of these results support the idea that DOACs are better than warfarin in preventing thromboembolic events in AF patients, especially in those with higher baseline risk, such as older adults, patients with unstable INR control and patients starting anticoagulation therapy for the first time. Following is a graph (Figure 2), showing comparison of DOACs vs warfarin in preventing thromboembolic events in AF patients. It is showing hazard ratios for thromboembolic event prevention, favoring DOACs over warfarin (values < 1.0).
Safety Results: Risk of Bleeding
When evaluating safety, DOACs consistently showed a better safety profile than warfarin, especially when it came to lower the risk of major bleeding and intracranial hemorrhage (ICH). In patients aged ≥75 years, apixaban decreased the risk of ICH by almost two- thirds (HR for ICH: 0.34) and decreased major bleeding by about 34% in the ARISTOTLE trial, making it a safer option for patients of all ages [5]. The ROCKET-AF trial offered detailed information about the bleeding profile of rivaroxaban. In centres with low TTR, rivaroxaban demonstrated a bleeding advantage; however, in centres with excellent INR control (TTR ≥70%), where warfarin was linked to fewer bleeding events (HR 1.25), this advantage was reversed. This implies that the safety profile of rivaroxaban is less reliant on the quality of INR management at the centre level, providing consistency in situations where it is difficult to maintain therapeutic INR [15]. A large population-based cohort study of 125,195 Ontario patients with AF, who were using warfarin, further highlighted the safety concern by revealing that major bleeding happened at a rate of 3.8% per person-year, with the highest risk occurring within the first 30 days of initiation (11.8% per person-year). The hemorrhage rate for patients aged ≥75 with a CHADS2 score ≥4 was 17.3% per person-year [2].
These results highlight the susceptibility of older warfarin users, especially in the initiation of warfarin therapy. Additionally, safety profiles specific to each region were noted. Rivaroxaban was linked to a significantly higher incidence of gastrointestinal (GI) bleeding than warfarin (HR 5.9; p=0.001) in an Oman study, but the rates of ischemic stroke and non-GI bleeding were comparable between the groups [9]. However, data from the U.S. Medicare system confirmed apixaban's superiority, demonstrating that it was linked to lower major bleeding rates than both dabigatran (HR 0.79) and rivaroxaban (HR 0.68), further establishing apixaban as the most advantageous DOAC in terms of safety [7]. Overall, DOACs, and apixaban in particular, have a much better safety profile than warfarin. They also reduce potentially fatal bleeding events, especially intracranial hemorrhage, which has a big impact on patient outcomes and clinical practice. Following table (Table 1) shows outcome and safety profile comparison of warfarin with DOACs.
|
Comparison |
Outcome |
Hazard Ratio (HR) / Key Findings |
|
Warfarin only [2] |
Overall major bleeding: 3.8% per person-year Highest risk in first 30 days: 11.8% Age ≥75 + CHADS2 ≥4: 17.3% |
High early-phase bleeding risk, especially in elderly |
|
Apixaban vs Warfarin [5] |
Apixaban ↓ Intracranial hemorrhage (ICH), ↓ Major bleeding |
HR for ICH: 0.34 (favoring apixaban) Major bleeding ↓ by 34% |
|
Rivaroxaban vs Warfarin [15] |
In centers with low TTR, rivaroxaban had a safety advantage In high-TTR centers, warfarin safer |
HR in high-TTR centers: 1.25 (favoring warfarin) |
|
Rivaroxaban vs Warfarin [9] |
↑ Gastrointestinal (GI) bleeding with rivaroxaban Similar result in ischemic stroke & non-GI bleeding |
HR for GI bleeding: 5.9 (p = 0.001) (favoring warfarin) |
Table 1
Subgroup Analysis
Across several high-risk and under-represented subgroups, safety and effectiveness of DOAC remained favorable. Apixaban showed long-lasting benefits in lowering the risk of stroke and bleeding in patients aged ≥80 years, which makes it especially appropriate for the elderly [5]. The difficulties of starting warfarin in this population, however, were highlighted by the startlingly high bleeding risks experienced by older warfarin users, particularly those who were new to therapy [2]. Time in Therapeutic Range (TTR) was a significant factor influencing effectiveness of warfarin. According to studies, the effectiveness and safety of warfarin are compromised when TTR drops below 60% because both stroke and bleeding rates sharply rise at that point [3], [4]. By providing reliable anticoagulation without requiring INR monitoring, DOACs completely avoid this problem. Differences by sex were also observed. Women who used rivaroxaban had significantly better stroke prevention outcomes than men (HR 0.57 vs. 1.02) [10]. This suggests that there may be sex-specific pharmacodynamic differences that need more research.
Numerous studies have emphasized the advantages of moving from warfarin to DOACs. Apixaban was found to be the most efficient and secure choice in the analysis of 171,000 switchers conducted [7]. Furthermore, the ORBIT-AF registry discovered that most common reasons for stopping warfarin were bleeding episodes, patient refusal, physician preference and worries about monitoring and adherence (10.1% at one year) [16]. Lastly, crucial context is provided by expert consensus and clinical guidelines. For the majority of AF patients, the 2020 ESC Guidelines suggest DOACs over VKAs due to their superior safety and usability [1]. Off-label DOAC dose reductions, which are frequently performed in elderly or frail patients, can paradoxically raise the risk of stroke, according to the ANMCO position paper, which stresses appropriate dosing [11]. In the end, these subgroup analysis support wider adoption in routine clinical care by demonstrating that DOACs not only benefit the entire population but also improve outcomes in more traditionally vulnerable groups, such as the elderly, patients with poor INR control and those switching from warfarin.
Table below shows subgroup comparison of anticoagulant drugs (Table 2).
|
Subgroup |
Key Findings |
Notes |
|
Elderly (≥80 years) |
Apixaban significantly reduced stroke and bleeding risks [5] |
Preferred DOAC in elderly due to lower ICH |
|
Patients with poor INR control |
TTR <60% associated with increased stroke and bleeding rates [3, 4] |
DOACs bypass INR monitoring, maintaining stable anticoagulation |
|
Sex differences (Female vs Male) |
Rivaroxaban more effective in women (HR 0.57) vs men (HR 1.02) [10] |
Potential pharmacodynamic variation; requires further investigation |
|
Warfarin switchers (to DOACs) |
Apixaban most effective and safest in >171,000 patients who switched [7] |
Reinforces real-world effectiveness of DOACs in transition scenarios |
|
Patients stopping warfarin |
Discontinuation often due to bleeding, monitoring difficulties, and patient/physician preference (10.1% at 1 year) [16] |
Monitoring burden is a major reason for discontinuation |
|
Dose reduction risks |
Off-label dose reductions in elderly may paradoxically increase stroke risk [11] |
Emphasizes need for correct DOAC dosing rather than empirical dose cuts |
Table 2
Discussion
A comprehensive epidemiological analysis from the Global Burden of Disease Study shows that between 1990 and 2021, the incidence and prevalence of atrial fibrillation (AF) increased by more than 120% and 130%, respectively, while the number of deaths from it increased by almost 200% during the same time period. Major modifiable risk factors of AF, such as high systolic blood pressure and elevated body mass index, were found to be prevalent in aging populations, with significant variations by sex and geographic location [17]. This raises the need for safer and more efficient anticoagulation techniques. In order to compare the effectiveness and safety of direct oral anticoagulants (DOACs) versus warfarin in patients with non-valvular atrial fibrillation (NVAF), this systematic review brings together data from 18 sources, including observational cohort studies, registry data, randomised controlled trials (RCTs) and clinical guidelines. A key component of preventive cardiology is optimizing anticoagulation therapy because AF is still the world leading cause of ischemic stroke in the world. This review emphasizes how well DOACs perform in comparison to warfarin across a number of patient subgroups and clinical outcomes, bringing about a paradigm shift in stroke prevention strategies.
All types of evidences consistently show that DOACs are at least as effective as warfarin at preventing stroke and systemic embolism, and often more so. The ARISTOTLE trial, a seminal investigation contrasting apixaban and warfarin, demonstrated a noteworthy decrease in stroke or systemic embolism with apixaban (HR 0.79), which held true for patients aged ≥80 years [5]. The effectiveness of high-dose edoxaban in East Asian patients, especially those with suboptimal INR control, was also validated by the ENGAGE AF-TIMI 48 sub-analysis, which showed a 69% decrease in the risk of stroke and systemic embolism when compared to warfarin [14]. These conclusions are further supported by real-world data, such as retrospective cohort studies from the Medicare and MarketScan databases. In a sizable cohort of patients who had never taken an anticoagulant, rivaroxaban decreased the risk of ischemic stroke (HR 0.75) in comparison to warfarin [10]. Using Medicare claims data, study showed that among patients switching from warfarin, apixaban was linked to the lowest rates of stroke and systemic embolism when compared to rivaroxaban and dabigatran [7]. These results support DOACs as a viable alternative for a range of patient populations. The quality of warfarin therapy is frequently assessed by time in therapeutic range (TTR). The limitations of warfarin were brought to light by community-based studies where TTR frequently drops below 60% [3], [4]. In contrast, DOACs are consistently effective regardless of INR control, which makes them especially useful in situations where the continuous monitoring of warfarin is not possible [15].
When choosing anticoagulants, safety considerations, particularly bleeding risks are just as significant as efficacy. The safety profile of DOACs as a class is consistently superior to that of warfarin, especially when it comes to lowering intracranial hemorrhage (ICH), the most dreaded side effect of anticoagulation treatment. In the ARISTOTLE trial, apixaban significantly reduced major bleeding (HR ~0.66–0.77) and ICH (HR 0.34 in patients ≥75 years) compared to warfarin [5]. These benefits were confirmed in real-world studies, reporting that apixaban had the lowest rates of both major bleeding and stroke/systemic embolism among elderly patients switching from warfarin [7]. Nevertheless, not every DOAC was consistently better for every safety outcome. Despite its effectiveness in preventing stroke, rivaroxaban has been linked to an increased risk of gastrointestinal bleeding (GI bleeding), particularly in elderly patients [9], [10]. This emphasizes the significance of choosing a customized course of treatment, particularly for patients who have a high risk of bleeding or pre-existing GI pathology.
The bleeding risk is significantly higher, especially in first few months of treatment with warfarin. During the first 30 days of warfarin therapy, it has been seen in patients of 75 years of age and older, with high CHA2DS2-VASc score that the rate of major hemorrhage is significantly elevated, i.e up to 17.3% per person-year [2]. Because of it, warfarin showed low adherence by patients, leading to suboptimal stroke prevention. In contrast, DOACs therapy is linked with increased adherence and low discontinuation rates [18]. This is because of their ease of use, fewer food-drug interactions and fixed doses. Moreover, continuous monitoring of INR is not required. The recommended option for elderly, frail or cognitively impaired patients are DOACs as they are safer and more effective for them. As its benefits for elders aged ≥80 years, first choice of many clinicians treating older adults with NVAF is apixaban [5]. The 2020 European Society of Cardiology (ESC) guidelines strongly recommend DOACs as the preferred agents for stroke prevention in NVAF, unless they are contraindicated due to severe mitral stenosis or mechanical valves [1]. This recommendation is in line with the corpus of research showing the efficacy and safety of DOACs, including RCTs and empirical data.
ANMCO 2022 consensus further stresses proper dosing procedures for DOACs. To lower the risk of bleeding, off-label underdosing is frequently used, which increases the risk of stoke. It needs to be avoided unless required clinically [11]. To comprehend how characteristics of patient alter treatment outcomes, subgroup analysis is essential. Senior Citizens have a higher baseline risk of bleeding and stroke, especially those over 75, and they benefit more from DOACs. Apixaban offers a safer and more effective profile for this subgroup [2]. Moreover, patients with impaired renal function need to have their doses adjusted because renal clearance influences the metabolism of DOACs. In contrast to dabigatran and rivaroxaban, which are more renally excreted, apixaban is notable for being both safe and effective, even in patients with moderate renal dysfunction [5]. As shown in ENGAGE AF-TIMI 48, East Asian patients typically have poor warfarin TTR, which increases their benefit from DOACs, especially edoxaban [14]. Participants who switched from warfarin to DOACs, particularly apixaban, had lower rates of bleeding and stroke. Medicare data verified that these switchers respond better than those who continued taking warfarin [7].
This systematic review has a number of limitations that should be noted. First, because included studies varied in terms of study designs, populations, outcome definitions and statistical reporting, a formal meta-analysis was not carried out. The results are therefore based on qualitative synthesis, which could be biased in its interpretation. Furthermore, direct comparability is limited by variations in follow-up durations, baseline risk profiles, and region-specific practices (e.g variation in TTR for warfarin), even though the studies covered a wide time period (2008–2025) and included real-world data, RCTs, and registry analysis. Furthermore, since studies with positive results for DOACs might have a higher chance of being published, publication bias cannot be completely ruled out. Finally, a large number of real-world studies used administrative claim data, which may be inaccurate or devoid of specific clinical information like INR levels, the use of over the counter medications or adherence patterns. By concentrating on under-represented subgroups, such as patients with severe renal impairment, extreme body weight, active malignancy, those undergoing cardioversion or ablation, future research should try to close these gaps. Prospective, head-to-head comparisons of individual DOACs in a variety of populations are also necessary, particularly for patients with complicated comorbidities. More detailed information about the efficacy of treatments in clinical practice may be provided by studies that integrate patient reported outcomes and real-time adherence monitoring. Furthermore, in order to promote fair access to DOACs worldwide, formal cost-effectiveness analysis across healthcare systems, particularly in low and middle-income nations are crucial.
In short, the increasing amount of data demonstrating the superiority of DOACs over warfarin in treating patients with non-valvular atrial fibrillation is highlighted by this systematic review. In addition to offer comparable or better stroke prevention, DOACs have important safety benefits, especially when it comes to lower potentially fatal bleeding events like intracranial hemorrhage. As long as proper dosage, patient selection and real- world monitoring are given top priority, DOACs are expected to continue to be the anticoagulants of choice for the majority of patients.
Conclusion
This thorough analysis provides compelling evidence for the safety and effectiveness of direct oral anticoagulants (DOACs) over warfarin in preventing stroke in patients with non- valvular atrial fibrillation (NVAF). DOACs have consistently demonstrated same effect as warfarin in the reduction of stroke and systemic embolism incidence. There is stronger evidence for the benefits of apixaban and edoxaban in the elderly and higher-risk subgroups. Furthermore, DOACs have a more stable anticoagulation profile and do not require the maintenance of INRs within the narrow therapeutic range required for warfarin therapy. DOACs are further favored by safety, a key distinction between the two drug classes. DOACs, particularly apixaban, were linked to noticeably lower rates of major bleeding and intracranial hemorrhage in several studies. Even though some medications, like rivaroxaban, have been associated with increased gastrointestinal bleeding, this risk is typically outweighed by decreases in potentially fatal bleeding and increased patient convenience. Significantly, there have been reports of improved adherence and persistence with DOAC therapy, most likely as a result of fewer drug–food interactions and the removal of routine INR monitoring. In both outpatient and resource constrained settings, this bolsters position of DOAC as a more sensible and patient-friendly choice. However, careful consideration must be given to factors like patient age, comorbidities, stroke and bleeding risk scores (e.g CHA2DS2-VASc, HAS-BLED, SAMe-TT2R2), renal function and prior bleeding history, while prescribing any anticoagulant. Furthermore, improper DOAC dosing can decrease their effectiveness, as demonstrated in a number of real-world studies, underscoring the necessity of cautious dose selection and continuous patient education. DOACs are well-positioned to continue to be the mainstay of anticoagulation therapy as the prevalence of atrial fibrillation rises worldwide, especially in older populations, as long as their use is informed by evidence-based, personalized decision-making.
References
- Hindricks, et al. “2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio- Thoracic Surgery (EACTS),” Eur. Heart J vol 42 (2021): 373–498.
- T Gomes, MM Mamdani, AM Holbrook, et al. “Rates of hemorrhage during warfarin therapy for atrial fibrillation,” Med. Assoc J 185 (2013): E121–E127.
- WL Baker, DA Cios, SD Sander, et al. “Meta-Analysis to Assess the Quality of Warfarin Control in Atrial Fibrillation Patients in the United States,” Manag. Care Pharm 15 (2009): 244–252.
- J Rose, A Ozonoff, LE Henault, et al. “Warfarin for atrial fibrillation in community-based practise,” Thromb. Haemost 6 (2008): 1647–1654.
- S Halvorsen et al. “Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial,” Heart J 35 (2014): 1864–1872.
- Martínez-Rubio et al. “Using Direct Oral Anticoagulants in Patients with Atrial Fibrillation: Assessment, Monitoring and Treatment Reversal,” Cardiol. Rev 11 (2016): 118.
- N Atreja et al. “Effectiveness and Safety in Patients with Non-Valvular Atrial Fibrillation Who Switched from Warfarin to Direct Oral Anticoagulants in Medicare Population,” Ther 42 (2025): 1462–1483.
- E Bertaglia et al. “Atrial high-rate episodes: prevalence, stroke risk, implications for management, and clinical gaps in evidence,” EP Eur 21 (2019): 1459–1467.
- MS Al-Maawali, HH Al-Naamani, LN Mokadem, et al. “Comparative Effectiveness and Safety of Rivaroxaban and Warfarin for Stroke Prevention in Patients with Non-Valvular Atrial Fibrillation in an Omani Tertiary Care Hospital,” Open Cardiovasc. Med. J 16 (2022): e187419242202281.
- FL Norby et al. “Comparative effectiveness of rivaroxaban versus warfarin or dabigatran for the treatment of patients with non-valvular atrial fibrillation,” BMC Cardiovasc. Disord 17 (2017): 238.
- D Mocini et al. “ANMCO position paper ‘Appropriateness of prescribing direct oral anticoagulants in stroke and systemic thromboembolism prevention in adult patients with non-valvular atrial fibrillation,’” Heart J. Suppl 24 (2022): C278– C288.
- Methavigul A, Yindeengam and Krittayaphong R. “Efficacy and safety outcomes of patients with atrial fibrillation compared between warfarin and non-vitamin K antagonist oral anticoagulants based on SAMe-TT2R2 score,” BMC Cardiovasc. Disord 23 (2023): 43.
- T Andersson et al. “Patients with atrial fibrillation and outcomes of cerebral infarction in those with treatment of warfarin versus no warfarin with references to CHA2DS2-VASc score, age and sex - A Swedish nationwide observational study with 48 433 patients,” PLOS ONE 12 (2017): e0176846.
- YJ Shimada et al. “Effects of Regional Differences in Asia on Efficacy and Safety of Edoxaban Compared with Warfarin – Insights from the ENGAGE AF-TIMI 48 Trial –,” J 79 (2015): 2560–2567.
- P Piccini et al. “Relationship Between Time in Therapeutic Range and Comparative Treatment Effect of Rivaroxaban and Warfarin: Results from the ROCKET AF Trial,” Am. Heart Assoc 3 (2014): e000521.
- EC O’Brien et al. “Reasons for warfarin discontinuation in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF),” Heart J 168 (2014): 487–494.
- S Cheng et al, “Global burden of atrial fibrillation/atrial flutter and its attributable risk factors from 1990 to 2021,” Europace 26 (2024): euae195.
- D Ko et al. “Trends in Use of Oral Anticoagulants in Older Adults with Newly Diagnosed Atrial Fibrillation, 2010-2020,” JAMA Netw. Open 5 (2022): e2242964.


 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 