Virtual Intensivist Support Model reducing Intensive Care Unit admission: Erie Shores HealthCare Experience
Michael Riley Jackson Jakob1,2, Alexandrea Gow1, Jaefer Mohamad3, Nima Andre Malakoti-Negad3, Angela Ciotoli1, Nadia Pedri4, Neelu Sehgal1, Matt Bessey1, Deepa Chawla1, Tazmeen Yekinni5, Munira Sultana*,1,5
1Erie Shores HealthCare, 194 Talbot St. W. Leamington, Ontario N8H 1N9
2Windsor Regional Hospital, Ouellette Campus, 1030 Ouellette Ave, Windsor Ontario N9A 1E1
3Schulich School of Medicine & Dentistry, London, Ontario, Canada, N6A 3K7
4University of Windsor, 401 Sunset Ave, Windsor, ON N9B 3P4
5WE SPARK HEALTH Institute, 401 Sunset Ave, Windsor, ON N9B 3P4
*Corresponding author: Munira Sultana, Erie Shores HealthCare, 194 Talbot St. W. Leamington, Ontario N8H 1N9.
Received: 29 July 2025; Accepted: 05 August 2025; Published: 04 September 2025
Article Information
Citation: Michael Riley Jackson Jakob, Alexandrea Gow, Jaefer Mohamad, Nima Andre Malakoti-Negad, Angela Ciotoli, Nadia Pedri, Neelu Sehgal, Matt Bessey, Deepa Chawla, Tazmeen Yekinni, Munira Sultana. Virtual Intensivist Support Model reducing Intensive Care Unit admission: Erie Shores HealthCare Experience. Fortune Journal of Health Sciences. 8 (2025): 845-851.
View / Download Pdf Share at FacebookAbstract
This study evaluated the impact of a Virtual Intensivist Support (VIS) Model on Intensive Care Unit (ICU) clinical outcomes at a rural Canadian hospital, Erie Shores HealthCare, where intensivist resources were previously limited. The model involved daily virtual rounds by off-site intensivists on all ICU patients and any other patients with critical care needs. A retrospective-prospective comparison of the ICU data, focusing on ICU admissions, antibiotic use, venous thromboembolism prophylaxis, patient transfers, and physiotherapy orders indicated a significant decrease in antibiotic-free days (OR = 0.56; p < 0.001), while challenges in patient transfers were mitigated as intensivists provided real-time updates and advocated for proper escalation. We also noticed higher transfer rates and physiotherapy orders and lower venous thromboembolism prophylaxis orders. The findings suggest that the VIS Model may influence clinical practice patterns, highlighting its potential to enhance ICU workflows in rural settings.
Keywords
<p>Virtual Intensivist, Intensive Care Unit, Rural healthcare, Care model</p>
Article Details
Introduction
Ensuring the timely recognition and management of critically ill patients remains a persistent challenge in hospital medicine. Intensive care units (ICUs), by their very nature, are designed to manage the most acutely unwell individuals. However, patients at risk of clinical deterioration often reside outside of these units, on general wards, where early warning signs may be overlooked or inadequately addressed due to limited access to specialized personnel, protocols, or resources [1]. This failure to intervene early can result in preventable morbidity, cardiac arrest, unplanned ICU admissions, or even death [2]. To address this issue, many hospitals have implemented Critical Care Outreach Teams (CCOTs), interdisciplinary teams, typically composed of ICU-trained personnel who respond to and proactively monitor patients exhibiting signs of clinical decline on general wards, following Comprehensive Critical Care guidelines [3]. The team represents a system-level response to the limitations of traditional hospital care models, aiming to deliver "critical care without walls" by bringing ICU expertise to the bedside, regardless of the patient's location within the hospital [4,5]. While CCOTs have shown promise in improving clinical outcomes and facilitating earlier interventions, the majority of existing evidence comes from large urban centers and tertiary academic hospitals [6]. Their implementation and efficacy in smaller, rural hospitals remain relatively underexplored.
Rural and community hospitals in Canada and globally face unique structural and resource-based constraints. These institutions often lack round-the-clock Intensivist coverage, have fewer ICU beds, and experience challenges in recruiting and retaining specialized staff [6-8]. The delivery of complex interventions such as physiotherapy or antimicrobial stewardship may be inconsistent, and timely access to higher levels of care—whether through consultation or patient transfer—is often logistically complicated [6]. In such contexts, CCOTs may play a vital role, not only by providing immediate clinical support but also by acting as bridges between general care teams and critical care specialists, enforcing protocols, and improving continuity of care [9,10]. The deployment of Critical Care Outreach Teams (CCOTs) in rural healthcare settings is a largely unexplored area, presenting a critical gap in our understanding. Rural hospitals frequently face a shortage of essential specialists, such as intensivists, infectious disease experts, and full-time physiotherapists [7]. Consequently, generalist teams are often left to manage complex, high-acuity patients with insufficient support. In many instances, these facilities operate without dedicated intensivists, relying solely on internal medicine and hospitalist teams to care for critically ill patients. Research [8,11] demonstrates that targeted interventions, including outreach education and integration of physiotherapy teams, can significantly enhance care processes. Thus, the potential of comprehensive CCOTs to revolutionize care quality—particularly in terms of antibiotic stewardship, venous thromboembolism (VTE) prophylaxis, physiotherapy utilization, and patient transfer practices—demands urgent and thorough investigation. The benefits of improved care delivery in rural settings could be transformative for both patients and the healthcare system. Considering the knowledge gap surrounding the use of CCOT in rural Canadian hospitals, this study aimed to evaluate the implementation of a Virtual Intensivist Support (VIS) Model at a rural Canadian hospital. This innovative model involved daily virtual rounds conducted by off-site intensivists, covering all ICU patients and any other individuals with critical care needs. We were also interested in evaluating whether the implementation of a CCOT at Erie Shores HealthCare (ESHC), a rural community hospital, influenced key markers of clinical practice and patient management in the ICU. The hypothesis underlying this investigation was that implementing a CCOT would lead to measurable changes in the clinical domains, particularly reflecting improved adherence to guidelines and optimized resource use. For instance, we hypothesized that CCOT involvement would be associated with increased oversight of VTE prophylaxis and physiotherapy engagement, more rational antibiotic prescribing, and more judicious decisions regarding patient transfers. To the best of our knowledge, VIS model in rural Ontario is a novel initiative.
Method
Study Design and Setting
This study employed a retrospective-prospective comparative case series design to evaluate the impact of a VIS model on key clinical outcomes within a rural community hospital (ESHC). The hospital operates a general ICU with limited specialty resources, characteristic of rural hospital settings, serving a diverse patient population from the surrounding communities. The model was introduced in April 2024, providing ICU-trained personnel to assist in the identification and management of critically ill patients both within and outside the ICU environment.
Data Sources
Clinical data were extracted from the hospital’s Cerner electronic medical records system, encompassing all ICU patient encounters from April 2021 to November 2024. The pre-intervention period included ICU admissions from April 2021 to March 2024, while the post-intervention period covered April to November 2024. The study was approved by the University of Windsor’s Research Ethics Board (REB). The hospital records used for the retrospective chart review were de-identified. No direct patient recruitment or consent was required, as the study exclusively utilized de-identified data from routine clinical records.
Intervention
The VIS model comprised of a multidisciplinary CCOT at ESHC led by a virtual Intensivist (first author), designed to support the recognition and management of patient deterioration across hospital units, including ward follow-ups and consultations for at-risk patients. The team's role included early identification of clinical decline, facilitation of timely interventions, support for guideline-directed therapies, and promotion of interdisciplinary care coordination, including physiotherapy engagement and antimicrobial stewardship.
Outcomes
The primary outcomes included: (1) antibiotic use patterns, particularly antibiotic-free days (AFD) as a marker of stewardship; (2) utilization of VTE prophylaxis; (3) frequency of interhospital transfers to higher-level centers; and (4) ordering patterns of physiotherapy (PT) consultations in the ICU. The secondary outcome was a change (if any) in the number of ICU admissions. We defined AFD as the number of ICU patient days during which no antibiotics were administered, normalized per 100 ICU patient days; VTE prophylaxis events as the number of patients receiving either pharmacological or mechanical prophylaxis during ICU admission; .transfers to higher levels of care as the number of ICU patients transferred to tertiary care centers for specialized management; and PT orders as the number of consult orders placed for physiotherapy services during ICU admission. These outcomes were selected to reflect both direct care processes (antibiotic use, prophylaxis, rehabilitation engagement) and broader systems coordination (interhospital transfers).
Statistical Analysis
The AFD reported with odds ratios (ORs) with corresponding 95% confidence intervals (CIs) using Microsoft Excel. Fisher’s exact test was applied for comparing proportions of AFD between the pre- and post-intervention periods. For VTE prophylaxis events, transfers to higher-level care, and PT orders, comparisons were performed using Poisson rate analyses, calculating rate ratios (RRs) with 95% CIs. These analyses accounted for the number of events per 1,000 ICU patient days. Poisson rate comparisons were conducted using OpenEpi software (version 3.01). A two-sided p-value of <0.05 was considered statistically significant for all tests. No power calculation was performed given the exploratory nature of this case series design and the available data from the hospital electronic medical records.
Results
With the implementation of the VIS Model in April 2024, a significant improvement was seen in transfer coordination. Intensivists with cross-site familiarity at both ESHC and receiving institutions began virtually rounding on ICU and critically ill patients. This change substantially reduced barriers, such as transfer delays due to physician refusals or hesitancy from tertiary centers unfamiliar with the ESHC setting and capabilities, as well as prolonged interhospital transfers resulting from logistical complexity and inconsistent patient acceptance. The improved transfer coordination instilled confidence in the efficiency of the VIS Model, and acceptance by tertiary sites significantly improved. Intensivists were able to provide real-time clinical updates, advocate for appropriate escalation, and facilitate early triage decisions. The Virtual Intensivist model contributed to a smoother, more reliable, and timelier transfer process. For example, after implementation, the number of AFD significantly decreased compared to the immediate pre-intervention year (2023–2024). The odds of an ICU patient day being antibiotic-free were significantly lower post-intervention (OR = 0.56; 95% CI: 0.45–0.69; p < 0.001). When aggregating all pre-intervention years (2021–2024), this effect remained significant (OR = 0.60; 95% CI: 0.50–0.71; p < 0.001). The rate of PT orders also increased along with changes in VTE prophylaxis (Rate Ratio = 0.73; 95% CI: 0.37–1.44; p = 0.36) and transfers to higher levels of care (Rate Ratio = 0.81; 95% CI: 0.56–1.17; p = 0.26). However, ICU admissions were not decreased as expected.
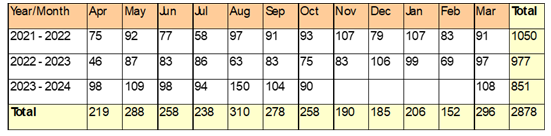
AFD
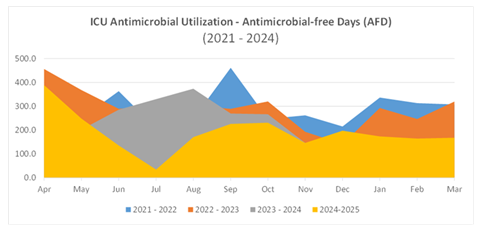
From April 2021 to March 2022 (pre-VIS model implementation), the ESHC ICU had a total of 1050 patient days with 313 AFD, averaging 298 AFD per 100 ICU patient days. The highest monthly AFD was observed in September 2021 (42 days), and the lowest in July 2021 (12 days). From April 2022 to March 2023, ICU patient days totaled 977, with 278 AFD recorded, averaging 284.5 per 100 ICU patient days. The highest monthly AFD was in May 2022 (32 days), while the lowest was in December 2022 (15 days). For April 2023 to March 2024, data available for eight months indicated 851 ICU patient days with 264 AFD, averaging 310.2 AFD per 100 ICU patient days. Post-CCOT, for the period April to November 2024, there were 963 ICU patient days and 194 AFD recorded, averaging 201.5 AFD per 100 ICU patient days, with April 2024 having the highest AFD (49 days) and July 2024 the lowest (4 days). The projected average AFD per 100 ICU patient days for the full 2024-2025 year is 190, lower compared to previous years (Figure 1). Comparing ICU patient days in the year immediately preceding CCOT implementation (2023–2024) to the post-CCOT period (April–November 2024), the odds of an ICU day being antibiotic-free were significantly lower post-implementation (OR = 0.56; 95% CI: 0.45–0.69; p < 0.001).When combining all pre-VIS model ICU days from 2021 to 2024, the odds of an ICU day being antibiotic-free remained significantly lower after VIS model implementation (OR = 0.60; 95% CI: 0.50–0.71; p < 0.001).
VTE Prophylaxis
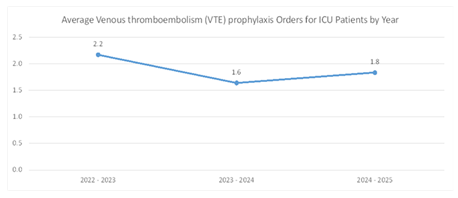
In the 2022-2023 pre-VIS model implementation period, ESHC ICU had a total of 26 patients receiving VTE prophylaxis, averaging 2.2 patients per month. Monthly counts ranged from 1 to 4 patients. During the 2023-2024 period, the number decreased slightly to 18 patients, averaging 1.6 patients monthly, with monthly counts ranging from 0 to 5 patients. In the post-VIS model period (April to November 2024), 11 patients received prophylaxis, maintaining a monthly average of 1.8, with monthly counts ranging from 1 to 3 patients (Figure 2). VTE prophylaxis events were analyzed using a Poisson rate comparison. Pre-VIS model, there were 36 events across 1738 ICU patient days (rate = 20.7 per 1000 ICU days). Post-VIS model, there were 11 events over 723 ICU patient days (rate = 15.2 per 1000 ICU days). The difference was not statistically significant (Rate Ratio = 0.73; 95% CI: 0.37 to 1.44; p = 0.36).
Transfers to Higher Levels of Care
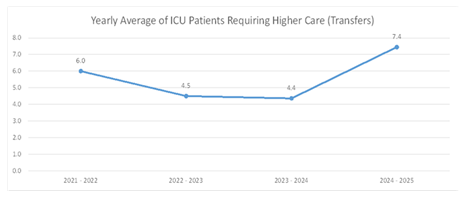
In the pre-VIS model period of 2021-2022, ESHC transferred 72 patients to higher levels of care, averaging 6.0 patients monthly, with transfers ranging from 2 to 11 patients per month. In 2022-2023, total transfers decreased to 54, averaging 4.5 per month (range 1-8). For the period of 2023-2024, there were 48 transfers averaging 4.4 monthly (range 2-7). Following the implementation of the VIS model, from April to November 2024, transfers increased to 67 patients total, averaging 7.4 per month, with monthly transfers ranging from 3 to 14 (Figure 3). Transfers to higher levels of care were analyzed using a Poisson rate comparison. Pre-VIS model, there were 153 transfers across 2788 ICU patient days (rate = 54.9 per 1000 ICU days). Post-VIS model, there were 32 transfers over 723 ICU patient days (rate = 44.3 per 1000 ICU days). The difference was not statistically significant (Rate Ratio = 0.81; 95% CI: 0.56 to 1.17; p = 0.26).
PT Orders
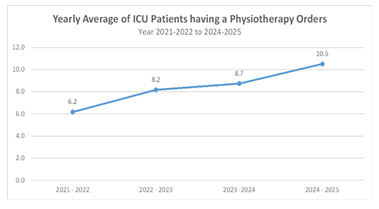
In the pre-VIS model period of 2021-2022, ICU patients had 74 PT orders, averaging 6.2 per month, ranging from 2 to 12 monthly. In 2022-2023, PT orders increased to 98, averaging 8.2 monthly, ranging from 5 to 11 monthly. In the 2023-2024 period, PT orders totaled 96, averaging 8.7 monthly, with a range from 5 to 15. From April to November 2024, PT orders further increased to 63, with an average of 10.5 per month, ranging from 6 to 15 monthly (Figure 4). The PT orders per ICU patient day were compared using a Poisson rate comparison. In the pre-VIS model period (2021–2024), there were 268 PT orders over 2878 ICU patient days. In the post-VIS model period (April–November 2024), there were 63 PT orders over 963 ICU patient days. The PT orders increased significantly in the post-VIS model period (OR = 0.55, p < 0.001).
Overall, a negative trend was noticed in ICU admissions (Table 1). However, the data does not confirm a reduction in ICU admissions as we expected.
Table 1: Trend in ICU admissions from 2021-22 to 2023-24
Discussion
This study evaluated the impact of a VIS model on clinical practice within a rural Canadian ICU, focusing on antibiotic utilization, physiotherapy engagement, VTE prophylaxis, and interhospital transfers. The findings demonstrate that the model implementation was associated with significant changes in the antibiotic use while trends in VTE prophylaxis, PT orders, and patient transfers, though not statistically significant, revealed important implications for care delivery in resource-limited settings. Previous research supports the clinical and operational benefits of standard CCOTs [4,6] demonstrating improved survival to discharge and reduced unplanned ICU readmissions. Garcea and colleagues [10] reported a significant reduction in 30-day post-ICU mortality after CCOT rollout. While outcomes vary, studies have associated CCOTs with earlier identification of deterioration, improved management of at-risk patients, and enhanced confidence among ward staff [5,12]. However, evidence for the reduction in ICU admissions over time in our hospital is not confirmed. The higher diagnosis rate of deteriorating patients with the VIS model is a possible confounding factor. We believe a randomized controlled trial with robust sample size is needed to confirm any reduction in ICU admissions echoing available research findings.
The most prominent change observed following VIS model implementation was a significant reduction in AFD. A decrease in AFD reflects improved timeliness and appropriateness of therapy, particularly in the context of rural ICUs where early recognition and management of sepsis remain a critical challenge. Previous studies have demonstrated that delays in antimicrobial initiation correlate with increased mortality in sepsis [7,13]. By facilitating early detection of infection and supporting evidence-based clinical decision-making, VIS model may bridge knowledge and resource gaps typically encountered in non-tertiary settings [14]. However, the absence of data regarding the appropriateness, duration, or spectrum of antibiotic therapy limits the interpretation of these findings in the context of stewardship. The literature emphasizes that optimal stewardship outcomes require not only timely initiation but also appropriate de-escalation and duration control [13]. PT orders increased significantly in the post-VIS model period, consistent with the role of CCOTs in supporting early mobilization and recovery-focused ICU care. This is particularly notable in rural hospitals, where staffing constraints often delay or limit access to allied health services. The integration of physiotherapy into ICU pathways has been associated with reduced ventilator days, lower incidence of ICU-acquired weakness, and improved functional outcomes [11]. Our findings suggest that VIS models can play a critical role in activating these interventions by embedding rehabilitation considerations into daily care routines aligning with literature. For example, Burrell and colleagues [15] described how CCOTs staffed with advanced practice providers promoted timely mobilization and streamlined care planning, reducing ICU transfers and promoting continuity of care.
Although not statistically significant (OR = 0.53, p = 0.132), the post-VIS model period revealed a modest increase in VTE prophylaxis usage. This suggests potential improvements in adherence to preventive care protocols, a domain in which lapses are well documented, especially during transitions of care or in institutions with limited staff training in critical care guidelines [16]. Previous evaluations have highlighted CCOTs as facilitators of protocol compliance and systems integration [17], and our findings support the hypothesis that even in rural settings, outreach teams may reinforce such preventive measures with VIS model. The number of interhospital transfers increased in the post-intervention period (OR = 1.25, p = 0.288), though the difference was not statistically significant. Importantly, this trend may not indicate a negative outcome. Instead, it likely reflects more timely recognition of patient deterioration and proactive triage decisions, a function that is increasingly attributed to CCOTs in both urban and rural settings [15,18]. Transfers from rural hospitals are often complex, requiring coordination, stabilization, and communication with tertiary centers. In this regard, the VIS model may serve as vital intermediaries, ensuring that patients are safely and efficiently escalated to appropriate levels of care. While our study did not assess transfer-related outcomes such as mortality or length of stay, future research may incorporate these endpoints to better understand the clinical impact of increased transfers.
Limitations
We acknowledge several limitations such as 1) the study design, a retrospective-prospective design, introducing potential confounding factors like unmeasured changes in hospital policy or concurrent quality improvement initiatives; 2) the relatively short post-intervention period (eight months) may not capture seasonal trends or long-term effects; 3) outcome measures focusing on process indicators instead of clinical endpoints like mortality, ICU length of stay, or functional status post-discharge due to the length of study period; and 4) the study being a single-centered challenging comparison and generalizability.
Conclusion
This study assessed the impact of the VIS model implementation at ESHC by evaluating key clinical outcomes in the ICU and revealed a statistically significant reduction in AFD and ICU PT orders. These findings suggest a positive change in clinical management patterns, potentially reflecting altered antibiotic stewardship practices and physiotherapy engagement post-intervention. The relevance of this study lies in hospital resource allocation and clinical decision-making. Understanding the shift of care models is crucial for optimizing patient care protocols, particularly in rural and resource-limited hospital settings. Future research may explore the underlying factors contributing to these observed changes and consider incorporating additional clinical outcomes to provide a more comprehensive evaluation of VIS model efficacy. By focusing on a rural ICU setting, this study contributes novel insight to the literature, exploring the real-world utility of VIS models beyond the high-resource tertiary environments in which CCOTs have typically been studied. The findings provide evidence on the feasibility, scope, and outcomes of VIS model implementation in community settings, informing policy and operational decisions in similar hospitals across Canada and internationally.
Declarations
All authors contributed to critical revision for important intellectual content and approval of final version of the manuscript. The work has not been published before nor is it being considered for publication in another journal. Authors do not have potential conflicts of interest. The publication activity was approved by the hospital’s Office of Research’s (https://www.erieshoreshealthcare.ca/research) internal ethics committee in accord with the ethical standards on Human Experimentation of the institution in which the experiments were done or in accord with the Helsinki Declaration of 1975.
References
- Hall KK, Lim A, Gale B. Failure to rescue. In: Hall KK, Shoemaker-Hunt S, Hoffman L, et al. Making Healthcare Safer III: A Critical Analysis of Existing and Emerging Patient Safety Practices [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US) (2020).
- Wilton P, Carbery C, Farrimond J, et al. Impact of critical care outreach on cardiac arrest rates and readmission rates in an adult cardiothoracic centre. Crit Care 9 (2005): P268.
- The Faculty of Intensive Care Medicine. Guidelines for the provision of intensive care services. Ed 2 (2019).
- Ball C, Kirkby M, Williams S. Effect of the critical care outreach team on patient survival to discharge from hospital and readmission to critical care: non-randomised population-based study 327 (2003): 1014.
- Williams TA, Leslie G, Finn J, et al. Clinical effectiveness of a critical care nursing outreach service in facilitating discharge from the intensive care unit. Am J Crit Care 19 (2010): e63-e72.
- Canadian Institutes of Health Research (CIHR). Impact Story: Tapping the research potential of community hospitals (2025).
- Bhatt J, Smith B, Neuhauser MM, et al. Collaborative Solutions to Antibiotic Stewardship in Small Community and Critical Access Hospitals. Acad Med 94 (2019): 1419-1421.
- Hendryx MS, Fieselmann JF, Bock MJ, et al. Outreach education to improve quality of rural ICU care. Results of a randomized trial. Am J Respir Crit Care Med 158 (1998): 418-423.
- Jeddian A, Sayadi L, Jafari N. Critical care outreach services for caring acutely ill patients in general wards. NPT 3 (2016): 1-4.
- Garcea G, Thomasset S, McClelland L, et al. Impact of a critical care outreach team on critical care readmissions and mortality. Acta Anaesthesiol Scand 48 (2004): 1096-1100.
- Vegh LA, Blunt AM, Wishart LR, et al. Managing deteriorating patients with a physiotherapy critical care outreach service: A mixed-methods study. Aust Crit Care 36 (2023): 223-231.
- Baker-McClearn D & Carmel S. Impact of critical care outreach services on the delivery and organization of hospital care. Journal of Health Services Research & Policy 13 (2008): 132–137.
- Kaki R, Elligsen M, Walker S, et al. Impact of antimicrobial stewardship in critical care: a systematic review. J Antimicrob Chemother 66 (2011): 1223-1230.
- Rowe K, Fletcher SJ. Critical care outreach: a review of current practice and evidence. Acute Med 9 (2010): 8-12.
- Burrell E, Fitzsimmons J, Shipley K, et al. Novel Critical Care Outreach Team model improving rapid response outcomes 50 (2022): 147.
- Chicote-Álvarez E, Mainar-Gil I, Íñiguez-de Diego A, et al. Effect on the time of admission to the Intensive Care Unit of the start-up of an Critical Care Outreach Team. J Healthc Qual Res 39 (2024): 50-54.
- Losurdo HL, Cook HJ, Wells B. Developing a critical care outreach team to improve patient outcomes and promote healthy work environments (2019).
- Messina A, Pradella A, Alicino V, et al. Critical Care Outreach Team During COVID-19: Ventilatory Support in the Ward and Outcomes. Respir Care 66 (2021): 928-935.


 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 